The Clinical Relevance of Overnight Oximetry in the Diagnosis of Intermittent Desaturations and the Need for Home Oxygen in the Near-Term and Term Infant
Abstract
1. Introduction
2. Methods of the Review
3. Defining Intermittent Hypoxia
4. Diagnosis of Intermittent Hypoxia in Near-Term to Term Infants
5. Intermittent Hypoxia and Impaired Neurodevelopment in Near-Term to Term Infants
6. Current Approaches to the Management of IH in the Near-Term to Term Newborn
6.1. Home Oxygen Therapy
6.2. Caffeine Therapy for Near-Term Infants
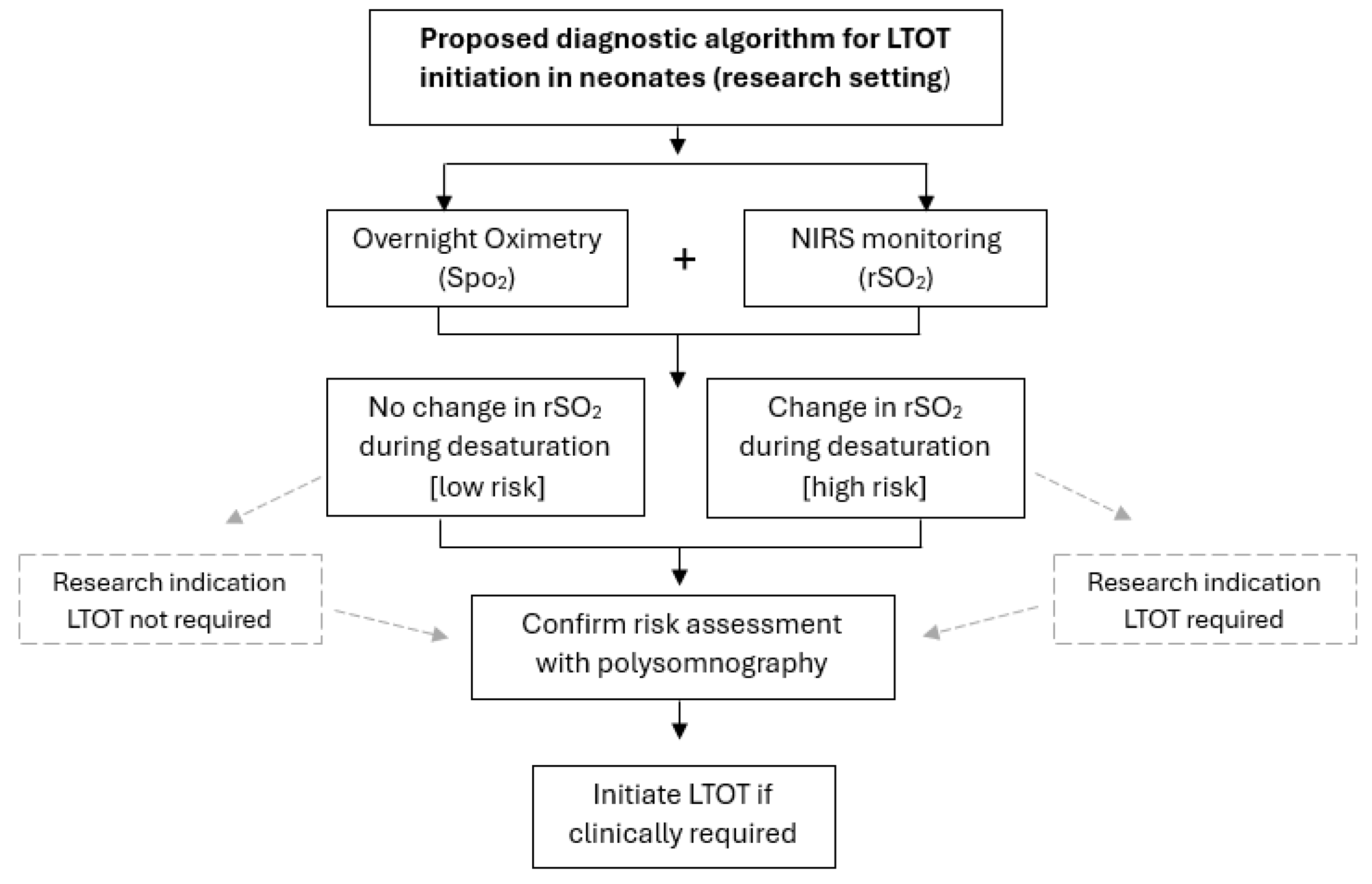
6.3. Is the Current Approach to Diagnosis of IH in the Near-Term to Term Newborn Appropriate?
7. Design Considerations for Future Clinical Studies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IH | Intermittent Hypoxia |
| SpO2 | Peripheral Arterial Oxygen Saturation |
| LTOT | Low-Flow Oxygen Therapy |
| NIRS | Near-Infrared Spectroscopy |
| REM | Rapid Eye Movement |
| EEG | Electroencephalogram |
| EOG | Electro-Oculograms |
| EMG | Electromyogram |
| ECG | Electrocardiogram |
| CHIME | Collaborative Home Infant Monitoring Evaluation |
| PMA | Postmenstrual Age |
| ASA | Australasian Sleep Association |
| OSA | Obstructive Sleep Apnoea |
| CSA | Central Sleep Apnoea |
| BSID | Bayley Scales of Infant and Toddler Development |
| MDI | Mental Developmental Index |
| NICU | Neonatal Intensive Care Unit |
| BPD | Bronchopulmonary Dysplasia |
| CAP | Caffeine for Apnoea of Prematurity |
| COT | Canadian Oxygen Trial |
| rSO2 | Cerebral Oxygen Tissue Saturation |
References
- Poets, C.F.; Stebbens, V.A.; Lang, J.A.; O’Brien, L.M.; Boon, A.W.; Southall, D.P. Arterial oxygen saturation in healthy term neonates. Eur. J. Pediatr. 1996, 155, 219–223. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; MacFarlane, P.M.; Martin, R.J. Intermittent Hypoxemia in Preterm Infants. Clin. Perinatol. 2019, 46, 553–565. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; Shah, V.; Patwardhan, A.; Sattar, A.; Wang, S.; Raffay, T.; Martin, R.J.; Jawdeh, E.G.A. Prematurity and postnatal alterations in intermittent hypoxaemia. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 557–559. [Google Scholar] [CrossRef]
- Balfour-Lynn, I.M.; Primhak, R.A.; Shaw, B.N. Home oxygen for children: Who, how and when? Thorax 2005, 60, 76–81. [Google Scholar] [CrossRef]
- Ellsbury, D.L.; Acarregui, M.J.; McGuinness, G.A.; Eastman, D.L.; Klein, J.M. Controversy surrounding the use of home oxygen for premature infants with bronchopulmonary dysplasia. J. Perinatol. 2004, 24, 36–40. [Google Scholar] [CrossRef]
- Lagatta, J.M.; Clark, R.H.; Brousseau, D.C.; Hoffmann, R.G.; Spitzer, A.R. Varying patterns of home oxygen use in infants at 23–43 weeks’ gestation discharged from United States neonatal intensive care units. J. Pediatr. 2013, 163, 976–982.e2. [Google Scholar] [CrossRef]
- Twiss, J.; Chawla, J.; Davey, M.J.; Edwards, E.A.; Elder, D.; Francis, A.; Griffiths, M.A.; Pamula, Y.; Suresh, S.; Verginis, N.; et al. Overnight oximetry for evaluating paediatric obstructive sleep apnoea: Technical specifications and interpretation guidelines. J. Paediatr. Child Health 2019, 55, 1279. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A.; Di Fiore, J.M.; Ambalavanan, N.; Bancalari, E.; Carroll, J.L.; Claure, N.; Hamvas, A.; Hibbs, A.M.; Indic, P.; Kemp, J.; et al. Pre-Vent: The prematurity-related ventilatory control study. Pediatr. Res. 2019, 85, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, K.D.; Nagraj, V.P.; Sullivan, B.A.; Moorman, J.R.; Lake, D.E. Oxygen desaturations in the early neonatal period predict development of bronchopulmonary dysplasia. Pediatr. Res. 2019, 85, 987–993. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; Bloom, J.N.; Orge, F.; Schutt, A.; Schluchter, M.; Cheruvu, V.K.; Walsh, M.; Finer, N.; Martin, R.J. higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 2010, 157, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, J.M.; Kaffashi, F.; Loparo, K.; Sattar, A.; Schluchter, M.; Foglyano, R.; Martin, R.J.; Wilson, C.G. The relationship between patterns of intermittent hypoxia and retinopathy of prematurity in preterm infants. Pediatr. Res. 2012, 72, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, J.M.; Arko, M.K.; Miller, M.J.; Krauss, A.; Betkerur, A.; Zadell, A.; Kenney, S.R.; Martin, R.J. Cardiorespiratory events in preterm infants referred for apnea monitoring studies. Pediatrics 2001, 108, 1304–1308. [Google Scholar] [CrossRef]
- Flint, A.; August, D.; Lai, M.; Chawla, J.; Ballard, E.; Davies, M.W. Determining reference data for overnight oximetry in neonates: A pilot study. Early Hum. Dev. 2022, 168, 105571. [Google Scholar] [CrossRef]
- Al-Hathlol, K.; Idiong, N.; Hussain, A.; Kwiatkowski, K.; Alvaro, R.; Weintraub, Z.; Cates, D.B.; Rigatto, H. A study of breathing pattern and ventilation in newborn infants and adult subjects. Acta. Paediatr. 2000, 89, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.J.; Karunatilleke, A.S.; Grantham-Hill, S.; Gavlak, J.C. A cohort study reporting normal oximetry values in healthy infants under 4 months of age using Masimo technology. Arch. Dis. Child. 2018, 103, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.; Corwin, M.J.; Hunt, C.E.; Lister, G.; Tinsley, L.R.; Baird, T.; Silvestri, J.M.; Crowell, D.H.; Hufford, D.; Martin, R.J.; et al. Cardiorespiratory events recorded on home monitors: Comparison of healthy infants with those at increased risk for SIDS. JAMA 2001, 285, 2199–2207. [Google Scholar] [CrossRef]
- Baddock, S.A.; Galland, B.C.; Bolton, D.P.G.; Williams, S.M.; Taylor, B.J. Hypoxic and Hypercapnic Events in Young Infants During Bed-sharing. Pediatrics 2012, 130, 237–244. [Google Scholar] [CrossRef]
- Gaultier, C. Cardiorespiratory adaptation during sleep in infants and children. Pediatr. Pulmonol. 1995, 19, 105–117. [Google Scholar] [CrossRef]
- Horne, R.S.C. Cardio-respiratory control during sleep in infancy. Paediatr. Respir. Rev. 2014, 15, 163–169. [Google Scholar] [CrossRef]
- Stark, A.R.; Cohlan, B.A.; Waggener, T.B.; Frantz, I.D., 3rd; Kosch, P.C. Regulation of end-expiratory lung volume during sleep in premature infants. J. Appl. Physiol. 1987, 62, 1117–1123. [Google Scholar] [CrossRef]
- Stothers, J.K.; Warner, R.M. Oxygen consumption and neonatal sleep states. J. Physiol. 1978, 278, 435–440. [Google Scholar] [CrossRef]
- Albani, M.; Bentele, K.H.; Budde, C.; Schulte, F.J. Infant sleep apnea profile: Preterm vs. term infants. Eur. J. Pediatr. 1985, 143, 261–268. [Google Scholar] [CrossRef]
- Williams, L.Z.J.; McNamara, D.; Alsweiler, J.M. Intermittent Hypoxemia in Infants Born Late Preterm: A Prospective Cohort Observational Study. J. Pediatr. 2019, 204, 89–95.e1. [Google Scholar] [CrossRef]
- Vesoulis, Z.A.; Lust, C.E.; Liao, S.M.; Trivedi, S.B.; Mathur, A.M. Early hyperoxia burden detected by cerebral near-infrared spectroscopy is superior to pulse oximetry for prediction of severe retinopathy of prematurity. J. Perinatol. 2016, 36, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Vesoulis, Z.A.; Mintzer, J.P.; Chock, V.Y. Neonatal NIRS monitoring: Recommendations for data capture and review of analytics. J. Perinatol. 2021, 41, 675–688. [Google Scholar] [CrossRef]
- Kyriacou, P.A.; Charlton, P.H.; Al-Halawani, R.; Shelley, K.H. Inaccuracy of pulse oximetry with dark skin pigmentation: Clinical implications and need for improvement. Br. J. Anaesthesia 2023, 130, e33–e36. [Google Scholar] [CrossRef]
- Tin, W.; Lal, M. Principles of pulse oximetry and its clinical application in neonatal medicine. Semin. Fetal Neonatal Med. 2015, 20, 192–197. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Manja, V.; Mathew, B.; Suresh, G.K. Oxygen targeting in preterm infants: A physiological interpretation. J. Perinatol. 2015, 35, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.D. Cerebral oximetry monitoring with near infrared spectroscopy detects alterations in oxygenation before pulse oximetry. J. Intensive Care. Med. 2008, 23, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Davies, M.W. The use of overnight oximetry in neonates: A literature review. J. Paediatr. Child Health 2018, 54, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.; Nixon, G.; Robinson, P.; Massie, J.; Prentice, B.; Wilson, A.; Schilling, S.; Twiss, J.; Fitzgerald, D.A. Respiratory management of infants with chronic neonatal lung disease beyond the NICU: A position statement from the Thoracic Society of Australia and New Zealand. Respirology 2020, 25, 880–888. [Google Scholar] [CrossRef]
- Askie, L.M.; Henderson-Smart, D.J. Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2001, 4, CD001077. [Google Scholar]
- Askie, L.M.; Darlow, B.A.; Finer, N.; Schmidt, B.; Stenson, B.; Tarnow-Mordi, W.; Davis, P.G.; Carlo, W.A.; Brocklehurst, P.; Davies, L.C.; et al. Association Between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. JAMA. 2018, 319, 2190–2201. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.S.; Hakak, H.; Mohamed, A.; Shah, J.; Young, J.; Kelly, E. Oxygen saturation profile in late-preterm and term infants: A prospective cohort study. J. Perinatol. 2014, 34, 917–920. [Google Scholar] [CrossRef]
- Hunt, C.E.; Corwin, M.J.; Weese-Mayer, D.E.; Ward, S.L.; Ramanathan, R.; Lister, G.; Tinsley, L.R.; Heeren, T.; Rybin, D.; Collaborative Home Infant Monitoring Evaluation (CHIME) Study Group. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. J. Pediatr. 2011, 159, 377–383.e1. [Google Scholar] [CrossRef]
- Hunt, C.E.; Corwin, M.J.; Lister, G.; Weese-Mayer, D.E.; Neuman, M.R.; Tinsley, L.; Baird, T.M.; Keens, T.G.; Cabral, H.J. Longitudinal assessment of hemoglobin oxygen saturation in healthy infants during the first 6 months of age. Collaborative Home Infant Monitoring Evaluation (CHIME) Study Group. J. Pediatr. 1999, 135, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, J.M.; Raffay, T.M. The relationship between intermittent hypoxemia events and neural outcomes in neonates. Exp. Neurol. 2021, 342, 113753. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Janvier, A.; Khairy, M.; Kokkotis, A.; Cormier, C.; Messmer, D.; Barrington, K.J. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J. Perinatol. 2004, 24, 763–768. [Google Scholar] [CrossRef]
- Pillekamp, F.; Hermann, C.; Keller, T.; von Gontard, A.; Kribs, A.; Roth, B. Factors influencing apnea and bradycardia of prematurity-implications for neurodevelopment. Neonatology 2007, 91, 155–161. [Google Scholar] [CrossRef]
- Taylor, H.G.; Klein, N.; Schatschneider, C.; Hack, M. Predictors of early school age outcomes in very low birth weight children. J. Dev. Behav. Pediatr. 1998, 19, 235–243. [Google Scholar] [CrossRef]
- Greene, M.M.; Patra, K.; Khan, S.; Karst, J.S.; Nelson, M.N.; Silvestri, J.M. Cardiorespiratory events in extremely low birth weight infants: Neurodevelopmental outcome at 1 and 2 years. J. Perinatol. 2014, 34, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Poets, C.F.; Roberts, R.S.; Schmidt, B.; Whyte, R.K.; Asztalos, E.V.; Bader, D.; Bairam, A.; Moddemann, D.; Peliowski, A.; Rabi, Y.; et al. Association Between Intermittent Hypoxemia or Bradycardia and Late Death or Disability in Extremely Preterm Infants. JAMA 2015, 314, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.E.; Corwin, M.J.; Baird, T.; Tinsley, L.R.; Palmer, P.; Ramanathan, R.; Crowell, D.H.; Schafer, S.; Martin, R.J.; Hufford, D.; et al. Cardiorespiratory events detected by home memory monitoring and one-year neurodevelopmental outcome. J. Pediatr. 2004, 145, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Piteo, A.M.; Kennedy, J.D.; Roberts, R.M.; Martin, A.J.; Nettelbeck, T.; Kohler, M.J.; Lushington, K. Snoring and cognitive development in infancy. Sleep. Med. 2011, 12, 981–987. [Google Scholar] [CrossRef]
- Montgomery-Downs, H.E.; Gozal, D. Snore-associated sleep fragmentation in infancy: Mental development effects and contribution of secondhand cigarette smoke exposure. Pediatrics 2006, 117, e496–e502. [Google Scholar] [CrossRef]
- Smith, C.B.; Walker, K.; Badawi, N.; Waters, K.A.; MacLean, J.E. Impact of sleep and breathing in infancy on outcomes at three years of age for children with cleft lip and/or palate. Sleep 2014, 37, 919–925. [Google Scholar] [CrossRef]
- Don Hayes, J.; Wilson, K.C.; Krivchenia, K.; Hawkins, S.M.M.; Balfour-Lynn, I.M.; Gozal, D.; Panitch, H.B.; Splaingard, M.L.; Rhein, L.M.; Kurland, G.; et al. Home Oxygen Therapy for Children. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2019, 199, e5–e23. [Google Scholar] [CrossRef]
- Zysman-Colman, Z.; Tremblay, G.M.; Bandeali, S.; Landry, J.S. Bronchopulmonary dysplasia-trends over three decades. Paediatr. Child Health 2013, 18, 86–90. [Google Scholar] [CrossRef]
- Dassios, T.; Williams, E.E.; Hickey, A.; Greenough, A. Duration of mechanical ventilation and prediction of bronchopulmonary dysplasia and home oxygen in extremely preterm infants. Acta. Paediatr. 2021, 110, 2052–2058. [Google Scholar] [CrossRef]
- Chow, S.S.W.; Creighton, P.; Chambers, G.M.; Lui, K. Report of the Australian and New Zealand Neonatal Network; Australian and New Zealand Neonatal Network: Sydney, Australia, 2022. [Google Scholar]
- Bird, T.M.; Bronstein, J.M.; Hall, R.W.; Lowery, C.L.; Nugent, R.; Mays, G.P. Late preterm infants: Birth outcomes and health care utilization in the first year. Pediatrics. 2010, 126, e311–e319. [Google Scholar] [CrossRef] [PubMed]
- Ghirardo, S.; Amaddeo, A.; Griffon, L.; Khirani, S.; Fauroux, B. Central apnea and periodic breathing in children with underlying conditions. J. Sleep Res. 2021, 30, e13388. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.C.; Jang, J.; Rhein, L.M. Apnea in the Otherwise Healthy, Term Newborn: National Prevalence and Utilization during the Birth Hospitalization. J. Pediatr. 2017, 181, 67–73.e1. [Google Scholar] [CrossRef]
- Patrinos, M.E.; Martin, R.J. Apnea in the term infant. Semin. Fetal Neonatal Med. 2017, 22, 240–244. [Google Scholar] [CrossRef]
- Xiao, L.; Sunkonkit, K.; Chiang, J.; Narang, I. Unexplained Significant Central Sleep Apnea in Infants: Clinical Presentation and Outcomes. Sleep Breath. 2023, 27, 255–264. [Google Scholar] [CrossRef]
- Urquhart, D. Investigation and management of childhood sleep apnoea. Hippokratia 2013, 17, 196–202. [Google Scholar] [PubMed]
- Hayashi, A.; Suresh, S.; Kevat, A.; Robinson, J.; Kapur, N. Central sleep apnea in otherwise healthy term infants. J. Clin. Sleep Med. 2022, 18, 2813–2817. [Google Scholar] [CrossRef]
- Ali, S.K.M.; Mohammed, N.; Qureshi, N.; Gupta, S. Oxygen therapy in preterm infants: Recommendations for practice. Paediatr. Child Health 2021, 31, 1–6. [Google Scholar] [CrossRef]
- STOP-ROP Multicenter Study Group. Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP-ROP), a randomized, controlled trial. I: Primary outcomes. Pediatrics 2000, 105, 295–310. [Google Scholar] [CrossRef]
- Jobe, A.H.; Kallapur, S.G. Long term consequences of oxygen therapy in the neonatal period. Semin. Fetal Neonatal Med. 2010, 15, 230–235. [Google Scholar] [CrossRef]
- Pirr, S.; Peter, C. Home oxygen therapy after hospital discharge. Semin. Fetal Neonatal Med. 2020, 25, 101082. [Google Scholar] [CrossRef]
- McAndrew, S.; Acharya, K.; Westerdahl, J.; Brousseau, D.C.; Panepinto, J.A.; Simpson, P.; Leuthner, J.; Lagatta, J.M. A Prospective Study of Parent Health-Related Quality of Life before and after Discharge from the Neonatal Intensive Care Unit. J. Pediatr. 2019, 213, 38–45.e3. [Google Scholar] [CrossRef]
- Solis, A.; Harrison, G.; Shaw, B.N. Assessing oxygen requirement after discharge in chronic lung disease: A survey of current practice. Eur. J. Pediatr. 2002, 161, 428–430. [Google Scholar] [CrossRef]
- Saletti, A.; Stick, S.; Doherty, D.; Simmer, K. Home oxygen therapy after preterm birth in Western Australia. J. Paediatr. Child Health 2004, 40, 519–523. [Google Scholar] [CrossRef]
- Simoes, E.A.; Rosenberg, A.A.; King, S.J.; Groothuis, J.R. Room air challenge: Prediction for successful weaning of oxygen-dependent infants. J. Perinatol. 1997, 17, 125–129. [Google Scholar]
- Thoracic Society of Australia and New Zealand; Fitzgerald, D.A.; Massie, R.J.H.; Nixon, G.M.; Jaffe, A.; Wilson, A.; Landau, L.I.; Twiss, J.; Smith, G.; Wainwright, C.; et al. Infants with chronic neonatal lung disease: Recommendations for the use of home oxygen therapy. Med. J. Aust. 2008, 189, 578–582. [Google Scholar] [CrossRef]
- Mitchell, L.; MacFarlane, P.M. Mechanistic actions of oxygen and methylxanthines on respiratory neural control and for the treatment of neonatal apnea. Respir. Physiol. Neurobiol. 2020, 273, 103318. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.; Pohl, M.; Hummler, H.D.; Schmid, M.B. Cerebral oxygenation and desaturations in preterm infants-a longitudinal data analysis. J. Neonatal Perinat. Med. 2017, 10, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W.; Caffeine for Apnea of Prematurity Trial Group. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2007, 357, 1893–1902. [Google Scholar] [PubMed]
- He, X.; Qiu, J.C.; Lu, K.Y.; Guo, H.L.; Li, L.; Jia, W.W.; Ni, M.-M.; Liu, Y.; Xu, J.; Chen, F.; et al. Therapy for Apnoea of Prematurity: A Retrospective Study on Effects of Standard Dose and Genetic Variability on Clinical Response to Caffeine Citrate in Chinese Preterm Infants. Adv. Ther. 2021, 38, 607–626. [Google Scholar] [CrossRef]
- Moschino, L.; Zivanovic, S.; Hartley, C.; Trevisanuto, D.; Baraldi, E.; Roehr, C.C. Caffeine in preterm infants: Where are we in 2020? ERJ Open Res. 2020, 6, 00330-2019. [Google Scholar] [CrossRef] [PubMed]
- Saroha, V.; Patel, R.M. Caffeine for preterm infants: Fixed standard dose, adjustments for age or high dose? Semin. Fetal Neonatal Med. 2020, 25, 101178. [Google Scholar] [CrossRef]
- Grainge, S.; Nair, V.; Kannan Loganathan, P. National survey on caffeine use in neonatal units across the United Kingdom. Acta Paediatr. 2023, 112, 1865–1869. [Google Scholar] [CrossRef]
- Schmidt, B.; Anderson, P.J.; Doyle, L.W.; Dewey, D.; Grunau, R.E.; Asztalos, E.V.; Davis, P.G.; Tin, W.; Moddemann, D.; Solimano, A.; et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 2012, 307, 275–282. [Google Scholar] [CrossRef]
- Rhein, L.M.; Dobson, N.R.; Darnall, R.A.; Corwin, M.J.; Heeren, T.C.; Poets, C.F.; McEntire, B.L.; Hunt, C.E.; Caffeine Pilot Study Group. Effects of caffeine on intermittent hypoxia in infants born prematurely: A randomized clinical trial. JAMA Pediatr. 2014, 168, 250–257. [Google Scholar] [CrossRef]
- Oliphant, E.A.; McKinlay, C.J.; McNamara, D.; Cavadino, A.; Alsweiler, J.M. Caffeine to prevent intermittent hypoxaemia in late preterm infants: Randomised controlled dosage trial. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 106–113. [Google Scholar] [CrossRef]
- Queensland Health (Ed.). Queensland Clinical Guidelines: Neonatal monograph Caffeine Citrate. In Clinical Excellence Queensland; Queensland Health, Queensland Government: Queensland, Australia, 2023; p. 3. [Google Scholar]
- Ramsey, R.; Mehra, R.; Strohl, K.P. Variations in physician interpretation of overnight pulse oximetry monitoring. Chest 2007, 132, 852–859. [Google Scholar] [CrossRef]
- Cheung, A.; Tu, L.; Macnab, A.; Kwon, B.K.; Shadgan, B. Detection of hypoxia by near-infrared spectroscopy and pulse oximetry: A comparative study. J. Biomed. Opt. 2022, 27, 077001. [Google Scholar] [CrossRef]
- Pellicer, A.; Bravo, C. Near-infrared spectroscopy: A methodology-focused review. Semin. Fetal Neonatal Med. 2011, 16, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Marin, T.; Moore, J. Understanding near-infrared spectroscopy. Adv. Neonatal Care 2011, 11, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Sood, B.G.; McLaughlin, K.; Cortez, J. Near-infrared spectroscopy: Applications in neonates. Semin. Fetal Neonatal Med. 2015, 20, 164–172. [Google Scholar] [CrossRef]
- Ozawa, J.; Watanabe, T.; Ito, M.; Miyake, F.; Nagano, N.; Ogawa, R.; Matsumura, S.; Araki, R.; Tamura, M.; Namba, F. Defining the reference range of regional cerebral tissue oxygen saturation using a new portable near-infrared spectroscopy device for term infants. Early Hum. Dev. 2020, 141, 104941. [Google Scholar] [CrossRef]
- Kato, R.; Hosono, S.; Takahashi, S. Reference Value of Brain Tissue Oxygen Saturation in Newborns Immediately After Birth. Adv. Exp. Med. Biol. 2020, 1232, 19–24. [Google Scholar]
- Pichler, G.; Binder, C.; Avian, A.; Beckenbach, E.; Schmölzer, G.M.; Urlesberger, B. Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J. Pediatr. 2013, 163, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Bernal, N.P.; Hoffman, G.M.; Ghanayem, N.S.; Arca, M.J. Cerebral and somatic near-infrared spectroscopy in normal newborns. J. Pediatr. Surg. 2010, 45, 1306–1310. [Google Scholar] [CrossRef]
- Carnicero, L.B.; Carbonero, S.C. Reference Ranges for Regional Cerebral Oxygen Saturation with Masimo O3 after Birth and Differences with Other Devices. Am. J. Perinatol. 2024, 41, 1736–1742. [Google Scholar] [CrossRef]
- Lemmers, P.M.; Toet, M.C.; van Bel, F. Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics 2008, 121, 142–147. [Google Scholar] [CrossRef]
- Seidel, D.; Bläser, A.; Gebauer, C.; Pulzer, F.; Thome, U.; Knüpfer, M. Changes in regional tissue oxygenation saturation and desaturations after red blood cell transfusion in preterm infants. J. Perinatol. 2013, 33, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Davie, S.N.; Grocott, H.P. Impact of extracranial contamination on regional cerebral oxygen saturation: A comparison of three cerebral oximetry technologies. Anesthesiology 2012, 116, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.; Diggs, S.; Pfeiffer, M.; Gerst, A.; Brumfiel, A.; Vesoulis, Z. Racial Variation in Cerebral Near Infrared Spectroscopy Accuracy Among Infants in a Cardiac Intensive Care Unit. medRxiv 2025. [Google Scholar] [CrossRef]
- Rodríguez, M.J.; Corredera, A.; Martínez-Orgado, J.; Arruza, L. Interference between cerebral NIRS and conjugated bilirubin in extremely low birth weight neonates. An. Pediatr. (Eng. Ed.) 2021, 95, 371–373. [Google Scholar] [CrossRef]
- Yoshitani, K.; Kawaguchi, M.; Miura, N.; Okuno, T.; Kanoda, T.; Ohnishi, Y.; Kuro, M. Effects of Hemoglobin Concentration, Skull Thickness, and the Area of the Cerebrospinal Fluid Layer on Near-infrared Spectroscopy Measurements. Anesthesiology 2007, 106, 458–462. [Google Scholar] [CrossRef]
- Ostojic, D.; Jiang, J.; Isler, H.; Kleiser, S.; Karen, T.; Wolf, M.; Scholkmann, F. Impact of Skull Thickness on Cerebral NIRS Oximetry in Neonates: An in silico Study. In Oxygen Transport to Tissue XLI; Ryu, P.-D., LaManna, J.C., Harrison, D.K., Lee, S.-S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 33–38. [Google Scholar]
- Demel, A.; Feilke, K.; Wolf, M.; Poets, C.F.; Franz, A.R. Correlation between skin, bone, and cerebrospinal fluid layer thickness and optical coefficients measured by multidistance frequency-domain near-infrared spectroscopy in term and preterm infants. J. Biomed. Opt. 2014, 19, 17004. [Google Scholar] [CrossRef]
- Kishi, K.; Kawaguchi, M.; Yoshitani, K.; Nagahata, T.; Furuya, H. Influence of Patient Variables and Sensor Location on Regional Cerebral Oxygen Saturation Measured by INVOS 4100 Near-Infrared Spectrophotometers. J. Neurosurg. Anesthesiol. 2003, 15, 302–306. [Google Scholar] [CrossRef]
- Eichhorn, L.; Erdfelder, F.; Kessler, F.; Doerner, J.; Thudium, M.O.; Meyer, R.; Ellerkmann, R.K. Evaluation of near-infrared spectroscopy under apnea-dependent hypoxia in humans. J. Clin. Monit. Comput. 2015, 29, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Ullman, N.; Anas, N.G.; Izaguirre, E.; Haugen, W.; Ortiz, H.; Arguello, O.; Nickerson, B.; Mink, R.B. Usefulness of cerebral NIRS in detecting the effects of pediatric sleep apnea. Pediatr. Pulmonol. 2014, 49, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
| Article | Aim | Sample | Methods | Main Findings | Strengths and Limitations |
|---|---|---|---|---|---|
| Poets et al. (1996) [1] | Determining oxygen saturation levels for healthy term infants in the first four weeks of life. | Healthy term infants > 37 weeks’ gestation. n = 60. | Systemic sampling procedure. Nellcore pulse oximeter (Nellcore Inc., Hayward, CA, USA). | Median baseline SpO2, measured during regular breathing was 97.6% (range 92–100) during week 1 versus 98.0% (86.6–100) during week 2–4. | Sample included healthy term infants (2 weeks–24 months). Recording performed in nursey and home environment. Did not collect data on sleep/wake periods. Data limited to device used. |
| Askie et al. (2018) [33] | To compare the effects of different pulse oximeter oxygen saturation target ranges on death or major morbidity. | Infants born < 28 weeks’ gestation. n = 4965. | Prospectively planned individual participant data meta-analysis of five randomised clinical trials (conducted 2005–2014). Masimo pulse oximeter. | Saturation target ranges showed no difference in the primary composite outcome (death or major disability at 18–24 months). Higher targets reduced death and NEC, while lower targets reduced ROP. | Meta-analysis, prospective five trial design. Large sample size. Two trials stopped early. Relatively short follow-up. Device specificity. |
| Shah et al. (2014) [34] | Determine oxygen saturation profile of healthy late-preterm and term neonates between 12 and 48 h after birth. | Full term (>37 weeks’ gestation) and late-preterm (35–36 weeks’ gestation) infants between 12 and 48 h postnatal age. n = 40 term infants. n = 20 late-preterm infants. | Prospective cohort study. 6 h monitoring period using Nellcor OxiMax N-600X pulse oximeter (Covidien, Mansfield, MA, USA) and PROFOX software. | Late-preterm infants spent an average 7% of the time and term infants an average of 4% of the time with SpO2 ≤ 90%. | Sample included term infants. Small sample size. Device specificity. |
| Hunt et al. (2011) [35] | Report longitudinal home recordings of haemoglobin O2 saturation by pulse oximetry during sleep in preterm and term infants. | Data from CHIME study. Preterm infants < 1750 g and <35 weeks PMA and healthy term infants. n = 103 preterm. n = 99 term. | Longitudinal cohort study. Ohmeda Minx pulse oximeter (Ohmeda Corp, Liberty Center, New Jersey, USA). | Median baseline SpO2 was approximately 98% for both the preterm and term groups. | Multicentre study (five clinical sites). Only 3 min recordings from each hour. Movement artefact was present in 41% of recordings. Device specificity. |
| Hunt et al. (1999) [36] | Describe SpO2 in the first 25 postnatal weeks and assess the relationships among SpO2, breathing pattern, heart rate, and sleep position in healthy term infants. | Healthy term infants enrolled in the CHIME study born at 38–42 weeks’ gestation, ≤30 days at enrolment. n = 64 study group. n = 150 healthy term infants. | Longitudinal cohort study. Ohmeda Minx pulse oximeter (Ohmeda Corp, Liberty Center, New Jersey, USA). | Median baseline SpO2 was 97.9%. Acute decreases in SpO2 occurred in 59% of infants, and are correlated with younger age, periodic breathing and apnoea appear to be part of normal breathing and oxygenation behaviour. | Multicentre study (five clinical sites). Term infants included in sample. Only 3 min recordings from each hour. Device specificity. |
| Evans et al. (2018) [15] | To determine sleeping saturation indices in healthy infants using a modern pulse oximeter with motion artefact extraction technology. | Healthy term infants (>37 weeks’ gestation). n = 45. | Prospective cohort study. Nocturnal pulse oximetry at home at 1 month of age and repeated at age 3–4 months. Masimo Rad-8 Oximeter (USA). | Mean oxygen saturation at 1 month is 97.05% (96.59 to 97.52) and 97.65% (97.19 to 98.12) at 3–4 months. Desaturation indices are higher in young infants than older children, and decrease by 3–4 months of age but are still higher than older children. | First to report both 4% and 3% desaturation indices in young infants. Parent-led in the home environment. Sleep/wake interpretation is limited. 4 h minimum recordings. Loss of follow-up. Device specificity. |
| Article | Aim | Sample | Methods | Main Findings | Strengths and Limitations |
|---|---|---|---|---|---|
| Poets et al. (2015) [43] | To determine the association between intermittent hypoxemia or bradycardia and late death or disability. | Infants born 23–27+6 weeks’ gestational age who survived to postmenstrual age of 36 weeks. n = 1019. | Post hoc analysis of data from the inception cohort assembled for the Canadian Oxygen Trial. Bayley Scales of Infant and Toddler Development III at corrected 18 months. Masimo pulse oximeter. | Prolonged hypoxemic episodes (at least 1 min) during the first 2 to 3 months after birth were associated with adverse 18-month outcomes (late death or disability). | 16 s averaging time. Post hoc design. Socioeconomic variables not considered. Device specificity. |
| Hunt et al. (2004) [44] | To determine if infants with cardiorespiratory events detected by home memory monitoring during early infancy have decreased neurodevelopmental performance at 1 year of age. | Data from CHIME study. Preterm infants < 1750 g and ≤34 weeks healthy term infants. n = 138 term. n = 118 preterm. | Home monitoring of cardiorespiratory events. Bayley Scales of Infant Development II at 92 weeks’ post-conceptual age. Ohmeda Minx pulse oximeter(Ohmeda Corp, Liberty Center, New Jersey, USA). | Having 5+ conventional events is associated with lower adjusted mean differences in MDI in term and preterm infants. | Multicentre study (five clinical sites). Interrater reliability tested. Sample includes term infants. The influence of demographic factors on low follow-up rates. Home monitoring limitations. Event thresholds may miss clinically relevant desaturations. Device specificity. |
| Piteo et al. (2011) [45] | To assess the influence of snoring and sleep duration on developmental outcomes in 6 month old infants. | Infants aged between 0 and 3 months. Non-snoring controls. Snored within the first month of life and continued until 6 months of age. n = 88 controls. n = 16 snoring infants. | Longitudinal cohort study. Parental-reported snoring. Bayley Scales of Infant and Toddler Development Edition III at 6 months of age. | Snoring during the first 6 months of life was associated with lower cognitive development scores. | Previous protocol lists term infants as sample. Socioeconomic variables analysed. Gestational age at birth not recorded. Parental-reported snoring. Degree of sleep disturbances or desaturations not reported. Small sample of snoring infants. |
| Montgomery-Downs et al. (2006) [46] | To test the potential association between snoring and developmental performance among infants. | Healthy infants (8.2 ± 0.4 months), born 38.8 (1.5) weeks’ gestational age. n = 35. | Longitudinal prospective cohort study. Initial screening survey completed over 6 sites. Bayley Scales of Infant Development II and polysomnography at 8 months of age. Arousals were scored manually defined by the American Sleep Disorders Association Task Force report. | In 8 month old infants, snoring without apnoea or hypopnoea is linked to lower MDI scores when it leads to sleep fragmentation, and is exacerbated by exposure to cigarette smoke. | Gold-standard polysomnography. Correlated against exposure to environmental smoke. Small sample size, limits generalisability. First survey response rate is unknown. |
| Smith et al. (2014) [47] | To evaluate the relationship between sleep-disordered breathing in early infancy and outcomes at 3 years of age in children with cleft lip and/or palate. | Children with cleft lip and/or palate. Healthy term controls (from a previously published sample). n = 33 infants with cleft lip and/or palate. n = 156 controls. | Longitudinal prospective cohort study. Diagnostic polysomnography at infancy. Bayley Scales of Infant and Toddler Development III at 3 year follow- up. | Polysomnography in infancy was correlated with outcomes at 3 years of age; lower percentage of AS/REM sleep was associated with lower cognition score; more obstructive events were associated with lower global behaviour ITQOL score; and higher number of respiratory events in infancy was associated with reduced weight z-score. | Extended follow-up (3 years). Results only generalisable to infants with clef lip and/or palate. Possible selection bias. Males overrepresented in follow-up cohort. |
| 1 | SpO2 is to be maintained within age-appropriate targets over time. |
| 2 | SpO2 should be assessed by overnight oximetry in the days prior to discharge and have the first clinical review in a 4–6-week window. |
| 3 | Thereafter, SpO2 is recommended to be reassessed on a 4–8 week basis to ensure adequacy of the supplemental oxygen and allow weaning or increase as appropriate. |
| 4 | Twenty four hour oxygen therapy is usually recommended. Some older infants may not tolerate daytime supplemental oxygen and compromising to sleep oxygen therapy may be appropriate to maximise the number of hours per day at target SpO2. |
| 5 | Supplemental oxygen for infants is usually prescribed in steps including 0.5 (1/2), 0.25 (1/4), and 0.125 (1/8) L/min. In some centres, lower flow rates may be available and appropriately utilised. |
| 6 | In general, an overnight oximetry study should be performed on the current prescription first. If a previous oximetry on this flow was recent and found to be at or above target, it may be appropriate to go straight to an assessment on a lower prescription. |
| 7 | Whilst SpO2 targets are the main guide, notes should also be taken of the infant’s overall clinical picture including work of breathing, feeding, growth, and any co-morbid cardiac disease. |
| 8 | Oxygen can be discontinued when target saturation is achieved without it. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noone, A.N.; Andersen, C.C.; Crawford, T.M.; Stark, M.J. The Clinical Relevance of Overnight Oximetry in the Diagnosis of Intermittent Desaturations and the Need for Home Oxygen in the Near-Term and Term Infant. Children 2025, 12, 1341. https://doi.org/10.3390/children12101341
Noone AN, Andersen CC, Crawford TM, Stark MJ. The Clinical Relevance of Overnight Oximetry in the Diagnosis of Intermittent Desaturations and the Need for Home Oxygen in the Near-Term and Term Infant. Children. 2025; 12(10):1341. https://doi.org/10.3390/children12101341
Chicago/Turabian StyleNoone, Amelia N., Chad C. Andersen, Tara M. Crawford, and Michael J. Stark. 2025. "The Clinical Relevance of Overnight Oximetry in the Diagnosis of Intermittent Desaturations and the Need for Home Oxygen in the Near-Term and Term Infant" Children 12, no. 10: 1341. https://doi.org/10.3390/children12101341
APA StyleNoone, A. N., Andersen, C. C., Crawford, T. M., & Stark, M. J. (2025). The Clinical Relevance of Overnight Oximetry in the Diagnosis of Intermittent Desaturations and the Need for Home Oxygen in the Near-Term and Term Infant. Children, 12(10), 1341. https://doi.org/10.3390/children12101341





