TPMT and HLA-DQ Allelic Variants in Relation to Drug Response, Safety and Need for Therapy Optimization in Pediatric Inflammatory Bowel Disease
Abstract
Highlights
- Pharmacogenetic variants shape treatment response to azathioprine and anti-TNF agents in pediatric IBD.
- Genetic differences influence both efficacy and risk of adverse events.
- Evidence remains limited and heterogeneous.
- Pharmacogenetics paves the way for personalized IBD therapy in children, but further clinical validation is still required.
Abstract
1. Introduction
1.1. Pharmacogenetics of Azathioprine
1.2. Pharmacogenetics of Infliximab and Adalimumab
2. Materials and Methods
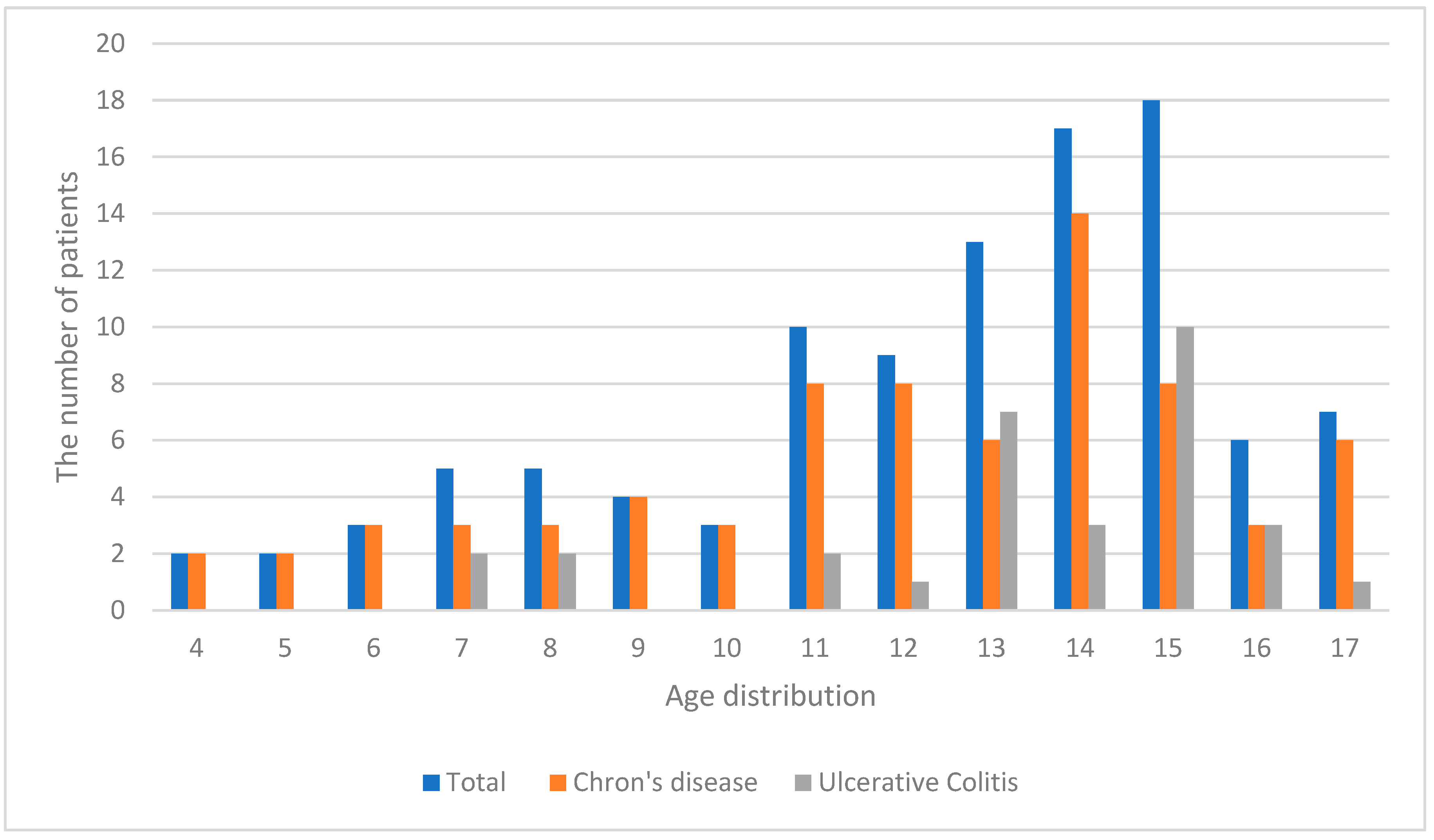
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | Crohn’s disease |
| CPIC | Clinical Pharmacogenetics Implementation Consortium |
| DNA | Deoxyribonucleic Acid |
| FDA | Food and Drug Administration |
| GIT | Gastrointestinal Tract |
| HLA | Human Leukocyte Antigen |
| IBD | Inflammatory Bowel Disease |
| PANTS | Personalizing Anti-TNF Therapy in Crohn’s Disease Study |
| RNA | Ribonucleic Acid |
| TNF | Tumor Necrosis Factor |
| TPMT | Thiopurine Methyltransferase |
| UC | Ulcerative Colitis |
References
- Oates, J.T.; Lopez, D. Pharmacogenetics: An Important Part of Drug Development with A Focus on Its Application. Int. J. Biomed. Investig. 2018, 1, 111. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Adamji, M.; Day, A.S. An overview of the role of exclusive enteral nutrition for complicated Crohn’s disease. Intest. Res. 2019, 17, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Stocco, G.; Pelin, M.; Franca, R.; De Iudicibus, S.; Cuzzoni, E.; Favretto, D.; Martelossi, S.; Ventura, A.; Decorti, G. Pharmacogenetics of azathioprine in inflammatory bowel disease: A role for glutathione-S-transferase? World J. Gastroenterol. 2014, 20, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, T.; Paradowska-Gorycka, A. Pharmacogenomics of Anti-TNF Treatment Response Marks a New Era of Tailored Rheumatoid Arthritis Therapy. Int. J. Mol. Sci. 2022, 23, 2366. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.E. Pharmacogenomics of azathioprine and 6-mercaptopurine in gastroenterologic therapy. Rev. Gastroenterol. Disord. 2003, 3, 150–157. [Google Scholar] [PubMed]
- Relling, M.V.; Schwab, M.; Whirl-Carillo, M.; Suarez-Kurtz, G.; Pui, C.H.; Stein, C.M.; Moyer, A.M.; Evans, W.E.; Klein, T.E.; Antillon-Klussmann, F.G.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin. Pharmacol. Ther. 2019, 105, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.; Stachelski, J.; Dervieux, T.; Dubinsky, M. Failure to Achieve Target Drug Concentrations During Induction and Not HLA-DQA1*05 Carriage Is Associated with Antidrug Antibody Formation in Patients with Inflammatory Bowel Disease. Gastroenterology 2022, 162, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Bosca-Watts, M.M.; Minguez, M.; Planelles, D.; Navarro, S.; Rodriguez, A.; Santiago, J.; Tosca, J.; Mora, F. HLA-DQ: Celiac disease vs inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Fink, L. The Human Genome Project. Alcohol Health Res. World 1995, 19, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Luna, M.; Maté, J.; González-Guijarro, L.; Cara, C.; Pajares, J.M. Choice of azathioprine or 6-mercaptopurine dose based on thiopurine methyltransferase (TPMT) activity to avoid myelosuppression. A prospective study. Hepatogastroenterology 2006, 53, 399–404. [Google Scholar] [PubMed]
- Schwab, M.; Schäffeler, E.; Marx, C.; Fischer, C.; Lang, T.; Behrens, C.; Gregor, M.; Eichelbaum, M.; Zanger, U.M.; Kaskas, B.A.; et al. Azathioprine therapy and adverse drug reactions in patients with inflammatory bowel disease: Impact of thiopurine S-methyltransferase polymorphism. Pharmacogenetics 2002, 12, 429–436. [Google Scholar] [CrossRef]
- Tavano, F.; Palmieri, O.; Latiano, M.; Gioffreda, D.; Latiano, T.; Guerra, M.; Martino, G.; Valvano, M.R.; Bossa, F.; Perri, F.; et al. Examination of the TPMT and NUDT15*3 Variants to Predict the Response to Thiopurines in an Italian Cohort of Patients with Inflammatory Bowel Disease. Int. J. Mol. Sci. 2025, 26, 7860. [Google Scholar] [CrossRef] [PubMed]
- Sazonovs, A.; Kennedy, N.A.; Moutsianas, L.; Heap, G.A.; Rice, D.L.; Reppell, M.; Bewshea, C.M.; Chanchlani, N.; Walker, G.J.; Perry, M.H.; et al. HLA-DQA1*05 Carriage Associated with Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients with Crohn’s Disease. Gastroenterology 2020, 158, 189–199. [Google Scholar] [CrossRef]
- Davis González, M.R.; Ballester, M.P.; Romero-González, E.; Sánchez-Pardo, A.M.; Marti-Aguado, D.; Tosca, J.; Suria, C.; Ausejo, R.A.; Moreno, I.P.; Planelles Silvestre, M.D.; et al. Biological treatment interruption in inflammatory bowel disease: Motivation and predictive factors. Gastroenterol. Hepatol. 2023, 46, 671–681. [Google Scholar] [CrossRef] [PubMed]
- DelBaugh, R.M.; Cook, L.J.; Siegel, C.A.; Tsongalis, G.J.; Khan, W.A. Validation of a rapid HLA-DQA1*05 pharmacogenomics assay to identify at-risk resistance to anti-tumor necrosis factor therapy among patients with inflammatory bowel disease. Am. J. Clin. Pathol. 2023, 160, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Lauro, R.; Mannino, F.; Irrera, N.; Squadrito, F.; Altavilla, D.; Squadrito, G.; Pallio, G.; Bitto, A. Pharmacogenetics of Biological Agents Used in Inflammatory Bowel Disease: A Systematic Review. Biomedicines 2021, 9, 1748. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.K.; Glapa-Nowak, A.; Banaszkiewicz, A.; Iwańczak, B.; Kwiecień, J.; Szaflarska-Popławska, A.; Grzybowska-Chlebowczyk, U.; Osiecki, M.; Kierkuś, J.; Hołubiec, M.; et al. HLA-DQA1*05 Associates with Extensive Ulcerative Colitis at Diagnosis: An Observational Study in Children. Genes 2021, 12, 1934. [Google Scholar] [CrossRef] [PubMed]
- Maksic, M.; Corovic, I.; Maksic, T.; Zivic, J.; Zivic, M.; Zdravkovic, N.; Begovic, A.; Medovic, M.; Kralj, D.; Todorovic, Z.; et al. Molecular Insight into the Role of HLA Genotypes in Immunogenicity and Secondary Refractoriness to Anti-TNF Therapy in IBD Patients. Int. J. Mol. Sci. 2025, 26, 7274. [Google Scholar] [CrossRef] [PubMed]
- Pau, A.; Galliano, I.; Barnini, E.; Dini, M.; Pizzol, A.; Ponte, A.; Gambarino, S.; Calvo, P.L.; Bergallo, M. Involvement of HLADQA1*05 in Patients with Inflammatory Bowel Disease Treated with Anti-TNF Drugs. Medicina 2025, 61, 102. [Google Scholar] [CrossRef] [PubMed]




| Azathioprine | Anti-TNF Agents (Infliximab/Adalimumab) | Combination Therapy (Aza + Anti-TNF) | |
|---|---|---|---|
| Mechanism | Purine analog—inhibits lymphocyte proliferation | Monoclonal antibodies against TNF-α | Dual mechanism (immune suppression + cytokine neutralization) |
| Efficacy | Effective in maintenance, slower onset | High efficacy for induction and maintenance | Improved remission rates compared to monotherapy |
| Adverse effects | Myelosuppression, hepatotoxicity, pancreatitis | Infusion reactions, infections, immunogenicity | Reduced immunogenicity of anti-TNF, but higher risk of adverse events |
| Pharmacogenetics | TPMT variants important for dosing | HLA-DQA1*05 linked to anti-drug antibodies | Combination reduces antibody formation |
| IBD | ||||
|---|---|---|---|---|
| Crohn’s Disease | Ulcerative Colitis | Total | ||
| Gender | Male | 39 (37.5%) | 14 (13.4%) | 53 (50.9%) |
| Female | 34 (32.7%) | 17 (16.4%) | 51 (49.1%) | |
| Total | 73 (70.2%) | 31 (29.8%) | 104 (100%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojšić, M.; Ležakov, O.; Ćeranić, S.; Stojšić, N.; Rajković, M.; Marković, S.; Kovačević, M.; Brkić, N. TPMT and HLA-DQ Allelic Variants in Relation to Drug Response, Safety and Need for Therapy Optimization in Pediatric Inflammatory Bowel Disease. Children 2025, 12, 1334. https://doi.org/10.3390/children12101334
Stojšić M, Ležakov O, Ćeranić S, Stojšić N, Rajković M, Marković S, Kovačević M, Brkić N. TPMT and HLA-DQ Allelic Variants in Relation to Drug Response, Safety and Need for Therapy Optimization in Pediatric Inflammatory Bowel Disease. Children. 2025; 12(10):1334. https://doi.org/10.3390/children12101334
Chicago/Turabian StyleStojšić, Mirjana, Ognjen Ležakov, Sanja Ćeranić, Nikola Stojšić, Marko Rajković, Savina Marković, Milica Kovačević, and Nina Brkić. 2025. "TPMT and HLA-DQ Allelic Variants in Relation to Drug Response, Safety and Need for Therapy Optimization in Pediatric Inflammatory Bowel Disease" Children 12, no. 10: 1334. https://doi.org/10.3390/children12101334
APA StyleStojšić, M., Ležakov, O., Ćeranić, S., Stojšić, N., Rajković, M., Marković, S., Kovačević, M., & Brkić, N. (2025). TPMT and HLA-DQ Allelic Variants in Relation to Drug Response, Safety and Need for Therapy Optimization in Pediatric Inflammatory Bowel Disease. Children, 12(10), 1334. https://doi.org/10.3390/children12101334








