Pain Neuroscience Education in Children and Adolescents with Chronic Pain: A Systematic Review
Highlights
- Pain neuroscience education improves pain understanding, functionality, and self-efficacy in children and adolescents with chronic pain.
- Pain neuroscience education also influences emotional variables such as anxiety, catastrophizing, and kinesiophobia, although effects are often modest and short-lived.
- Combining pain neuroscience education with physical exercise and implementing school- or digital-based delivery formats may enhance effectiveness and broaden the reach of interventions.
- Including parents and caregivers in pain neuroscience education programs could improve clinical outcomes and support a more comprehensive and sustainable approach to pediatric chronic pain.
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Review Protocol Registration
2.2. Eligibility Criteria
2.3. Design and Search Strategy
2.4. Data Extraction
2.5. Study Quality and Assessment of Risk of Bias
2.6. Synthesis and Effect Measures
3. Results
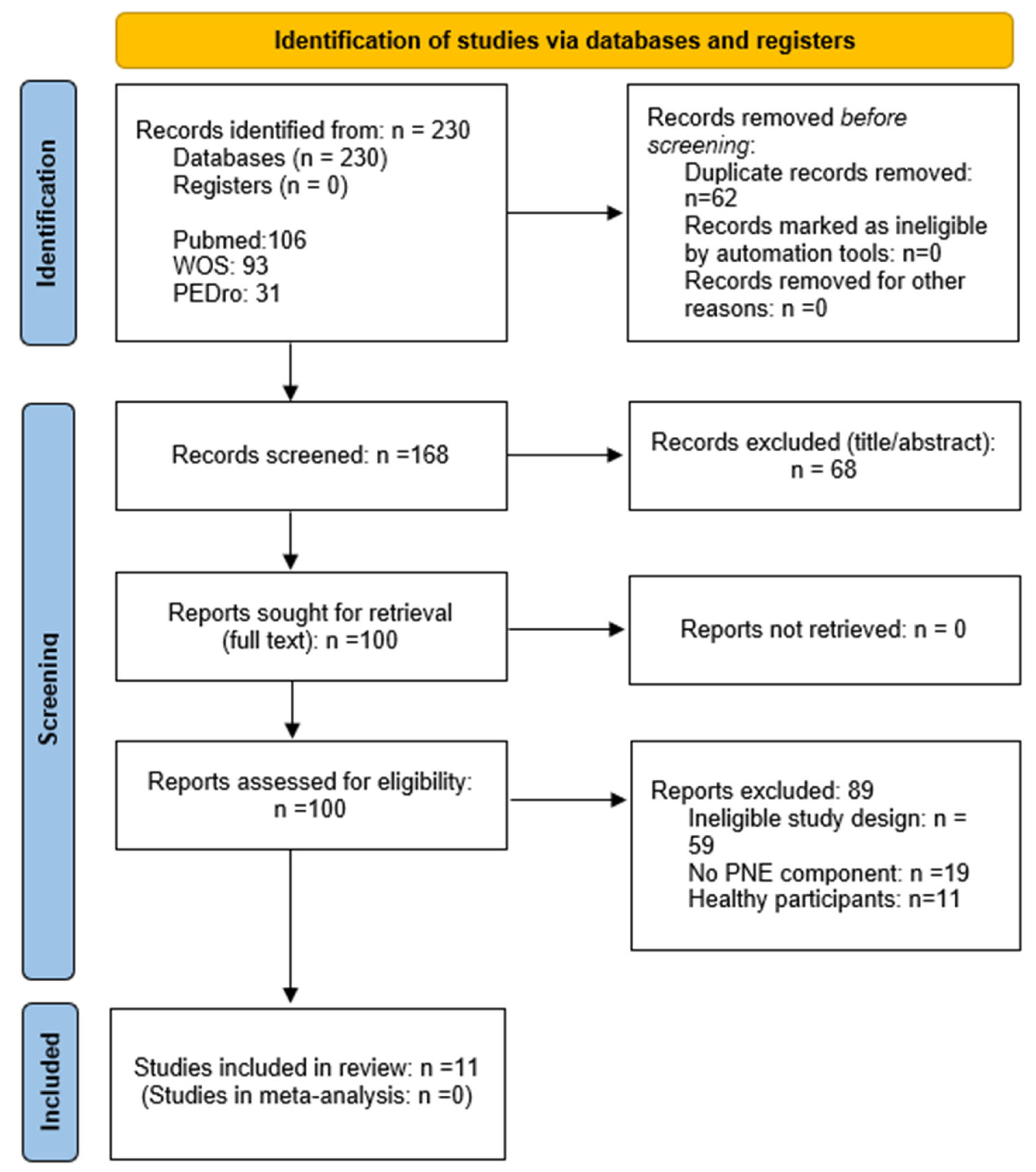
3.1. Search Results
3.2. Study Quality and Risk-of-Bias Assessment
3.3. Description of Included Studies
3.3.1. Sample Characteristics
3.3.2. Type of Intervention
3.3.3. Assessment Tools and Outcomes Obtained
Pain Intensity and the Severity of Pain-Related Symptoms
Pain Modulation
Emotional Function
Pain-Related Knowledge
Functionality
Sleep
Pain Coping
Medication Use
Satisfaction
Parental Assessment in the Included Studies
4. Discussion
4.1. Study Quality and Risk of Bias
4.2. Sample and Setting
4.3. Formats to Deliver PNE
4.4. Outcome Measures and Assessment Tools
4.5. Follow-Up
4.6. Implications for Clinical Practice
4.7. Limitations
4.8. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBT | Cognitive Behavioral Therapy |
| CP | Chronic Pain |
| CS | Central Sensitization |
| CSI | Central Sensitization Inventory |
| EC | Control Group |
| EG | Experimental Group |
| FAPD | Functional Abdominal Pain |
| HICP | High-Impact Chronic Pain |
| MeSH | Medical Subject Headings |
| PA | Physical Activity |
| PCP | Pediatric Chronic Pain |
| PedIMMPACT | Pediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials |
| PGIC | Patient Global Impression of Change |
| PNE | Pain Neuroscience Education |
| RCTs | Randomized Controlled Trials |
| VR | Virtual Reality |
| Web-MAP2 | Web-based Management of Adolescent Pain program |
| WOS | Web of Science |
Appendix A. PRISMA 2020 Checklist
| Section/Topic | Item | Checklist Item (Short Description) | Where Reported | Notes |
|---|---|---|---|---|
| Title | 1 | Identify the report as a systematic review. | Title page (“…A Systematic Review”) | |
| Abstract | 2 | Provide a structured summary per PRISMA 2020 for abstracts. | Abstract (Background/Objectives; Methods; Results; Conclusions; PROSPERO) | |
| Introduction | 3 | Rationale—describe the rationale for the review. | Introduction, para 1–3 | |
| Introduction | 4 | Objectives—provide an explicit statement of the objective(s). | Introduction, last paragraph | |
| Methods | 5 | Eligibility criteria—specify inclusion and exclusion criteria. | Section 2.2 Eligibility Criteria | |
| Methods | 6 | Information sources—describe all sources (databases, registers, other), dates of last search. | Section 2.3 Design and Search Strategy (PubMed, WOS, PEDro; last search 21 July 2025); 2.4 Data Extraction | |
| Methods | 7 | Search strategy—present full search strategies for all databases, including filters/limits. | Appendix C (Full Electronic Search Strategies) | |
| Methods | 8 | Selection process—specify how studies were selected, how many reviewers, independence, automation tools. | Section 2.4 Data Extraction (two reviewers; third reviewer for consensus; no automation tools) | |
| Methods | 9 | Data collection process—who extracted data and how; any contacting of authors. | Section 2.4 Data Extraction (two reviewers; contacted authors as needed) | |
| Methods | 10a | Data items—list and define all outcomes for which data were sought, specifying if all time points were sought. | Section 2.4 Data Extraction (prespecified outcomes and time points; PedIMMPACT priority) | |
| Methods | 10b | Data items—list and define all other variables for which data were sought (e.g., participant/setting, funding). | Section 2.4 Data Extraction (study characteristics, intervention/comparator, follow-up) | |
| Methods | 11 | Study risk of bias assessment—specify methods used to assess risk of bias in included studies. | Section 2.5 Study quality and assessment of risk of bias (RoB-2/CASP/NIH tools) | |
| Methods | 12 | Effect measures—specify for each outcome the effect measure(s) used. | Section 2.6 Synthesis and effect measures | Narrative synthesis; effect measures not applicable; reported as in studies. |
| Methods | 13a–f | Synthesis methods—describe methods for analyses and synthesis; handling of data; subgroup/sensitivity analyses; meta-bias. | Section 2.6 Synthesis and effect measures | No meta-analysis planned due to heterogeneity. |
| Methods | 14 | Reporting bias assessment—describe methods to assess risk of bias due to missing results (publication bias). | Appendix E (Publication bias columns marked ‘Unclear’ per GRADE) | No formal small-study/publication bias test performed. |
| Methods | 15 | Certainty assessment—describe methods used to assess certainty/certainty (e.g., GRADE). | Section 2.5 (GRADE) and Appendix D/E | |
| Results | 16a | Study selection—describe results of search and selection; ideally present in a flow diagram. | Section 3.1 Search results; Figure 1 (PRISMA flow diagram) | |
| Results | 16b | Cite studies excluded at full-text with reasons. | Appendix C (Figure A1 Full-text articles excluded with primary reason) | |
| Results | 17 | Study characteristics—cite characteristics for each included study. | Section 3.3 Description of Included Studies; Appendix F (Table A11) | |
| Results | 18 | Risk of bias in studies—present assessments for each included study. | Section 3.2 Study Quality and Risk-of-Bias Assessment; Appendix D (Table A3, Table A4 and Table A5) | |
| Results | 19 | Results of individual studies—present results for all outcomes considered for each study. | Results subsections (Pain intensity, Functionality, etc.); Appendix F | |
| Results | 20a–d | Results of syntheses—summarize syntheses; explore heterogeneity; sensitivity analyses; risk of bias on syntheses. | Results (narrative by domain); Discussion (heterogeneity) | Meta-analysis not conducted (not applicable). |
| Results | 21 | Reporting biases—present assessments of risk of bias due to missing results. | Appendix E (Publication bias: ‘Unclear’) | Not formally assessed; acknowledged in certainty tables. |
| Results | 22 | Certainty of evidence—present assessments of certainty for each outcome. | Section 3.2 (GRADE summary); Appendix E (SoF and Evidence Profiles) | |
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Section 4. Discussion (opening paragraphs) | |
| Discussion | 23b | Discuss limitations of the evidence included. | Section 4. Discussion (risk of bias/heterogeneity/small samples) | |
| Discussion | 23c | Discuss limitations of the review processes used. | Section 4. Discussion (limitations paragraph: databases only; no gray literature/registries; no automation) | |
| Discussion | 23d | Discuss implications of the results for practice, policy, and future research. | Section 4. Discussion (implications and future directions) | |
| Other information | 24a–c | Registration and protocol—provide registration information; protocol access; amendments. | Section 2.1 Protocol Registration (PROSPERO CRD420251062922); no amendments | |
| Other information | 25 | Support—describe sources of financial or non-financial support. | Funding: ‘no external funding’ | |
| Other information | 26 | Competing interests—declare any competing interests. | Conflicts of Interest: ‘none declared’ | |
| Other information | 27 | Availability of data, code, and other materials. | Data Availability Statement (‘All data are in the article/Supplementary Information’) |
Appendix B. Full-Text Articles Excluded with Primary Reason
| N | Author | Year | Title | Journal | DOI/PMID | Primary Reason (PRISMA) |
|---|---|---|---|---|---|---|
| 1 | Caverius U, Åkerblom S, Lexell J, Fischer MR. | 2025 | Characteristics of Children With Persistent Pain and Their Parents in a Tertiary Interdisciplinary Pain Clinic. | Paediatr Neonatal Pain. | 10.1002/pne2.70005 | No PNE component |
| 2 | Bogard I, Ayre J, Smith J, Pate JW, Sortwell A, Gorringe J, Gordon G, Kamper SJ, Yamato TP. | 2024 | Exploring Adolescents’ Understanding, Experiences and Beliefs About Pain: A Qualitative Study. | Health Expect | 10.1111/hex.70132 | No PNE component |
| 3 | Geremek A, Ruby L, Lindner C, Niederberger U, Schild U, Jung M, Soyka O, Siniatchkin M. | 2023 | Child and adolescent psychiatry staff’s knowledge on pain management. | Clin Child Psychol Psychiatry | 10.1177/13591045221125334 | No PNE component |
| 4 | Wakefield EO, Belamkar V, Litt MD, Puhl RM, Zempsky WT. | 2022 | “There’s Nothing Wrong With You”: Pain-Related Stigma in Adolescents With Chronic Pain. | J Pediatr Psychol. | 10.1093/jpepsy/jsab122 | No PNE component |
| 5 | Foxen-Craft E, Bourchtein E, Kaplan C, Clauw DJ, Scott E. | 2023 | Pain Widespreadedness, and Not Primary Pain Location, is Associated With Comorbid Symptoms in Children With Chronic Pain. | Clin J Pain. | 10.1097/AJP.0000000000001083 | No PNE component |
| 6 | Bale P, Easton V, Bacon H, Jerman E, Watts L, Barton G, Clark A, Armon K, MacGregor AJ. | 2019 | The effectiveness of a multidisciplinary intervention strategy for the treatment of symptomatic joint hypermobility in childhood: a randomised, single Centre parallel group trial (The Bendy Study). | Pediatr Rheumatol Online J. | 10.1186/s12969-018-0298-x | No PNE component |
| 7 | Korterink JJ, Ockeloen LE, Hilbink M, Benninga MA, Deckers-Kocken JM. | 2016 | Yoga Therapy for Abdominal Pain-Related Functional Gastrointestinal Disorders in Children: A Randomized Controlled Trial. | J Pediatr Gastroenterol Nutr. | 10.1097/MPG.0000000000001230 | No PNE component |
| 8 | Ahlqwist A, Hagman M, Kjellby-Wendt G, Beckung E. | 2008 | Physical therapy treatment of back complaints on children and adolescents. | Spine (Phila Pa 1976). | 10.1097/BRS.0b013e318182c347 | No PNE component |
| 9 | Sikka I, Chawla C, Seth S, Alghadir AH, Khan M. | 2020 | Effects of Deep Cervical Flexor Training on Forward Head Posture, Neck Pain, and Functional Status in Adolescents Using Computer Regularly. | Biomed Res Int. | 10.1155/2020/8327565 | No PNE component |
| 10 | Vidal J, Borràs PA, Ponseti FJ, Cantallops J, Ortega FB, Palou P. | 2013 | Effects of a postural education program on school backpack habits related to low back pain in children. | Eur Spine J. | 10.1007/s00586-012-2558-7 | No PNE component |
| 11 | Vidal J, Borras PA, Ortega FB, Cantallops J, Ponseti X, Palou P. | 2011 | Effects of postural education on daily habits in children. | Int J Sports Med. | 10.1055/s-0030-1270469 | No PNE component |
| 12 | Dolphens M, Cagnie B, Danneels L, De Clercq D, De Bourdeaudhuij I, Cardon G. | 2011 | Long-term effectiveness of a back education programme in elementary schoolchildren: an 8-year follow-up study. | Eur Spine J. | 10.1007/s00586-011-1856-9 | No PNE component |
| 13 | Geldhof E, Cardon G, De Bourdeaudhuij I, De Clercq D. | 2007 | Back posture education in elementary schoolchildren: a 2-year follow-up study. | Eur Spine J. | 10.1007/s00586-006-0227-4 | No PNE component |
| 14 | Pate JW, Harrison LE, Hess CW, Moseley GL, Rush G, Heathcote LC, Simons LE. | 2023 | Targeting Pain Science Education in Youth With Chronic Pain: What Are the Sticking Points for Youth and Their Parents? | Clin J Pain. | 10.1097/AJP.0000000000001088 | No PNE component |
| 15 | Morris MC, Bruehl S, Stone AL, Garber J, Smith C, Palermo TM, Walker LS. | 2021 | Does Quantitative Sensory Testing Improve Prediction of Chronic Pain Trajectories? A Longitudinal Study of Youth With Functional Abdominal Pain Participating in a Randomized Controlled Trial of Cognitive Behavioral Treatment. | Clin J Pain. | 10.1097/AJP.0000000000000956 | No PNE component |
| 16 | Hill JJ, Keating JL. | 2015 | Daily exercises and education for preventing low back pain in children: cluster randomized controlled trial. | Phys Ther. | 10.2522/ptj.20140273 | No PNE component |
| 17 | Andrews NE, Ireland D, Vijayakumar P, Burvill L, Hay E, Westerman D, Rose T, Schlumpf M, Strong J, Claus A. | 2023 | Acceptability of a Pain History Assessment and Education Chatbot (Dolores) Across Age Groups in Populations With Chronic Pain: Development and Pilot Testing. | JMIR Form Res. | 10.2196/47267 | No PNE component |
| 18 | Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, Passo MH, Schikler KN, Hashkes PJ, Spalding S, Lynch-Jordan AM, Banez G, Richards MM, Lovell DJ. | 2012 | Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: a multisite, single-blind, randomized, controlled clinical trial. | Arthritis Rheum. | 10.1002/art.30644 | No PNE component |
| 19 | Nilsson S, Wallbing U, Alfvén G, Dalenius K, Fors A, Golsäter M, Rosvall PÅ, Wigert H, Lundberg M. | 2019 | Development of the Help Overcoming Pain Early (HOPE) Programme Built on a Person-Centred Approach to Support School Nurses in the Care of Adolescents with Chronic Pain-A Feasibility Study. | Children (Basel) | 10.3390/children6090095 | No PNE component |
| 20 | Kashikar-Zuck S, Sil S, Lynch-Jordan AM, Ting TV, Peugh J, Schikler KN, Hashkes PJ, Arnold LM, Passo M, Richards-Mauze MM, Powers SW, Lovell DJ. | 2013 | Changes in pain coping, catastrophizing, and coping efficacy after cognitive-behavioral therapy in children and adolescents with juvenile fibromyalgia. | J Pain. | 10.1016/j.jpain.2012.12.019 | No PNE component |
| 21 | Mankelow J, Ryan CG, Skidmore N, Potter J, Ravindran D, Chattle R, Browne S, Suri S, Graham A, Pate JW, Newport R, Langford T, Martin D. | 2025 | An evaluation of a one-day pain science education event in a 16–18 years school setting targeting pain-related beliefs, knowledge, and behavioural intentions: A mixed-methods, non-randomised controlled trial. | Musculoskelet Sci Pract | 10.1016/j.msksp.2025.103385. | Healthy participants |
| 22 | Mankelow J, Ravindran D, Graham A, Suri S, Pate JW, Ryan CG, Martin D. | 2023 | An evaluation of a one-day pain science education event in a high school setting targeting pain related beliefs, knowledge, and behavioural intentions. | Musculoskelet Sci Pract. | 10.1016/j.msksp.2023.102818 | Healthy participants |
| 23 | Rheel E, Ickmans K, Wauters A, Van Ryckeghem DML, Barbé K, Malfliet A, Vervoort T. | 2022 | The Effect of a Pain Educational Video Upon Child Pain-Related Memory and the Moderating Role of Parental Pain- and Non-Pain-Attending Verbalizations: An Experimental Lab-Based Study. | J Pediatr Psychol | 10.1093/jpepsy/jsac044 | Healthy participants |
| 24 | Bacardit Pintó P, Ickmans K, Rheel E, Iwens M, Meeus M, Nijs J, Pas R. | 2021 | Do Parental Pain Knowledge, Catastrophizing, and Hypervigilance Improve Following Pain Neuroscience Education in Healthy Children? | Children (Basel). | 10.3390/children8050420 | Healthy participants |
| 25 | Martí L, Castarlenas E, Solé E, de la Vega R, Miró J. | 2021 | Video-based Pain Education in Schools: A Study With Adolescents. | Clin J Pain. | 10.1097/AJP.0000000000000906 | Healthy participants |
| 26 | Louw A, Podalak J, Zimney K, Schmidt S, Puentedura EJ. | 2018 | Can pain beliefs change in middle school students? A study of the effectiveness of pain neuroscience education. | Physiother Theory Pract | 10.1080/09593985.2017.1423142 | Healthy participants |
| 27 | Cardon GM, de Clercq DL, Geldhof EJ, Verstraete S, de Bourdeaudhuij IM. | 2007 | Back education in elementary schoolchildren: the effects of adding a physical activity promotion program to a back care program. | Eur Spine J. | 10.1007/s00586-006-0095-y | Healthy participants |
| 28 | Dullien S, Grifka J, Jansen P. | 2018 | Cluster-randomized, controlled evaluation of a teacher led multi factorial school based back education program for 10 to 12-year old children. | BMC Pediatr. | 10.1186/s12887-018-1280-y | Healthy participants |
| 29 | Louw A, Louw C, Podalak J, Zimney K, DeLorenzo J, Maiers N, Puentedura EJ, Mintken P. | 2023 | Pain Neuroscience Education in Elementary and Middle Schools. | Pediatr Phys Ther. | 10.1097/PEP.0000000000001018 | Healthy participants |
| 30 | Rheel E, Ickmans K, Wauters A, Van Ryckeghem DML, Malfliet A, Vervoort T. | 2021 | The effect of a pain educational video intervention upon child pain-related outcomes: A randomized controlled study. | Eur J Pain. | 10.1002/ejp.1822 | Healthy participants |
| 31 | Pas R, Meeus M, Malfliet A, Baert I, Oosterwijck SV, Leysen L, Nijs J, Ickmans K. | 2018 | Development and feasibility testing of a Pain Neuroscience Education program for children with chronic pain: treatment protocol. | Braz J Phys Ther. | 10.1016/j.bjpt.2018.02.004 | Healthy participants |
| 32 | Ciolan F, Bertoni G, Crestani M, Falsiroli Maistrello L, Coppola I, Rossettini G, Battista S. | 2025 | Perceived factors influencing the success of pain neuroscience education in chronic musculoskeletal pain: a meta-synthesis of qualitative studies. | Disabil Rehabil. | 10.1080/09638288.2024.2398141 | Ineligible study design |
| 33 | Thong HPA, Mardon AK, Evans S. | 2025 | Pelvic pain education—A short review on pelvic pain and endometriosis educational programs for adolescents. | Aust N Z J Obstet Gynaecol. | 10.1111/ajo.13856 | Ineligible study design |
| 34 | Rezende J, Acalantis L, Nogueira LC, Meziat-Filho N, Ickmans K, Reis FJJ. | 2024 | Contents and delivery methods of pain neuroscience education in pediatrics: A scoping review. | Musculoskelet Sci Pract. | 10.1016/j.msksp.2024.103182 | Ineligible study design |
| 35 | Kara OK, Gursen C, Ickmans K, Rheel E, Elma O, Cetin SY, Dogan M, Kutluk MG, Kara K. | 2024 | Enhancing pediatric pain management in Turkey: A modified Delphi study on culturally adapted pain neuroscience education for chronic pain in children. | J Pediatr Nurs. | 10.1016/j.pedn.2024.09.001 | Ineligible study design |
| 36 | Berryman C, Starr T, Ferencz N, Coakley R. | 2024 | Co-creation in healthcare and research to improve service delivery for young people with chronic pain. | Front Med (Lausanne). | 10.3389/fmed.2024.1431155 | Ineligible study design |
| 37 | Rodriguez-Restrepo A, AuBuchon JD. | 2024 | Chronic pain in pediatric patients: epidemiology, pathophysiology, and mitigation strategies. | Curr Opin Anaesthesiol. | 10.1097/ACO.0000000000001372 | Ineligible study design |
| 38 | GBD 2021 Diseases and Injuries Collaborators. | 2024 | Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. | Lancet. | 10.1016/S0140-6736(24)00757-8 | Ineligible study design |
| 39 | GBD 2021 Causes of Death Collaborators. | 2024 | Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. | Lancet. | 10.1016/S0140-6736(24)00367-2 | Ineligible study design |
| 40 | Schwerdt H, Christe G, Pate JW, Blake C, Smart KM. | 2024 | The prevalence of chronic pain in adolescents in Central Switzerland: A cross-sectional school-based study protocol. | PLoS One. | 10.1371/journal.pone.0297088 | Ineligible study design |
| 41 | Borucki AN, Benki CM, Peterson EE. | 2024 | Terminology for discussing chronic pain: Using metaphors to educate families on chronic pediatric pain. | J Pediatr Gastroenterol Nutr. | 10.1002/jpn3.12072 | Ineligible study design |
| 42 | Lin LH, Lin TY, Chang KV, Wu WT, Özçakar L. | 2024 | Pain neuroscience education for reducing pain and kinesiophobia in patients with chronic neck pain: A systematic review and meta-analysis of randomized controlled trials. | Eur J Pain. | 10.1002/ejp.2182 | Ineligible study design |
| 43 | Allaire C, Yong PJ, Bajzak K, Jarrell J, Lemos N, Miller C, Morin M, Nasr-Esfahani M, Singh SS, Chen I. | 2024 | Guideline No. 445: Management of Chronic Pelvic Pain | J Obstet Gynaecol Can. | 10.1016/j.jogc.2023.102283 | Ineligible study design |
| 44 | Darnall BD, Edwards KA, Courtney RE, Ziadni MS, Simons LE, Harrison LE. | 2023 | Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. | Front Pain Res (Lausanne). | 10.3389/fpain.2023.1223172 | Ineligible study design |
| 45 | Shaygan M, Jaberi A, Razavizadegan M, Shayegan Z. | 2023 | Prevalence of chronic pain and contributing factors: a cross-sectional population-based study among 2379 Iranian adolescents. | Korean J Pain. | 10.3344/kjp.22336 | Ineligible study design |
| 46 | Leake HB, Moseley GL, Murphy LK, Murray CB, Palermo TM, Heathcote LC. | 2023 | How does pain work? A qualitative analysis of how young adults with chronic pain conceptualize the biology of pain. | Eur J Pain. | 10.1002/ejp.2069 | Ineligible study design |
| 47 | Miró J, Solé E, Castarlenas E, Ingelmo P, Nolla MDC, Escribano J, Reinoso-Barbero F. | 2023 | The Treatment of Pediatric Pain in Spain: A Survey Study. | Int J Environ Res Public Health. | 10.3390/ijerph20032484 | Ineligible study design |
| 48 | Dale CM, Cioffi I, Novak CB, Gorospe F, Murphy L, Chugh D, Watt-Watson J, Stevens B. | 2023 | Continuing professional development needs in pain management for Canadian health care professionals: A cross sectional survey. | Can J Pain. | 10.1080/24740527.2022.2150156 | Ineligible study design |
| 49 | Ickmans K, Rheel E, Rezende J, Reis FJJ. | 2022 | Spreading the word: pediatric pain education from treatment to prevention. | Arch Physiother. | 10.1186/s40945-022-00151-4 | Ineligible study design |
| 50 | Baerg K, Tupper SM, Chu LM, Cooke N, Dick BD, Doré-Bergeron MJ, Findlay S, Ingelmo PM, Lamontagne C, Mesaroli G, Oberlander TF, Poolacherla R, Spencer AO, Stinson J, Finley GA. | 2022 | Canadian surveillance study of complex regional pain syndrome in children. | Pain. | 10.1097/j.pain.0000000000002482 | Ineligible study design |
| 51 | Hurley-Wallace AL, Nowotny E, Schoth DE, Liossi C. | 2021 | Online multidisciplinary interventions for paediatric chronic pain: A content analysis. | Eur J Pain. | 10.1002/ejp.1827 | Ineligible study design |
| 52 | Thacker L, Walsh RM, Shinyoung Song G, Khan HA, Parmar P, Vance KT, Grant G, Mesaroli G, Hunter J, Vader K. | 2021 | Exploring physiotherapy practice within hospital-based interprofessional chronic pain clinics in Ontario. | Can J Pain. | 10.1080/24740527.2021.1905508 | Ineligible study design |
| 53 | Salvat I, Adillón C, Andrés EM, Monterde S, Miró J. | 2021 | Development of the Conceptualization of Pain Questionnaire: A Measure to Study How Children Conceptualize Pain. | Int J Environ Res Public Health | 10.3390/ijerph18073821 | Ineligible study design |
| 54 | Mueri K, Kennedy M, Pavlova M, Jordan A, Lund T, Neville A, Belton J, Noel M. | 2021 | The sociocultural context of pediatric pain: an examination of the portrayal of pain in children’s popular media. | Pain | 10.1097/j.pain.0000000000002086 | Ineligible study design |
| 55 | Miró J, Micó JA, Reinoso-Barbero F. | 2021 | The management of pediatric chronic pain in Spain: a web-based survey study. | Curr Med Res Opin. | 10.1080/03007995.2020.1854208 | Ineligible study design |
| 56 | Emerson ND, Bursch B. | 2020 | Communicating with Youth about Pain: Developmental Considerations. | Children (Basel). | 10.3390/children7100184 | Ineligible study design |
| 57 | Koechlin H, Locher C, Prchal A. | 2020 | Talking to Children and Families about Chronic Pain: The Importance of Pain Education-An Introduction for Pediatricians and Other Health Care Providers. | Children (Basel). | 10.3390/children7100179 | Ineligible study design |
| 58 | Pack R MPT, OCS, Gilliland R PhD, Mecham A DPT. | 2020 | The treatment of central sensitization in an adolescent using pain neuroscience education and graded exposure to activity: A case report. | Physiother Theory Pract. | 10.1080/09593985.2018.1551454 | Ineligible study design |
| 59 | Velazquez Cardona C, Rajah C, Mzoneli YN, Friedrichsdorf SJ, Campbell F, Cairns C, Rodseth RN. | 2019 | An audit of paediatric pain prevalence, intensity, and treatment at a South African tertiary hospital. | Pain Rep. | 10.1097/PR9.0000000000000789 | Ineligible study design |
| 60 | Leake HB, Heathcote LC, Simons LE, Stinson J, Kamper SJ, Williams CM, Burgoyne LL, Craigie M, Kammers M, Moen D, Pate JW, Szeto K, Moseley GL. | 2019 | Talking to Teens about Pain: A Modified Delphi Study of Adolescent Pain Science Education. | Can J Pain | 10.1080/24740527.2019.1682934 | Ineligible study design |
| 61 | Kanstrup M, Jordan A, Kemani MK. | 2019 | Adolescent and Parent Experiences of Acceptance and Commitment Therapy for Pediatric Chronic Pain: An Interpretative Phenomenological Analysis. | Children (Basel). | 10.3390/children6090101 | Ineligible study design |
| 62 | Grasaas E, Fegran L, Helseth S, Stinson J, Martinez S, Lalloo C, Haraldstad K. | 2019 | iCanCope With Pain: Cultural Adaptation and Usability Testing of a Self-Management App for Adolescents With Persistent Pain in Norway. | JMIR Res Protoc. | 10.2196/12940 | Ineligible study design |
| 63 | Robins H, Perron V, Heathcote LC, Simons LE. | 2016 | Pain Neuroscience Education: State of the Art and Application in Pediatrics. | Children (Basel). | 10.3390/children3040043 | Ineligible study design |
| 64 | Manworren RC, Stinson J. | 2016 | Pediatric Pain Measurement, Assessment, and Evaluation. | Semin Pediatr Neurol. | 10.1016/j.spen.2016.10.001 | Ineligible study design |
| 65 | Louw A, Zimney K, Puentedura EJ, Diener I. | 2016 | The efficacy of pain neuroscience education on musculoskeletal pain: A systematic review of the literature. | Physiother Theory Pract. | 10.1080/09593985.2016.1194646 | Ineligible study design |
| 66 | Carr EC, Briggs EV, Briggs M, Allcock N, Black P, Jones D. | 2016 | Understanding factors that facilitate the inclusion of pain education in undergraduate curricula: Perspectives from a UK survey. | Br J Pain. | 10.1177/2049463716634377 | Ineligible study design |
| 67 | Maciel SC, Jennings F, Jones A, Natour J. | 2009 | The development and validation of a Low Back Pain Knowledge Questionnaire—LKQ. | Clinics (Sao Paulo). | 10.1590/S1807-59322009001200006 | Ineligible study design |
| 68 | Zhang Y, Yang C. | 2024 | Influence of pain neuroscience education and exercises for the management of neck pain: A meta-analysis of randomized controlled trials. | Medicine (Baltimore). | 10.1097/MD.0000000000040760 | Ineligible study design |
| 69 | Harrison LE, Pate JW, Richardson PA, Ickmans K, Wicksell RK, Simons LE. | 2019 | Best-Evidence for the Rehabilitation of Chronic Pain Part 1: Pediatric Pain. | J Clin Med. | 10.3390/jcm8091267 | Ineligible study design |
| 70 | Clinch J, Eccleston C. | 2009 | Chronic musculoskeletal pain in children: assessment and management. | Rheumatology (Oxford). | 10.1093/rheumatology/kep001 | Ineligible study design |
| 71 | O’Sullivan K, O’Keeffe M, Forster BB, Qamar SR, van der Westhuizen A, O’Sullivan PB. | 2019 | Managing low back pain in active adolescents. | Best Pract Res Clin Rheumatol. | 10.1016/j.berh.2019.02.005 | Ineligible study design |
| 72 | Fechner R, Verhagen A, Alcock M, Norton J, Stubbs PW, Harrison LE, Pate JW. | 2024 | The Effectiveness of Pain Science Education on Caregiver and Children’s Knowledge, Beliefs, Attitudes, and Behaviors-A Systematic Review and Meta-Analysis. | J Pain. | 10.1016/j.jpain.2024.104578 | Ineligible study design |
| 73 | Barrett MJ, Barnett PL. | 2016 | Complex Regional Pain Type 1. | Pediatr Emerg Care. | 10.1097/PEC.0000000000000731 | Ineligible study design |
| 74 | Lalloo C, Jibb LA, Rivera J, Agarwal A, Stinson JN. | 2015 | There’s a Pain App for That: Review of Patient-targeted Smartphone Applications for Pain Management. | Clin J Pain. | 10.1097/AJP.0000000000000171 | Ineligible study design |
| 75 | de la Vega R, Miró J. | 2024 | mHealth: a strategic field without a solid scientific soul. a systematic review of pain-related apps. | PLoS One. | 10.1371/journal.pone.0101312 | Ineligible study design |
| 76 | Anyachukwu CC, Amarah CC, Atueyi BC, Anthony I, Nweke M, Abaraogu U. | 2024 | Effectiveness of Back care education Programme among school children: a systematic review of randomized controlled trials. | BMC Pediatr. | 10.1186/s12887-024-04563-y | Ineligible study design |
| 77 | Yu H, Southerst D, Wong JJ, Verville L, Connell G, Ead L, Mior S, Hestbaek L, Swain M, Brunton G, Shearer HM, Papaconstantinou E, To D, Germann D, Pohlman K, Cedraschi C, Cancelliere C. | 2024 | Rehabilitation of back pain in the pediatric population: a mixed studies systematic review. | Chiropr Man Therap. | 10.1186/s12998-024-00538-z | Ineligible study design |
| 78 | Klausen SH, Rønde G, Tornøe B, Bjerregaard L. | 2019 | Nonpharmacological Interventions Addressing Pain, Sleep, and Quality of Life in Children and Adolescents with Primary Headache: A Systematic Review. | J Pain Res. | 10.2147/JPR.S216807 | Ineligible study design |
| 79 | van der Velde G, Yu H, Paulden M, Côté P, Varatharajan S, Shearer HM, Wong JJ, Randhawa K, Southerst D, Mior S, Sutton D, Jacobs C, Taylor-Vaisey A. | 2016 | Which interventions are cost-effective for the management of whiplash-associated and neck pain-associated disorders? A systematic review of the health economic literature by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. | Spine J. | 10.1016/j.spinee.2015.08.025 | Ineligible study design |
| 80 | Liegl G, Boeckle M, Leitner A, Pieh C. | 2016 | A meta-analytic review of brief guided self-help education for chronic pain. | Eur J Pain. | 10.1002/ejp.881 | Ineligible study design |
| 81 | Michaleff ZA, Kamper SJ, Maher CG, Evans R, Broderick C, Henschke N. | 2014 | Low back pain in children and adolescents: a systematic review and meta-analysis evaluating the effectiveness of conservative interventions. | Eur Spine J. | 10.1007/s00586-014-3461-1 | Ineligible study design |
| 82 | García-Moreno JM, Calvo-Muñoz I, Gómez-Conesa A, López-López JA. | 2025 | Therapeutic Exercise is Effective in Reducing the Intensity of Nonspecific Low Back Pain in Children and Adolescents: A Systematic Review and Network Meta-analysis. | Arch Phys Med Rehabil. | 10.1016/j.apmr.2024.11.002. | Ineligible study design |
| 83 | Dobe, M; Zernikow, B | 2019 | Inpatient Pain Treatment: Module 4 (Integrating the Family System) | springer nature | 10.1007/978-3-030-19201-3_11 | Ineligible study design |
| 84 | Serena Maria Dib, Gaelle Rached, Dimitri Fiani, Souraya Torbey, | 2021 | The Role of Pain Education in the Treatment of Pediatric Chronic Pain | J. Am. Acad. Child Adolesc. Psychiatry | 10.1016/j.jaac.2021.09.395 | Ineligible study design |
| 85 | Shankey-Smith, W | 2025 | Paediatric chronic pain | Anaesth. Intensive Care Med. | 10.1016/j.mpaic.2025.04.013 | Ineligible study design |
| 86 | Palahí-Calsina, I., Jubany, J., Sordo, L., Lorente, S., Espelt, A. y Borao, O. | 2024 | Effectiveness of pain neuroscience education among adults with chronic neck pain. Systematic review | Eur. J. Physiother. | 10.1080/21679169.2024.2365694 | Ineligible study design |
| 87 | Stinson JN, Lalloo C, Harris L, Isaac L, Campbell F, Brown S, Ruskin D, Gordon A, Galonski M, Pink LR, Buckley N, Henry JL, White M, Karim A. | 2014 | iCanCope with Pain™: User-centred design of a web- and mobile-based self-management program for youth with chronic pain based on identified health care needs. | Pain Res Manag. | 10.1155/2014/935278 | Ineligible study design |
| 88 | Sil S, Arnold LM, Lynch-Jordan A, Ting TV, Peugh J, Cunningham N, Powers SW, Lovell DJ, Hashkes PJ, Passo M, Schikler KN, Kashikar-Zuck S. | 2014 | Identifying treatment responders and predictors of improvement after cognitive-behavioral therapy for juvenile fibromyalgia. | Pain. | 10.1016/j.pain.2014.03.005 | Ineligible study design |
| 89 | Murray CB, de la Vega R, Loren DM, Palermo TM. | 2020 | Moderators of Internet-Delivered Cognitive-Behavioral Therapy for Adolescents With Chronic Pain: Who Benefits From Treatment at Long-Term Follow-Up? | J Pain. | 10.1016/j.jpain.2019.10.001 | Ineligible study design |
Appendix C. Full Electronic Search Strategies
Appendix D. Results of Risk of Bias
| Study | Variable | D1a | D1b | D2 | D3 | D4 | D5 | Overall |
|---|---|---|---|---|---|---|---|---|
| Randomization | Cluster Trial Part b | Deviations | Missing Data | Measurement | Reporting | |||
| Palermo [39] | Child—NRS-11 (pain intensity) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk |
| Child—BAPQ (anxiety) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Child—CALI (disability) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Child—ASWS (sleep) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Child—HHI-Pain (helplessness) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Child—TEI-SF (satisfaction) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Parent—BAPQ-PIQ (anxiety) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Parent—ARCS (protective behavior) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Parent—HHI-Pain (helplessness) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Parent—TEI-SF (satisfaction) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Andías [40] | EVA (pain intensity) | Some concerns | Not applicable | Some concerns | Low risk | High risk | Low risk | Some concerns |
| Muscle Assesment | Some concerns | Not applicable | Low risk | Low risk | High risk | Low risk | Some concerns | |
| STAIC (anxiety) | Some concerns | Not applicable | Low risk | Low risk | High risk | Low risk | Some concerns | |
| PCS (catastrophizing) | Some concerns | Not applicable | Low risk | Low risk | High risk | Low risk | Some concerns | |
| NPQ (knowledge) | Some concerns | Not applicable | Low risk | Low risk | High risk | Low risk | Some concerns | |
| PGIC (satisfaction) | Some concerns | Not applicable | Low risk | Low risk | Some concerns | Low risk | Some concerns | |
| Pas [41] | Child—FPS-R (pain intensity) | Low risk | Not applicable | High risk | Low risk | Low risk | Low risk | Some concerns |
| Parents—PCS-P (catastrophizing) | Low risk | Not applicable | High risk | Low risk | Low risk | Low risk | Some concerns | |
| Parents—FDI-P (function) | Low risk | Not applicable | High risk | Low risk | Low risk | Low risk | Some concerns | |
| Parents—FOPQ-P (fear of pain) | Low risk | Not applicable | High risk | Low risk | Low risk | Low risk | Some concerns | |
| Louw [42] | rNPQ (knowledge) | Low risk | Some concerns | Low risk | Some concerns | High risk | Some concerns | High risk |
| FABQ-PA (Kinesiophobia) | Low risk | Some concerns | Low risk | Some concerns | High risk | Some concerns | High risk | |
| Behavior change | Low risk | Some concerns | Low risk | Some concerns | High risk | Some concerns | High risk | |
| Medication use | Low risk | Some concerns | Low risk | Some concerns | High risk | Some concerns | High risk | |
| Kisling [43] | NRS (pain intensity) | Some concerns | Low risk | Low risk | Low risk | High risk | Low risk | Some concerns |
| PKQ-CH (knowledge) | Some concerns | Low risk | Low risk | Some concerns | High risk | Some concerns | High risk | |
| PPCI-r (passive coping) | Some concerns | Low risk | Low risk | Some concerns | Low risk | Some concerns | Some concerns | |
| PPDI (disability) | Some concerns | Low risk | Low risk | Some concerns | Low risk | Some concerns | Some concerns | |
| Walker [44] | CCSSI-24 and API (pain intensity) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk |
| TEI-SF (satisfaction) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Andías [45] | NPQ (knowledge) | Low risk | Not applicable | Low risk | Low risk | High risk | Some concerns | High risk |
| PCS (catastrophizing) | Low risk | Not applicable | Low risk | Low risk | Low risk | Some concerns | Some concerns | |
| TSK (kinesiophobia) | Low risk | Not applicable | Low risk | Low risk | Low risk | Some concerns | Some concerns | |
| FDI (function) | Low risk | Not applicable | Low risk | Low risk | Low risk | Some concerns | Some concerns | |
| CSES (self-efficacy) | Low risk | Not applicable | Low risk | Low risk | Low risk | Some concerns | Some concerns | |
| BaSIQS (insomnia) | Low risk | Not applicable | Low risk | Low risk | Low risk | Some concerns | Some concerns | |
| CSI (Central Sensitizacion) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk | |
| PGIC (Patient Global Impression of Change) | Low risk | Not applicable | Low risk | Low risk | Low risk | Low risk | Low risk | |
| Beach [46] | COPI (knowledge) | High risk | Not applicable | Low risk | Some concerns | Low risk | Low risk | High risk |
| Menes [47] | COPAQ (knowledge) | Some concerns | Not applicable | Some concerns | Some concerns | Low risk | Low risk | Some concerns |
| CASP Criteria | Neto [48] | |
|---|---|---|
| Variable | ||
| Relevance of Knowledge | Intervention Adequacy | |
| 1. Clear Study Aims | ✅ Fully addressed | ✅ Fully addressed |
| 2. Appropriate Methodology | ✅ Thematic analysis | ✅ Thematic analysis |
| 3. Research Design | ⚠️ Single-center | ⚠️ Single-center |
| 4. Recruitment Strategy | ⚠️ Homogeneous sample | ⚠️ Homogeneous sample |
| 5. Data Collection | ✅ Rigorous (recordings) | ✅ Rigorous (recordings) |
| 6. Researcher-Participant Relationship | ⚠️ Potential bias (therapists as researchers) | ⚠️ Potential bias (therapists as researchers) |
| 7. Ethical Considerations | ✅ Approved + consent | ✅ Approved + consent |
| 8. Data Analysis | ✅ Triangulation | ✅ Triangulation |
| 9. Findings | ✅ Rich quotes + quant data | ✅ Rich quotes + quant data |
| 10. Research Value | ✅ Novel insights | ✅ Novel insights |
| 11. Overall Rating | Excellent | Excellent |
| Wager [49] | ||
|---|---|---|
| Item | NIH Tool Question | Assessment |
| 1 | Was the research question or objective clearly stated? | Yes |
| 2 | Were eligibility/selection criteria for the study population prespecified and applied uniformly? | Partially |
| 3 | Was the study population representative of the intended population? | Unclear |
| 4 | Were all participants clearly described at each stage of the study? | Yes |
| 5 | Was the intervention clearly described and delivered consistently to all participants? | Yes |
| 6 | Were outcome measures assessed more than once before the intervention? | No |
| 7 | Were outcome measures assessed more than once after the intervention? | No |
| 8 | Were outcome measures clearly defined, valid, and reliable? | Partially |
| 9 | Were the outcome assessors blinded to the intervention status of participants? | No |
| 10 | Was loss to follow-up after baseline 20% or less? | Yes |
| 11 | Were all participants who were enrolled included in the analysis? | Yes |
| 12 | Were statistical methods appropriate for the study design? | Yes |
| 13 | Were potential confounding variables measured and adjusted for? | Partially |
| 14 | Are the results believable given the study design and limitations? | Yes, with caution |
| Overall Quality: Yes: 7; Partially: 3; No count: 3 | Fair | |
Appendix E. GRADE Summary of Findings and Evidence Profiles (By Informant)
| Outcome | Importance | No. of Studies | Direction of Effect | Certainty (GRADE) |
|---|---|---|---|---|
| Pain knowledge | Critical | 7 | Consistent and sustained improvement | Moderate |
| Disability/Function | Critical | 4 | Modest improvements; heterogeneous | Low |
| Pain intensity | Critical | 6 | Mixed results; 2/6 with sustained reduction | Low |
| Anxiety | Important | 2 | Minor effects/not sustained | Very low |
| Catastrophizing | Important | 2 | Decreases (significant in 1) | Low |
| Kinesiophobia | Important | 2 | Improvements in both groups; significant in 1 | Low |
| Sleep | Important | 2 | Slight improvement; in one RCT, favors CBT | Low |
| Self-efficacy | Important | 1 | Improvement in both groups | Very low |
| Medication use | Important | 1 | Reduction with PNE + reinforcements | Very low |
| Patient Global Impression of Change | Critical | 1 | Perceived improvement post-treatment | Low q |
| Satisfaction with treatment/care | Important | 3 | High/moderate; variability across studies | Low p r |
| Pain coping | Important | 1 | Small improvements/inconclusive | Very low |
| Central sensitization | Important | 1 | Reduction/inconclusive depending on instrument | Very low |
| Depression | Important | 1 | Small/not sustained effects | Very low |
| Miscarried helping | Important | 1 | Small/moderate reduction (if reported) | Very low |
| Adverse events | Critical | 0 | Not reported | — u |
| Outcome | Importance | No. of Studies | Direction of Effect | Certainty (GRADE) |
|---|---|---|---|---|
| Parental catastrophizing | Important | 1 | Small to moderate decreases | Low |
| Caregiver anxiety/stress | Important | 1 | Small/unsustained effects | Very low |
| Parental behaviors/family accommodation | Important | 2 | Small to moderate reductions; slight heterogeneity | Low |
| Disability/Function (proxy) | Critical | 2 | Mixed/inconclusive results | Low * ℓ |
| Pain intensity (proxy) | Critical | 2 | Mixed/inconclusive results | Low * ℓ |
| Satisfaction with treatment/care | Important | 2 | High/moderate; variability between studies | Very low p r |
| Outcome | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Other Considerations | Certainty |
|---|---|---|---|---|---|---|---|
| Pain knowledge | Some concerns | Not serious | Not serious | Serious | Unclear | - | Moderate |
| Disability/Function | Some concerns | Serious | Not serious | Serious | Unclear | - | Low |
| Pain intensity | Some concerns | Serious | Not serious | Serious | Unclear | - | Low |
| Anxiety | Low | Serious | Possible (comparators) | Very serious | Unclear | - | Very low |
| Catastrophizing | Some concerns | Not serious | Not serious | Serious | Unclear | - | Low |
| Patient Global Impression of Change | Some concerns | Not assessable | Not serious | Serious | Unclear | - | Low |
| Satisfaction (child/adolescent) | Some concerns (unvalidated measurement) | Serious | Not serious | Serious | Unclear | - | Low |
| Miscarried helping | Some concerns | Not assessable | Not serious | Very serious | Unclear | - | Very low |
| Pain coping | Some concerns | Not assessable | Not serious | Very serious | Unclear | - | Very low |
| Central sensitization | Some concerns | Not assessable | Not serious | Very serious | Unclear | - | Very low |
| Depression | Low | Not assessable | Not serious | Very serious | Unclear | - | Very low |
| Outcome | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Other Considerations | Certainty |
|---|---|---|---|---|---|---|---|
| Parental catastrophizing | Some concerns | Not assessable | Not serious | Serious | Unclear | - | Low |
| Caregiver anxiety/stress | Low | Serious | Not serious | Very serious | Unclear | - | Very low |
| Parental behaviors/family accommodation | Some concerns | Unclear | Not serious | Serious | Unclear | Heterogeneous instruments | Low |
| Satisfaction (parent/caregiver) | Some concerns (measurement unvalidated) | Serious | Not serious | Very serious | Unclear | - | Very low |
| Disability/Function (proxy) | Some concerns | Serious | Serious (proxy measurement) | Serious | Unclear | - | Low * |
| Pain intensity (proxy) | Some concerns | Serious | Serious (proxy measurement) | Serious | Unclear | - | Low * |
| Code | Description |
|---|---|
| m | If the program includes a parent-directed component, parent outcomes are direct (do not downgrade for indirectness merely for being parental). |
| n | Prioritize child self-report for subjective outcomes; use proxy only if self-report is unavailable. |
| p | Satisfaction measures are heterogeneous and often unvalidated in pediatrics. |
| q | PGIC is susceptible to expectation/non-blinding; few studies. |
| r | Distinguish satisfaction with the program/intervention vs. with services/care. |
| s | Proxy rows are shown only if ≥1 study contributes data; otherwise omitted from SoF and mentioned in text. |
| t | In the evidence profile, proxy outcomes are downgraded for INDIRECTNESS (≥1 level). |
| u | Adverse events: critical pre-specified outcome; not reported by included studies (kept as a row in SoF and noted in text). |
| ℓ | When a child’s outcome is measured by parent proxy, consider downgrading for INDIRECTNESS. |
Appendix F. Characteristics of the Included Studies
| Author | Sample | Intervention | Assessment | Results (GE Versus GC) |
|---|---|---|---|---|
| Palermo et al. [39] | n = 273 11–17 years
Parents included | Randomized controlled trial. Setting: Home. Duration: 4 weeks. Follow-up: 6 months. EG: Internet-delivered CBT. CG: Internet-delivered PNE. |
|
|
| Andías et al. [40] | n = 43 16–18 years
Cervical CP | Pilot randomized controlled study. Setting: School. Duration: 1 session/week for 4 weeks. Follow-up: Not performed. EG: PNE and exercises. CG: No intervention. |
|
|
| Neto et al. [48] This trial was conducted after the Andías 2018 study. | n = 43 16–18 years
| Qualitative randomized controlled trial. Setting: School. Duration: 1 session/week for 4 consecutive weeks. Follow-up: Not performed. EG: Exercises + PNE. CG: No intervention. | Relevance of the knowledge acquired and perception of the intervention: through 4 focus group interviews analyzed using the COREQ criteria (items 1–4, 9–21, 24–26, and 29–32). |
|
| Wager et al. [49] | n = 33 10–15 years
| Feasibility study. Setting: School. Duration: 45–50 min. Follow-up: Not performed. EG = CG = PNE (10-min educational film on CP management). |
|
|
| Pas et al. [41] | n = 28 6 y 12 years
Parents included | Comparative pilot randomized study. Setting: University Hospital. Duration: Once per week for 3 weeks. Follow-up: At 3 weeks. EG: 1 session of hypnotherapy + PNE (PNE4Kids). CG: 2 sessions of hypnotherapy. |
Function: FDI-P Fear of pain: FOPQ-P |
|
| Louw et al. [42] | n = 96 11–13 years
| Quasi-experimental clinical trial. Setting: School. Duration: 30-min lecture. Follow-up: 6 months. G1: Lecture on PNE. G2: Usual care (UC). G3: PNE + 2 booster sessions (PNEBoost). |
|
|
| Kisling et al. [43] | n = 108 9–14 years
| Randomized controlled trial. Setting: School. Duration: 4–5 weeks. Follow-up: At 4–5 weeks. EG = CG = PNE (10-min educational film on CP management). |
|
|
| Walker et al. [44] | n = 278 11–17 years
Parents included | Randomized controlled trial. Setting: Home. Duration: 8 weeks. Follow-up: At 6 and 12 months. EG: Internet-delivered CBT. CG: Internet-delivered PNE. |
|
|
| Andías et al. [45] | n = 127 15–18 years
| Randomized controlled trial. Setting: School. Duration: 8 weeks. Follow-up: At 6 months. EG: Exercise + PNE. CG: Exercise. |
|
|
| Beach et al. [46] | n = 17 12–18 years
Parents included | Pilot randomized controlled study Setting: University Pediatric Neurology Clinic. Duration: 10 min. Follow-up: At 6 months. EG = CG: PNE with a 3D brain model and a 10-min discussion. |
|
|
| Menés et al. [47] | n = 73 11–13 years
| Randomized controlled trial. Setting: School. Duration: Two sessions with a 4-week interval between them. Follow-up: At 7 and 13 months. EG: PNE. CG: Standard teaching program. |
|
|
References
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic Pain as a Symptom or a Disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Volcheck, M.M.; Graham, S.M.; Fleming, K.C.; Mohabbat, A.B.; Luedtke, C.A. Central Sensitization, Chronic Pain, and Other Symptoms: Better Understanding, Better Management. Cleve Clin. J. Med. 2023, 90, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.T.; Dol, J.; Tutelman, P.R.; Langley, C.L.; Parker, J.A.; Cormier, B.T.; Macfarlane, G.J.; Jones, G.T.; Chapman, D.; Proudfoot, N.; et al. The Prevalence of Chronic Pain in Children and Adolescents: A Systematic Review Update and Meta-Analysis. Pain 2024, 165, 2215–2234. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.; Steultjens, M.; Riskowski, J. Chronic Widespread Pain Prevalence in the General Population: A Systematic Review. Eur. J. Pain 2018, 22, 5–18. [Google Scholar] [CrossRef]
- Miró, J.; Roman-Juan, J.; Sánchez-Rodríguez, E.; Solé, E.; Castarlenas, E.; Jensen, M.P. Chronic Pain and High Impact Chronic Pain in Children and Adolescents: A Cross-Sectional Study. J. Pain 2023, 24, 812–823. [Google Scholar] [CrossRef]
- Kusi Amponsah, A.; Kyei-Dompim, J.; Kyei, E.F.; Oduro, E.; Afaya, R.A.; Ahoto, C.K. Final Year Nursing Students’ Knowledge and Attitudes Regarding Children’s Pain. Pain. Res. Manag. 2020, 2020, 7283473. [Google Scholar] [CrossRef]
- Forgeron, P.A.; Chambers, C.T.; Cohen, J.; Dick, B.D.; Finley, G.A.; Lamontagne, C. Dyadic Differences in Friendships of Adolescents with Chronic Pain Compared with Pain-Free Peers. Pain 2018, 159, 1103–1111. [Google Scholar] [CrossRef]
- Hjern, A.; Alfven, G.; Östberg, V. School Stressors, Psychological Complaints and Psychosomatic Pain. Acta Paediatr. Int. 2008, 97, 112–117. [Google Scholar] [CrossRef]
- Du, Y.; Knopf, H.; Zhuang, W.; Ellert, U. Pain Perceived in a National Community Sample of German Children and Adolescents. Eur. J. Pain 2011, 15, 649–657. [Google Scholar] [CrossRef]
- Williams, A.C.D.C.; Craig, K.D. Updating the Definition of Pain. Pain 2016, 157, 2420–2423. [Google Scholar] [CrossRef]
- Kröner-Herwig, B.; Gassmann, J.; Van Gessel, H.; Vath, N. Multiple Pains in Children and Adolescents: A Risk Factor Analysis in a Longitudinal Study. J. Pediatr. Psychol. 2011, 36, 420–432. [Google Scholar] [CrossRef]
- Moseley, G.L. Reconceptualising Pain According to Modern Pain Science. Phys. Ther. Rev. 2007, 12, 169–178. [Google Scholar] [CrossRef]
- Nijs, J.; Torres-Cueco, R.; Paul Van Wilgen, C.; Girbés, E.L.; Struyf, F.; Roussel, N.; Van Oosterwijck, J.; Daenen, L.; Kuppens, K.; Vanderweeën, L.; et al. Applying Modern Pain Neuroscience in Clinical Practice: Criteria for the Classification of Central Sensitization Pain. Pain Physician 2014, 17, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Pas, R.; Ickmans, K.; VanOosterwijck, S.; Van derCruyssen, K.; Foubert, A.; Leysen, L.; Nijs, J.; Meeus, M. Hyperexcitability of the Central Nervous Systemin Children with Chronic Pain: A Systematic Review. Pain Med. 2018, 19, 2504–2514. [Google Scholar] [CrossRef] [PubMed]
- Harte, S.E.; Harris, R.E.; Clauw, D.J. The Neurobiology of Central Sensitization. J. Appl. Biobehav. Res. 2018, 23, e12137. [Google Scholar] [CrossRef]
- Fine, J.G.; Sung, C. Neuroscience of Child and Adolescent Health Development. J. Couns. Psychol. 2014, 61, 521–527. [Google Scholar] [CrossRef]
- Tsao, J.C.; Seidman, L.C.; Evans, S.; Lung, K.C.; Zeltzer, L.K.; Naliboff, B.D. Conditioned Pain Modulation (CPM) in Children and Adolescents. J. Pain 2013, 14, 558–567. [Google Scholar] [CrossRef]
- Cáceres-Matos, R.; Gil-García, E.; Barrientos-Trigo, S.; Molina, E.; Porcel-Gálvez, A.M. Consequences of Chronic Pain in Childhood and Adolescence. Gac Sanit. 2019, 33, 272–282. [Google Scholar] [CrossRef]
- Roman-Juan, J.; Ceniza-Bordallo, G.; Sánchez-Rodríguez, E.; Jensen, M.P.; Miró, J. Fatigue, Sleep Disturbance, and Pain Interference in Children and Adolescents with Chronic Pain: A Longitudinal Study. Pain 2025, 166, 927–935. [Google Scholar] [CrossRef]
- Long, A.C.; Krishnamurthy, V.; Palermo, T.M. Sleep Disturbances in School-Age Children with Chronic Pain. J. Pediatr. Psychol. 2008, 33, 258–268. [Google Scholar] [CrossRef]
- Nelson, S.; Miller, J.V.; Timmers, I.; Simons, L.E.; Meldrum, K.; Noel, M. Paediatric Chronic Pain as a Catalyst for Toxic Stress. Lancet Child Adolesc. Health 2022, 6, 671–672. [Google Scholar] [CrossRef]
- Wrona, S.K.; Melnyk, B.M.; Hoying, J. Chronic Pain and Mental Health Co-Morbidity in Adolescents: An Urgent Call for Assessment and Evidence-Based Intervention. Pain Manag. Nurs. 2021, 22, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Solé, E.; Roman-Juan, J.; Sánchez-Rodríguez, E.; Castarlenas, E.; Jensen, M.P.; Miró, J. School Bullying and Peer Relationships in Children with Chronic Pain. Pain 2024, 165, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Szabo, E.; Chang, Y.C.; Shulman, J.; Sieberg, C.B.; Sethna, N.F.; Borsook, D.; Holmes, S.A. Lebel AA Alterations in the Structure and Function of the Brain in Adolescents with New Daily Persistent Headache: A Pilot MRI Study. Headache 2022, 62, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.; Dengler-Crish, C.M.; Rippel, S.; Bruehl, S. Functional Abdominal Pain in Childhood and Adolescence Increases Risk for Chronic Pain in Adulthood. Pain 2010, 150, 568–572. [Google Scholar] [CrossRef]
- Brna, P.; Dooley, J.; Gordon, K.; Dewan, T. He Prognosis of Childhood Headache: A 20-Year Follow-Up. Arch. Pediatr. Adolesc. Med. 2005, 159, 1157–1160. [Google Scholar] [CrossRef]
- Lo Cascio, A.; Cascino, M.; Dabbene, M.; Paladini, A.; Viswanath, O.; Varrassi, G.; Latina, R. Epidemiology of Pediatric Chronic Pain: An Overview of Systematic Reviews. Curr. Pain Headache Rep. 2025, 29, 71. [Google Scholar] [CrossRef]
- Zernikow, B.; Ruhe, A.; Stahlschmidt, L.; Schmidt, P.; Staratzke, T.; Frosch, M.; Wager, J. Clinical and Economic Long-Term Treatment Outcome of Children and Adolescents with Disabling Chronic Pain. Pain Med. 2017, 19, 16–28. [Google Scholar] [CrossRef]
- Wren, A.; Ross, A.; D’Souza, G.; Almgren, C.; Feinstein, A.; Marshall, A.; Golianu, B. Multidisciplinary Pain Management for Pediatric Patients with Acute and Chronic Pain: A Foundational Treatment Approach When Prescribing Opioids. Children 2019, 6, 33. [Google Scholar] [CrossRef]
- Rheel, E.; Ickmans, K.; Wauters, A.; Van Ryckeghem, D.M.L.; Malfliet, A.; Vervoort, T. The Effect of a Pain Educational Video Intervention upon Child Pain-Related Outcomes: A Randomized Controlled Study. Eur. J. Pain 2021, 25, 2094–2111. [Google Scholar] [CrossRef]
- Cuenda-Gago, J.D.; Espejo-Antunez, L. Efectividad de La Educacion Basada En NeurocienEffectiveness of Education Based on Neuroscience in the Treatment of Musculoskeletal Chronic Pain. Rev. Neurol. 2017, 65, 1–12. [Google Scholar]
- World Health Organization. Guidelines on the Management of Chronic Pain in Children; World Health Organization: Geneva, Switzerland, 2020; ISBN 13:978-92-4-001788-7. [Google Scholar]
- Eccleston, C.; Fisher, E.; Howard, R.F.; Slater, R.; Forgeron, P.; Palermo, T.M.; Birnie, K.A.; Anderson, B.J.; Chambers, C.T.; Crombez, G.; et al. Delivering Transformative Action in Paediatric Pain: A Lancet Child & Adolescent Health Commission. Lancet Child Adolesc. Health 2021, 5, 47–87. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Critical Appraisal Skills Programme (CASP). CASP Qualitative Checklist [Internet]; CASP: Oxford, UK, 2024; Available online: https://casp-uk.net/casp-checklists/CASP-checklist-qualitative-2024.pdf (accessed on 25 July 2025).
- National Heart, Lung, and Blood Institute (NHLBI). Study Quality Assessment Tools [Internet]; National Institutes of Health (NIH): Bethesda, MD, USA, 2018. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 25 July 2025).
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without Meta-Analysis (SWiM) in Systematic Reviews: Reporting Guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Palermo, T.M.; Law, E.F.; Fales, J.; Bromberg, M.H.; Jessen-Fiddick, T.; Tai, G. Internet-Delivered Cognitive-Behavioral Treatment for Adolescents with Chronic Pain and Their Parents: A Randomized Controlled Multicenter Trial. Pain 2016, 157, 174. [Google Scholar] [CrossRef]
- Andias, R.; Neto, M.; Silva, A.G. The Effects of Pain Neuroscience Education and Exercise on Pain, Muscle Endurance, Catastrophizing and Anxiety in Adolescents with Chronic Idiopathic Neck Pain: A School-Based Pilot, Randomized and Controlled Study. Physiother. Theory Pract. 2018, 34, 682–691. [Google Scholar] [CrossRef]
- Pas, R.; Rheel, E.; Van Oosterwijck, S.; Foubert, A.; De Pauw, R.; Leysen, L.; Roete, A.; Nijs, J.; Meeus, M.; Ickmans, K. Pain Neuroscience Education for Children with Functional Abdominal Pain Disorders: A Randomized Comparative Pilot Study. J. Clin. Med. 2020, 9, 1797. [Google Scholar] [CrossRef]
- Louw, A.; Landrus, R.; Podolak, J.; Benz, P.; Delorenzo, J.; Davis, C.; Rogers, A.; Cooper, K.; Louw, C.; Zimney, K.; et al. Behavior Change Following Pain Neuroscience Education in Middle Schools: A Public Health Trial. Int. J. Environ. Res. Public Health 2020, 17, 4505. [Google Scholar] [CrossRef]
- Kisling, S.; Claus, B.B.; Stahlschmidt, L.; Wager, J. The Efficacy of an Educational Movie to Improve Pain and Dysfunctional Behavior in School Children: A Randomized Controlled Trial. Eur. J. Pain 2021, 25, 1612–1621. [Google Scholar] [CrossRef]
- Walker, L.S.; Stone, A.L.; Han, G.T.; Garber, J.; Bruehl, S.; Smith, C.A.; Anderson, J.; Palermo, T.M. Internet-Delivered Cognitive Behavioral Therapy for Youth with Functional Abdominal Pain: A Randomized Clinical Trial Testing Differential Efficacy by Patient Subgroup. Pain 2021, 162, 2945–2955. [Google Scholar] [CrossRef]
- Andias, R.; Sa-Couto, P.; Silva, A.G. Blended-Learning Pain Neuroscience Education and Exercise in High School Students with Chronic Neck Pain: A Randomized Controlled Trial. Phys. Ther. 2022, 102, pzac048. [Google Scholar] [CrossRef]
- Beach, I.R.; Madhur, R.M.; Bingham, P.M. Pain Neuroscience Education: A Pilot Trial in Pediatric Primary Headache. Int. J. Pediatr. 2022, 10, 15919–15924. [Google Scholar] [CrossRef]
- Menés Fernández, L.; Salvat, I.; Adillón, C. Effectiveness of a Pain Science Education Programme in Middle School Students: A Randomised Controlled Trial. Front. Public Health 2024, 12, 1423716. [Google Scholar] [CrossRef] [PubMed]
- Neto, M.; Andias, R.; Silva, A.G. Pain Neuroscience Education and Exercise for Neck Pain: A Focus Group Study on Adolescents’ Views. Pediatr. Phys. Ther. 2018, 30, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Wager, J.; Stahlschmidt, L.; Heuer, F.; Troche, S.; Zernikow, B. The Impact of a Short Educational Movie on Promoting Chronic Pain Health Literacy in School: A Feasibility Study. Eur. J. Pain 2018, 22, 1142–1150. [Google Scholar] [CrossRef]
- Moseley, G.L.; Butler, D.S. Explaining Pain, 2nd ed.; Noigroup Publications: Adelaide, Australia, 2013. [Google Scholar]
- Louw, A.; Puentedura, E.; International Spine and Pain Institute. Therapeutic Neuroscience Education: Teaching Patients About Pain; Hand Therapy Press: Salt Lake City, UT, USA, 2014. [Google Scholar]
- Leake, H.B.; Heathcote, L.C.; Simons, L.E.; Stinson, J.; Kamper, S.J.; Williams, C.M.; Burgoyne, L.L.; Craigie, M.; Kammers, M.; Moen, D.; et al. Talking to Teens about Pain: A Modified Delphi Study of Adolescent Pain Science Education. Can. J. Pain 2019, 3, 200–208. [Google Scholar] [CrossRef]
- Louw, A. Why Do I Hurt? A Patient Book About the Neuroscience of Pain, 1st ed.; Orthopedic Physical Therapy Products: Minneapolis, MN, USA, 2013. [Google Scholar]
- López, C.C. Cuentos Analgésicos, 2nd ed.; Zérapi: Córdoba, Spain, 2018. [Google Scholar]
- Solano, E.G.; Gómez, M.A.B.; Algarra, R.R.; Aguirre, R.M.I.; Verdecho, M.A.C. Gender Determinants in the Approach to Chronic Pain. Rev. Soc. Esp. Dolor 2020, 27, 252–256. [Google Scholar]
- Gobina, I.; Villberg, J.; Välimaa, R.; Tynjälä, J.; Whitehead, R.; Cosma, A.; Brooks, F.; Cavallo, F.; Ng, K.; de Matos, M.G.; et al. Prevalence of Self-Reported Chronic Pain among Adolescents: Evidence from 42 Countries and Regions. Eur. J. Pain 2019, 23, 316–326. [Google Scholar] [CrossRef]
- Chmura Kraemer, H.; Mintz, J.; Noda, A.; Tinklenberg, J.; Yesavage, J.A. Caution Regarding the Use of Pilot Studies to Guide Power Calculations for Study Proposals. Arch. Gen. Psychiatry 2006, 63I, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Vetter, T.R.; Bridgewater Bsn Msn, C.L.; Ascherman, L.I.; Madan-Swain, A.; Mcgwin, G.L. Patient versus Parental Perceptions about Pain and Disability in Children and Adolescents with a Variety of Chronic Pain Conditions. Pain Res. Manag. 2014, 19, 7–14. [Google Scholar] [CrossRef]
- Hoftun, G.B.; Romundstad, P.R.; Rygg, M. Association of Parental Chronic Pain with Chronic Pain in the Adolescent and Young Adult: Family Linkage Data from the HUNT Study. Arch. Pediatr. Adolesc. Med. 2013, 167, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Valrie, C.R.; Karlson, C.W. Family and Parent Influences on Pediatric Chronic Pain: A Developmental Perspective. Am. Psychol. 2014, 69, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Sieberg, C.B.; Williams, S.; Simons, L.E. Do Parent Protective Responses Mediate the Relation between Parent Distress and Child Functional Disability among Children with Chronic Pain? J. Pediatr. Psychol. 2011, 36, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Alsaggaf, F.; Coyne, I. A Systematic Review of the Impact of Chronic Pain on Adolescents’ School Functioning and School Personnel Responses to Managing Pain in the Schools. J. Adv. Nurs. 2020, 76, 2005–2022. [Google Scholar] [CrossRef]
- Gainforth, H.L.; Berry, T.; Faulkner, G.; Rhodes, R.E.; Spence, J.C.; Tremblay, M.S.; Latimer-Cheung, A.E. Evaluating the Uptake of Canada’s New Physical Activity and Sedentary Behavior Guidelines on Service Organizations’ Websites. Transl. Behav. Med. 2013, 3, 172–179. [Google Scholar] [CrossRef]
- Buchbinder, R.; Jolley, D. Population Based Intervention to Change Back Pain Beliefs: Three Year Follow up Population Survey. BMJ 2004, 328, 321. [Google Scholar] [CrossRef][Green Version]
- Donnelly, T.J.; Palermo, T.M.; Newton-John, T.R.O. Parent Cognitive, Behavioural, and Affective Factors and Their Relation to Child Pain and Functioning in Pediatric Chronic Pain: A Systematic Review and Meta-Analysis. Pain 2020, 161, 1401–1419. [Google Scholar] [CrossRef]
- Andrews, N.E.; Ireland, D.; Vijayakumar, P.; Burvill, L.; Hay, E.; Westerman, D.; Rose, T.; Schlumpf, M.; Strong, J.; Claus, A. Acceptability of a Pain History Assessment and Education Chatbot (Dolores) Across Age Groups in Populations with Chronic Pain: Development and Pilot Testing. JMIR Form. Res. 2023, 7, e47267. [Google Scholar] [CrossRef]
- Jones, T.; Moore, T.; Choo, J. The Impact of Virtual Reality on Chronic Pain. PLoS ONE 2016, 11, 167523. [Google Scholar] [CrossRef]
- Logan, D.E.; Khanna, K.; Randall, E.; O’Donnell, S.; Reks, T.; McLennan, L. Centering Patient and Clinician Voices in Developing Tools to Address Pain Related School Impairment: A Phase I Study of a Virtual Reality School Simulation for Children and Adolescents with Chronic Pain. Children 2023, 10, 1644. [Google Scholar] [CrossRef]
- Kjeldgaard Pedersen, L.; Fisker, L.Y.V.; Rölfing, J.D.; Ahlburg, P.; Veien, M.; Vase, L.; Møller-Madsen, B. Virtual Reality Increases Pressure Pain Threshold and Lowers Anxiety in Children Compared with Control and Non-Immersive Control-A Randomized, Crossover Trial. Eur. J. Pain 2023, 27, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P. Integrated Theory of Health Behavior Change: Background and Intervention Development. Clin. Nurse Spec. 2009, 23, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lillo, S. Physical Medicine and Rehabilitation in Pediatric Chronic Pain. Rev. Med. Clin. Condes 2019, 30, 436–445. [Google Scholar] [CrossRef]
- Revivo, G.; Amstutz, D.K.; Gagnon, C.M.; McCormick, Z.L. Interdisciplinary Pain Management Improves Pain and Function in Pediatric Patients with Chronic Pain Associated with Joint Hypermobility Syndrome. PM&R 2019, 11, 150–157. [Google Scholar] [CrossRef]
- Nascimento Leite, M.; Kamper, S.J.; O’Connell, N.E.; Michaleff, Z.A.; Fisher, E.; Viana Silva, P.; Williams, C.M.; Yamato, T.P. Physical Activity and Education about Physical Activity for Chronic Musculoskeletal Pain in Children and Adolescents. Cochrane Database Syst. Rev. 2023, 2023, CD013527. [Google Scholar] [CrossRef]
- McGrath, P.J.; Walco, G.A.; Turk, D.C.; Dworkin, R.H.; Brown, M.T.; Davidson, K.; Eccleston, C.; Finley, G.A.; Goldschneider, K.; et al. Core Outcome Domains and Measures for Pediatric Acute and Chronic/Recurrent Pain Clinical Trials: PedIMMPACT Recommendations. J. Pain 2008, 9, 771–783. [Google Scholar] [CrossRef]
- Roldan-Jimenez, C.; Perez-Cruzado, D.; Neblett, R.; Gatchel, R.; Cuesta-Vargas, A. Central Sensitization in Chronic Musculoskeletal Pain Disorders in Different Populations: A Cross-Sectional Study. Pain Med. 2020, 21, 2958–2963. [Google Scholar] [CrossRef]
- Hwang, P.S.; Ma, M.L.; Spiegelberg, N.; Ferland, C.E. Current Methodological Approaches in Conditioned Pain Modulation Assessment in Paediatrics. J. Pain Res. 2017, 10, 2797–2802. [Google Scholar] [CrossRef][Green Version]
- Dudeney, J.; Aaron, R.V.; Hathway, T.; Bhattiprolu, K.; Bisby, M.A.; McGill, L.S.; Gandy, M.; Harte, N.; Dear, B.F. Anxiety and Depression in Youth with Chronic Pain: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2024, 178, 1114–1123. [Google Scholar] [CrossRef]
- Samwel, H.J.A.; Evers, A.W.M.; Crul, B.J.P.; Kraaimaat, F.W. The Role of Helplessness, Fear of Pain, and Passive Pain-Coping in Chronic Pain Patients. Clin. J. Pain 2006, 22, 245–251. [Google Scholar] [CrossRef]
- Miller, M.M.; Meints, S.M.; Hirsh, A.T. Catastrophizing, Pain, and Functional Outcomes for Children with Chronic Pain: A Meta-Analytic Review. Pain 2018, 159, 2442–2460. [Google Scholar] [CrossRef]
- Elbers, S.; Wittink, H.; Konings, S.; Kaiser, U.; Kleijnen, J.; Pool, J.; Köke, A.; Smeets, R. Longitudinal Outcome Evaluations of Interdisciplinary Multimodal Pain Treatment Programmes for Patients with Chronic Primary Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Eur. J. Pain 2022, 26, 310–335. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Law, E.; Churchill, S.S.; Walker, A. Longitudinal Course and Impact of Insomnia Symptoms in Adolescents with and without Chronic Pain. J. Pain 2012, 13, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.; Heathcote, L.C.; Eccleston, C.; Simons, L.E.; Palermo, T.M. Assessment of Pain Anxiety, Pain Catastrophizing, and Fear of Pain in Children and Adolescents with Chronic Pain: A Systematic Review and Meta-Analysis. J. Pediatr. Psychol. 2018, 43, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Landry, B.W.; Fischer, P.R.; Driscoll, S.W.; Koch, K.M.; Harbeck-Weber, C.; Mack, K.J.; Wilder, R.T.; Bauer, B.A.; Brandenburg, J.E. Managing Chronic Pain in Children and Adolescents: A Clinical Review. PM&R 2015, 7, S295-315. [Google Scholar] [CrossRef]
- Odell, S.; Logan, D.E. Pediatric Pain Management: The Multidisciplinary Approach. J. Pain Res. 2013, 6, 785–790. [Google Scholar] [CrossRef]
- Murray, C.B.; de la Vega, R.; Loren, D.M.; Palermo, T.M. Moderators of Internet-Delivered Cognitive-Behavioral Therapy for Adolescents with Chronic Pain: Who Benefits from Treatment at Long-Term Follow-Up? J. Pain 2020, 21, 603–615. [Google Scholar] [CrossRef]
- Ceniza-Bordallo, G.; Guerra-Armas, J.; Flores-Cortes, M.; Bermúdez-Ramirez, S. Multimodal Physiotherapist Intervention Program for Physical and Psychological Functioning in Children with Chronic Pain: Guiding Physiotherapy Intervention with the Pediatric Pain Screening Tool with Recommendations for Clinical Practice. J. Clin. Med. 2025, 14, 3629. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pico, M.; Matey-Rodríguez, C.; Domínguez-García, A.; Yubero, N.; Santos-Lozano, A. Pain Neuroscience Education in Children and Adolescents with Chronic Pain: A Systematic Review. Children 2025, 12, 1317. https://doi.org/10.3390/children12101317
Pico M, Matey-Rodríguez C, Domínguez-García A, Yubero N, Santos-Lozano A. Pain Neuroscience Education in Children and Adolescents with Chronic Pain: A Systematic Review. Children. 2025; 12(10):1317. https://doi.org/10.3390/children12101317
Chicago/Turabian StylePico, Mónica, Carmen Matey-Rodríguez, Ana Domínguez-García, Noemí Yubero, and Alejandro Santos-Lozano. 2025. "Pain Neuroscience Education in Children and Adolescents with Chronic Pain: A Systematic Review" Children 12, no. 10: 1317. https://doi.org/10.3390/children12101317
APA StylePico, M., Matey-Rodríguez, C., Domínguez-García, A., Yubero, N., & Santos-Lozano, A. (2025). Pain Neuroscience Education in Children and Adolescents with Chronic Pain: A Systematic Review. Children, 12(10), 1317. https://doi.org/10.3390/children12101317