Time and Mind: A State-of-the-Art Perspective on Time Perception and Cognitive–Motor Interactions in Children and Adolescents with Cerebral Palsy
Abstract
Highlights
- •
- In children and adolescents with cerebral palsy, time perception difficulties are not fully explained by motor impairments and likely reflect broader cognitive–perceptual disruption.
- •
- When studies minimize motor demands, selective time-perception deficits can still appear, indicating that timing can be affected independently of movement difficulties.
- •
- Temporal perception should be treated as a distinct, clinically relevant domain in cerebral palsy, with consequences for daily functioning and development.
- •
- Assessment and intervention should explicitly disentangle perceptual timing from motor execution, favoring motor-minimal paradigms to guide targeted rehabilitation.
Highlights
- •
- In children and adolescents with cerebral palsy, time-perception difficulties are not fully explained by motor impairments and may reflect broader cognitive–perceptual disruption.
- •
- Studies that minimize motor demands sometimes reveal selective time-perception deficits, indicating that timing can be affected independent of movement difficulties.
- •
- Temporal processing should be recognized as a distinct and clinically relevant domain in cerebral palsy, with consequences for daily functioning and development.
- •
- Assessment and intervention should explicitly disentangle perceptual timing from motor execution and favor motor-minimal paradigms to guide targeted rehabilitation.
Abstract
1. Introduction
Terminology and Scope
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TP | Time perception |
| CP | Cerebral palsy |
| SMT | Synchronized metronome training |
| TPA | Time-processing ability |
| SMA | Supplemental motor area |
| BG | Basal ganglia |
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A Report: The Definition and Classification of Cerebral Palsy April 2006. Dev. Med. Child. Neurol. Suppl. 2007, 109, 8–14. [Google Scholar]
- Stadskleiv, K. Cognitive Functioning in Children with Cerebral Palsy. Dev. Med. Child. Neurol. 2020, 62, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Laporta-Hoyos, O.; Pannek, K.; Pagnozzi, A.M.; Whittingham, K.; Wotherspoon, J.; Benfer, K.; Fiori, S.; Ware, R.S.; Boyd, R.N. Cognitive, Academic, Executive and Psychological Functioning in Children with Spastic Motor Type Cerebral Palsy: Influence of Extent, Location, and Laterality of Brain Lesions. Eur. J. Paediatr. Neurol. 2022, 38, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Zimonyi, N.; Kói, T.; Dombrádi, V.; Imrei, M.; Nagy, R.; Pulay, M.Á.; Lang, Z.; Hegyi, P.; Takacs, Z.K.; Túri, I. Comparison of Executive Function Skills between Patients with Cerebral Palsy and Typically Developing Populations: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 1867. [Google Scholar] [CrossRef]
- Delobel-Ayoub, M.; Klapouszczak, D.; van Bakel, M.M.E.; Horridge, K.; Sigurdardottir, S.; Himmelmann, K.; Arnaud, C. Prevalence and Characteristics of Autism Spectrum Disorders in Children with Cerebral Palsy. Dev. Med. Child. Neurol. 2017, 59, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Bøttcher, L.; Stadskleiv, K.; Berntsen, T.; Christensen, K.; Korsfelt, Å.; Kihlgren, M.; Ödman, P. Systematic Cognitive Monitoring of Children with Cerebral Palsy—The Development of an Assessment and Follow-up Protocol. Scand. J. Disabil. Res. 2017, 18, 304–315. [Google Scholar] [CrossRef]
- Compagnone, E.; Maniglio, J.; Camposeo, S.; Vespino, T.; Losito, L.; De Rinaldis, M.; Gennaro, L.; Trabacca, A. Functional Classifications for Cerebral Palsy: Correlations between the Gross Motor Function Classification System (GMFCS), the Manual Ability Classification System (MACS) and the Communication Function Classification System (CFCS). Res. Dev. Disabil. 2014, 35, 2651–2657. [Google Scholar] [CrossRef]
- Fluss, J.; Lidzba, K. Cognitive and Academic Profiles in Children with Cerebral Palsy: A Narrative Review. Ann. Phys. Rehabil. Med. 2020, 63, 447–456. [Google Scholar] [CrossRef]
- Stadskleiv, K.; Jahnsen, R.; Andersen, G.L.; von Tetzchner, S. Neuropsychological Profiles of Children with Cerebral Palsy. Dev. Neurorehabil 2018, 21, 108–120. [Google Scholar] [CrossRef]
- Critten, V.; Campbell, E.; Farran, E.; Messer, D. Visual Perception, Visual-Spatial Cognition and Mathematics: Associations and Predictions in Children with Cerebral Palsy. Res. Dev. Disabil. 2018, 80, 180–191. [Google Scholar] [CrossRef]
- Barca, L.; Frascarelli, F.; Pezzulo, G. Working Memory and Mental Imagery in Cerebral Palsy: A Single Case Investigation. Neurocase 2012, 18, 298–304. [Google Scholar] [CrossRef]
- Peeters, M.; Verhoeven, L.; de Moor, J. Predictors of Verbal Working Memory in Children with Cerebral Palsy. Res. Dev. Disabil. 2009, 30, 1502–1511. [Google Scholar] [CrossRef]
- Gagliardi, C.; Brenna, V.; Romaniello, R.; Arrigoni, F.; Tavano, A.; Romani, M.; Valente, E.M.; Borgatti, R. Cognitive Rehabilitation in a Child with Joubert Syndrome: Developmental Trends and Adaptive Changes in a Single Case Report. Res. Dev. Disabil. 2015, 47, 375–384. [Google Scholar] [CrossRef] [PubMed]
- White, D.A.; Christ, S.E. Executive Control of Learning and Memory in Children with Bilateral Spastic Cerebral Palsy. J. Int. Neuropsychol. Soc. 2005, 11, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.S.; Woollacott, M.H.; van Donkelaar, P.; Saavedra, S. The Interaction between Executive Attention and Postural Control in Dual-Task Conditions: Children with Cerebral Palsy. Arch. Phys. Med. Rehabil. 2008, 89, 834–842. [Google Scholar] [CrossRef]
- Surkar, S.M.; Edelbrock, C.; Stergiou, N.; Berger, S.; Harbourne, R. Sitting Postural Control Affects the Development of Focused Attention in Children with Cerebral Palsy. Pediatr. Phys. Ther. 2015, 27, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Lopes, S.; Magalhães, P.; Sampaio, A.; Chaleta, E.; Rosário, P. How Executive Functions Are Evaluated in Children and Adolescents with Cerebral Palsy? A Systematic Review. Front. Psychol. 2018, 9, 21. [Google Scholar] [CrossRef]
- Weierink, L.; Vermeulen, R.J.; Boyd, R.N. Brain Structure and Executive Functions in Children with Cerebral Palsy: A Systematic Review. Res. Dev. Disabil. 2013, 34, 1678–1688. [Google Scholar] [CrossRef]
- Mei, C.; Reilly, S.; Reddihough, D.; Mensah, F.; Pennington, L.; Morgan, A. Language Outcomes of Children with Cerebral Palsy Aged 5 Years and 6 Years: A Population-Based Study. Dev. Med. Child. Neurol. 2016, 58, 605–611. [Google Scholar] [CrossRef]
- Pirila, S.; van der Meere, J.; Pentikainen, T.; Ruusu-Niemi, P.; Korpela, R.; Kilpinen, J.; Nieminen, P. Language and Motor Speech Skills in Children with Cerebral Palsy. J. Commun. Disord. 2007, 40, 116–128. [Google Scholar] [CrossRef]
- Cabezas, M.; Carriedo, N. Inhibitory Control and Temporal Perception in Cerebral Palsy. Child. Neuropsychol. 2020, 26, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Olivier, I.; Baker, C.; Cordier, J.; Thomann, G.; Nougier, V. Cognitive and Motor Aspects of a Coincidence-Timing Task in Cerebral Palsy Children. Neurosci. Lett. 2015, 602, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Block, R.A.; Gruber, R.P. Time Perception, Attention, and Memory: A Selective Review. Acta Psychol. (AMST) 2014, 149, 129–133. [Google Scholar] [CrossRef]
- Zélanti, P.S.; Droit-Volet, S. Auditory and Visual Differences in Time Perception? An Investigation from a Developmental Perspective with Neuropsychological Tests. J. Exp. Child Psychol. 2012, 112, 296–311. [Google Scholar] [CrossRef]
- Hartcher-O’Brien, J.; Brighouse, C.; Levitan, C.A. A Single Mechanism Account of Duration and Rate Processing via the Pacemaker-Accumulator and Beat Frequency Models. Curr. Opin. Behav. Sci. 2016, 8, 268–275. [Google Scholar] [CrossRef]
- Treisman, M. The Information-Processing Model of Timing (Treisman, 1963): Its Sources and Further Development. Timing Time Percept. 2013, 1, 131–158. [Google Scholar] [CrossRef]
- Jones, M.R.; Boltz, M. Dynamic Attending and Responses to Time. Psychol. Rev. 1989, 96, 459–491. [Google Scholar] [CrossRef]
- Casini, L.; Pech-Georgel, C.; Ziegler, J.C. It’s about Time: Revisiting Temporal Processing Deficits in Dyslexia. Dev. Sci. 2018, 21. [Google Scholar] [CrossRef] [PubMed]
- Toplak, M.E.; Dockstader, C.; Tannock, R. Temporal Information Processing in ADHD: Findings to Date and New Methods. J. Neurosci. Methods 2006, 151, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, B.; Zou, X.; Jing, J.; Pen, G.; McAlonan, G.M.; Chan, R.C.K. Temporal Processing Impairment in Children with Attention-Deficit-Hyperactivity Disorder. Res. Dev. Disabil. 2012, 33, 538–548. [Google Scholar] [CrossRef]
- Giannotta, G.; Ruggiero, M.; Trabacca, A. Chronobiology in Paediatric Neurological and Neuropsychiatric Disorders: Harmonizing Care with Biological Clocks. J. Clin. Med. 2024, 13, 7737. [Google Scholar] [CrossRef] [PubMed]
- Ivry, R.B.; Spencer, R.M.C. The Neural Representation of Time. Curr. Opin. Neurobiol. 2004, 14, 225–232. [Google Scholar] [CrossRef]
- Merchant, H.; Harrington, D.L.; Meck, W.H. Neural Basis of the Perception and Estimation of Time. Annu. Rev. Neurosci. 2013, 36, 313–336. [Google Scholar] [CrossRef]
- Wittmann, M.; van Wassenhove, V. The Experience of Time: Neural Mechanisms and the Interplay of Emotion, Cognition and Embodiment. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1809–1813. [Google Scholar] [CrossRef]
- Wing, A.M.; Kristofferson, A.B. Response Delays and the Timing of Discrete Motor Responses. Percept. Psychophys. 1973, 14, 5–12. [Google Scholar] [CrossRef]
- Avanzino, L.; Pelosin, E.; Vicario, C.M.; Lagravinese, G.; Abbruzzese, G.; Martino, D. Time Processing and Motor Control in Movement Disorders. Front. Hum. Neurosci. 2016, 10, 631. [Google Scholar] [CrossRef]
- Johansson, A.-M.; Domellöf, E.; Rönnqvist, L. Timing Training in Three Children with Diplegic Cerebral Palsy: Short- and Long-Term Effects on Upper-Limb Movement Organization and Functioning. Front. Neurol. 2014, 5, 38. [Google Scholar] [CrossRef]
- Wiener, M.; Turkeltaub, P.; Coslett, H.B. The Image of Time: A Voxel-Wise Meta-Analysis. Neuroimage 2010, 49, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Block, R.A.; Hancock, P.A.; Zakay, D. How Cognitive Load Affects Duration Judgments: A Meta-Analytic Review. Acta Psychol. 2010, 134, 330–343. [Google Scholar] [CrossRef]
- Repp, B.H.; Su, Y.-H. Sensorimotor Synchronization: A Review of Recent Research (2006–2012). Psychon. Bull. Rev. 2013, 20, 403–452. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.; Nobre, A. Dissociating Explicit Timing from Temporal Expectation with fMRI. Curr. Opin. Neurobiol. 2008, 18, 137–144. [Google Scholar] [CrossRef]
- Masaki, H.; Sommer, W. Cognitive Neuroscience of Motor Learning and Motor Control. J. Phys. Fit. Sports Med. 2012, 1, 369–380. [Google Scholar] [CrossRef]
- Janeslätt, G.; Kottorp, A.; Granlund, M. Evaluating Intervention Using Time Aids in Children with Disabilities. Scand. J. Occup. Ther. 2014, 21, 181–190. [Google Scholar] [CrossRef]
- Sköld, A.; Janeslätt, G.K. Self-Rating of Daily Time Management in Children: Psychometric Properties of the Time-S. Scand. J. Occup. Ther. 2017, 24, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Grondin, S. Timing and Time Perception: A Review of Recent Behavioral and Neuroscience Findings and Theoretical Directions. Atten. Percept. Psychophys. 2010, 72, 561–582. [Google Scholar] [CrossRef] [PubMed]
- Fontes, R.; Ribeiro, J.; Gupta, D.S.; Machado, D.; Lopes-Júnior, F.; Magalhães, F.; Bastos, V.H.; Rocha, K.; Marinho, V.; Lima, G.; et al. Time Perception Mechanisms at Central Nervous System. Neurol. Int. 2016, 8, 5939. [Google Scholar] [CrossRef]
- Himmelmann, K.; Uvebrant, P. Function and Neuroimaging in Cerebral Palsy: A Population-Based Study. Dev. Med. Child. Neurol. 2011, 53, 516–521. [Google Scholar] [CrossRef]
- O’Regan, L.; Spapé, M.M.; Serrien, D.J. Motor Timing and Covariation with Time Perception: Investigating the Role of Handedness. Front. Behav. Neurosci. 2017, 11, 147. [Google Scholar] [CrossRef]
- Schubotz, R.I.; Friederici, A.D.; von Cramon, D.Y. Time Perception and Motor Timing: A Common Cortical and Subcortical Basis Revealed by fMRI. Neuroimage 2000, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Treisman, M.; Faulkner, A.; Naish, P.L. On the Relation between Time Perception and the Timing of Motor Action: Evidence for a Temporal Oscillator Controlling the Timing of Movement. Q. J. Exp. Psychol. A 1992, 45, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Szelag, E.; Kowalska, J.; Galkowski, T.; Pöppel, E. Temporal Processing Deficits in High-Functioning Children with Autism. Br. J. Psychol. 2004, 95, 269–282. [Google Scholar] [CrossRef]
- Allman, M.J.; Teki, S.; Griffiths, T.D.; Meck, W.H. Properties of the Internal Clock: First- and Second-Order Principles of Subjective Time. Annu. Rev. Psychol. 2014, 65, 743–771. [Google Scholar] [CrossRef] [PubMed]
- Hadzagic Catibusic, F.; Avdagic, E.; Zubcevic, S.; Uzicanin, S. Brain Lesions in Children with Unilateral Spastic Cerebral Palsy. Med. Arch. 2017, 71, 7–11. [Google Scholar] [CrossRef]
- Rosello, M.; Caro-Llopis, A.; Orellana, C.; Oltra, S.; Alemany-Albert, M.; Marco-Hernandez, A.V.; Monfort, S.; Pedrola, L.; Martinez, F.; Tomás, M. Hidden Etiology of Cerebral Palsy: Genetic and Clinical Heterogeneity and Efficient Diagnosis by next-Generation Sequencing. Pediatr. Res. 2021, 90, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Cui, M.; Liang, Q.; Zhu, D.; Liu, J.; Hu, J.; Ma, S.; Li, D.; Wang, J.; Wang, X.; et al. Cerebral Palsy Heterogeneity: Clinical Characteristics and Diagnostic Significance from a Large-Sample Analysis. Neuroepidemiology 2024, 58, 470–480. [Google Scholar] [CrossRef]
- Cheney, P.D. Pathophysiology of the Corticospinal System and Basal Ganglia in Cerebral Palsy. Ment. Retard. Dev. Disabil. Res. Rev. 1997, 3, 153–167. [Google Scholar] [CrossRef]
- Schmahmann, J.D. Disorders of the Cerebellum: Ataxia, Dysmetria of Thought, and the Cerebellar Cognitive Affective Syndrome. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 367–378. [Google Scholar] [CrossRef] [PubMed]
- García-Galant, M.; Blasco, M.; Laporta-Hoyos, O.; Berenguer-González, A.; Moral-Salicrú, P.; Ballester-Plané, J.; Caldú, X.; Miralbell, J.; Alonso, X.; Medina-Cantillo, J.; et al. A Randomized Controlled Trial of a Home-Based Computerized Executive Function Intervention for Children with Cerebral Palsy. Eur. J. Pediatr. 2023, 182, 4351–4363. [Google Scholar] [CrossRef]
- Blasco, M.; García-Galant, M.; Ballester-Plané, J.; Laporta-Hoyos, O.; Caldú, X.; Leiva, D.; Boyd, R.N.; Ortibus, E.; Pueyo, R. Clinic Practice Group Transferability of an Executive Function Intervention in Children with Cerebral Palsy: A Randomized Controlled Trial. Dev. Med. Child. Neurol. 2025, 67, 496–509. [Google Scholar] [CrossRef]
- Kalkantzi, A.; Kleeren, L.; Baeyens, D.; Decraene, L.; Crotti, M.; Klingels, K.; Van Campenhout, A.; Verheyden, G.; Ortibus, E.; Feys, H.; et al. Daily-Life Executive Functions and Bimanual Performance in Children with Unilateral Cerebral Palsy. Dev. Med. Child. Neurol. 2025, 67, 1290–1300. [Google Scholar] [CrossRef]
- Buzi, G.; Eustache, F.; Droit-Volet, S.; Desaunay, P.; Hinault, T. Towards a Neurodevelopmental Cognitive Perspective of Temporal Processing. Commun. Biol. 2024, 7, 987. [Google Scholar] [CrossRef] [PubMed]
- Krystecka, K.; Stanczyk, M.; Choinski, M.; Szelag, E.; Szymaszek, A. Time to Inhibit: P300 Amplitude Differences in Individuals with High and Low Temporal Efficiency. Cereb. Cortex 2025, 35, bhae500. [Google Scholar] [CrossRef] [PubMed]
- Nejati, V.; Peyvandi, A. The Impact of Time Perception Remediation on Cold and Hot Executive Functions and Behavioral Symptoms in Children with ADHD. Child. Neuropsychol. 2024, 30, 636–651. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Grant, M.J.; Booth, A. A Typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Olofsson, H.; Brolund, A.; Hellberg, C.; Silverstein, R.; Stenström, K.; Österberg, M.; Dagerhamn, J. Can Abstract Screening Workload Be Reduced Using Text Mining? User Experiences of the Tool Rayyan. Res. Synth. Methods 2017, 8, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Mondok, C.; Wiener, M. Selectivity of Timing: A Meta-Analysis of Temporal Processing in Neuroimaging Studies Using Activation Likelihood Estimation and Reverse Inference. Front. Hum. Neurosci. 2023, 16, 995. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a Critical Appraisal Tool to Assess the Quality of Cross-Sectional Studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Goswami, U. A Temporal Sampling Framework for Developmental Dyslexia. Trends Cogn. Sci. 2011, 15, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Noreika, V.; Falter, C.M.; Rubia, K. Timing Deficits in Attention-Deficit/Hyperactivity Disorder (ADHD): Evidence from Neurocognitive and Neuroimaging Studies. Neuropsychologia 2013, 51, 235–266. [Google Scholar] [CrossRef]
- Janeslätt, G.; Ahlström, S.W.; Granlund, M. Intervention in Time-Processing Ability, Daily Time Management and Autonomy in Children with Intellectual Disabilities Aged 10-17 Years—A Cluster Randomised Trial. Aust. Occup. Ther. J. 2019, 66, 110–120. [Google Scholar] [CrossRef]
- Janeslätt, G.; Granlund, M.; Kottorp, A. Measurement of Time Processing Ability and Daily Time Management in Children with Disabilities. Disabil. Health J. 2009, 2, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Morris, C. Definition and Classification of Cerebral Palsy: A Historical Perspective. Dev. Med. Child. Neurol. Suppl. 2007, 109, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Surveillance of Cerebral Palsy in Europe. Available online: http://scpe.edu.eacd.org (accessed on 16 September 2025).
- Bareš, M.; Apps, R.; Avanzino, L.; Breska, A.; D’Angelo, E.; Filip, P.; Gerwig, M.; Ivry, R.B.; Lawrenson, C.L.; Louis, E.D.; et al. Consensus Paper: Decoding the Contributions of the Cerebellum as a Time Machine. From Neurons to Clinical Applications. Cerebellum 2019, 18, 266–286. [Google Scholar] [CrossRef]
- Aravamuthan, B.R.; Waugh, J.L. Localization of Basal Ganglia and Thalamic Damage in Dyskinetic Cerebral Palsy. Pediatr. Neurol. 2016, 54, 11–21. [Google Scholar] [CrossRef]
- Ballester-Plané, J.; Schmidt, R.; Laporta-Hoyos, O.; Junqué, C.; Vázquez, É.; Delgado, I.; Zubiaurre-Elorza, L.; Macaya, A.; Póo, P.; Toro, E.; et al. Whole-Brain Structural Connectivity in Dyskinetic Cerebral Palsy and Its Association with Motor and Cognitive Function. Hum. Brain Mapp. 2017, 38, 4594–4612. [Google Scholar] [CrossRef]
- Lee, J.D.; Park, H.-J.; Park, E.S.; Oh, M.-K.; Park, B.; Rha, D.-W.; Cho, S.-R.; Kim, E.Y.; Park, J.Y.; Kim, C.H.; et al. Motor Pathway Injury in Patients with Periventricular Leucomalacia and Spastic Diplegia. Brain 2011, 134, 1199–1210. [Google Scholar] [CrossRef]
- Park, B.-H.; Park, S.-H.; Seo, J.-H.; Ko, M.-H.; Chung, G.-H. Neuroradiological and Neurophysiological Characteristics of Patients with Dyskinetic Cerebral Palsy. Ann. Rehabil. Med. 2014, 38, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Monbaliu, E.; Himmelmann, K.; Lin, J.-P.; Ortibus, E.; Bonouvrié, L.; Feys, H.; Vermeulen, R.J.; Dan, B. Clinical Presentation and Management of Dyskinetic Cerebral Palsy. Lancet Neurol. 2017, 16, 741–749. [Google Scholar] [CrossRef]
- Zhou, J.; Butler, E.E.; Rose, J. Neurologic Correlates of Gait Abnormalities in Cerebral Palsy: Implications for Treatment. Front. Hum. Neurosci. 2017, 11, 103. [Google Scholar] [CrossRef]
- Himmelmann, K.; Påhlman, M. The Panorama of Cerebral Palsy in Sweden Part XIII Shows Declining Prevalence in Birth-Years 2011–2014. Acta Paediatr. 2023, 112, 124–131. [Google Scholar] [CrossRef]
- Holmbeck, G.N.; Franks Bruno, E.; Jandasek, B. Longitudinal Research in Pediatric Psychology: An Introduction to the Special Issue. J. Pediatr. Psychol. 2006, 31, 995–1001. [Google Scholar] [CrossRef]
- Macchitella, L.; Accogli, G.; Barraco, G.; Nicolardi, V.; Pirani, G.; Ferrante, C.; Oliva, M.C.; Fanizza, I.; Gallo, I.; De Rinaldis, M.; et al. A Two-Step Neurorehabilitation Program Utilizing Extended Reality and Telerehabilitation for Children with Cerebral Palsy: A Pilot Study on Effectiveness, Adherence, and Technical Feasibility. Appl. Sci. 2024, 14, 11961. [Google Scholar] [CrossRef]
- Himmelmann, K.; McManus, V.; Hagberg, G.; Uvebrant, P.; Krägeloh-Mann, I.; Cans, C. SCPE collaboration Dyskinetic Cerebral Palsy in Europe: Trends in Prevalence and Severity. Arch. Dis. Child. 2009, 94, 921–926. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.; Mathur, M.; Chen, Y. Outcome-Wide Longitudinal Designs for Causal Inference: A New Template for Empirical Studies. Stat. Sci. 2020, 35, 437–466. [Google Scholar] [CrossRef]
- Bodimeade, H.L.; Whittingham, K.; Lloyd, O.; Boyd, R.N. Executive Function in Children and Adolescents with Unilateral Cerebral Palsy. Dev. Med. Child. Neurol. 2013, 55, 926–933. [Google Scholar] [CrossRef] [PubMed]
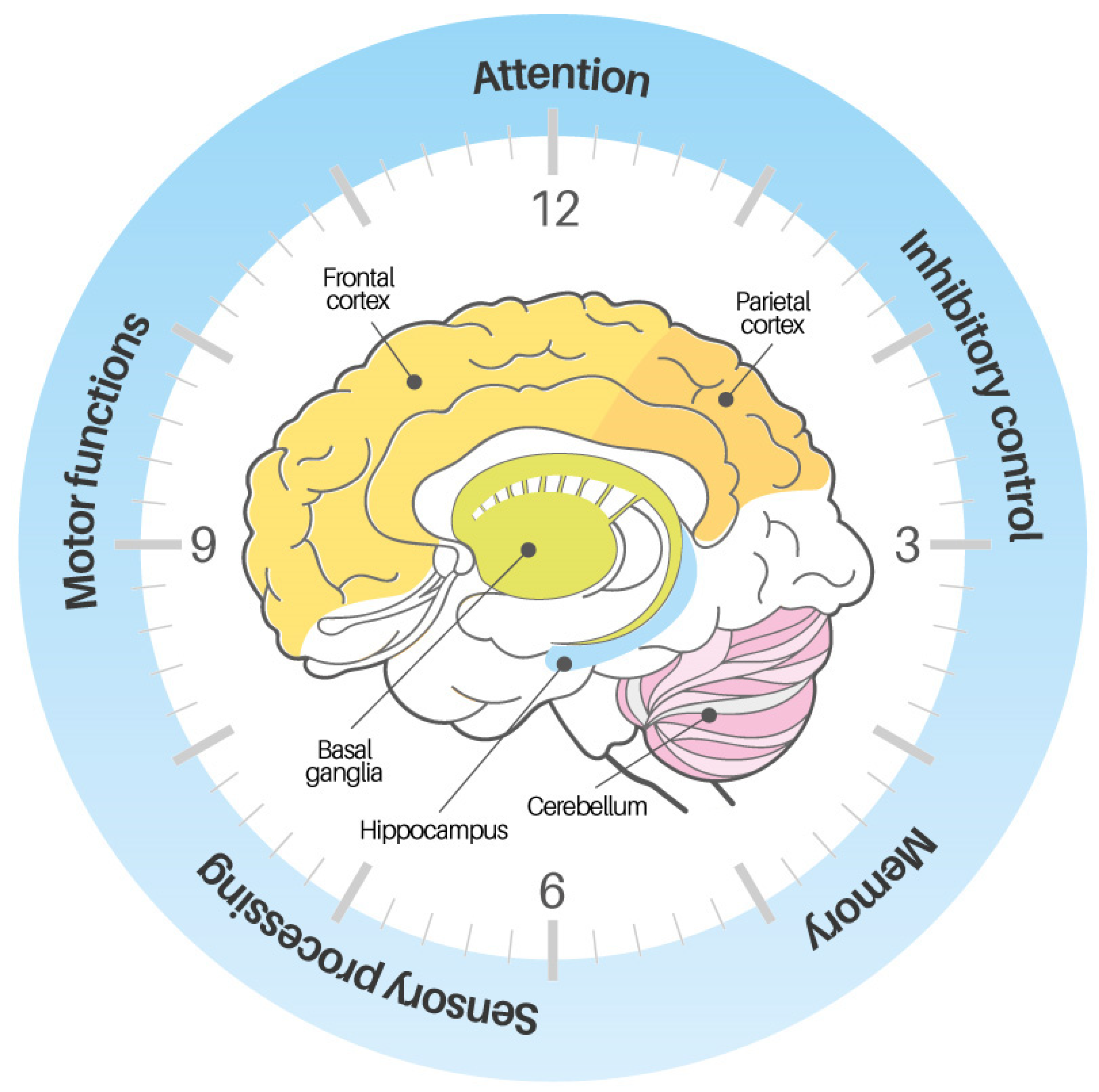

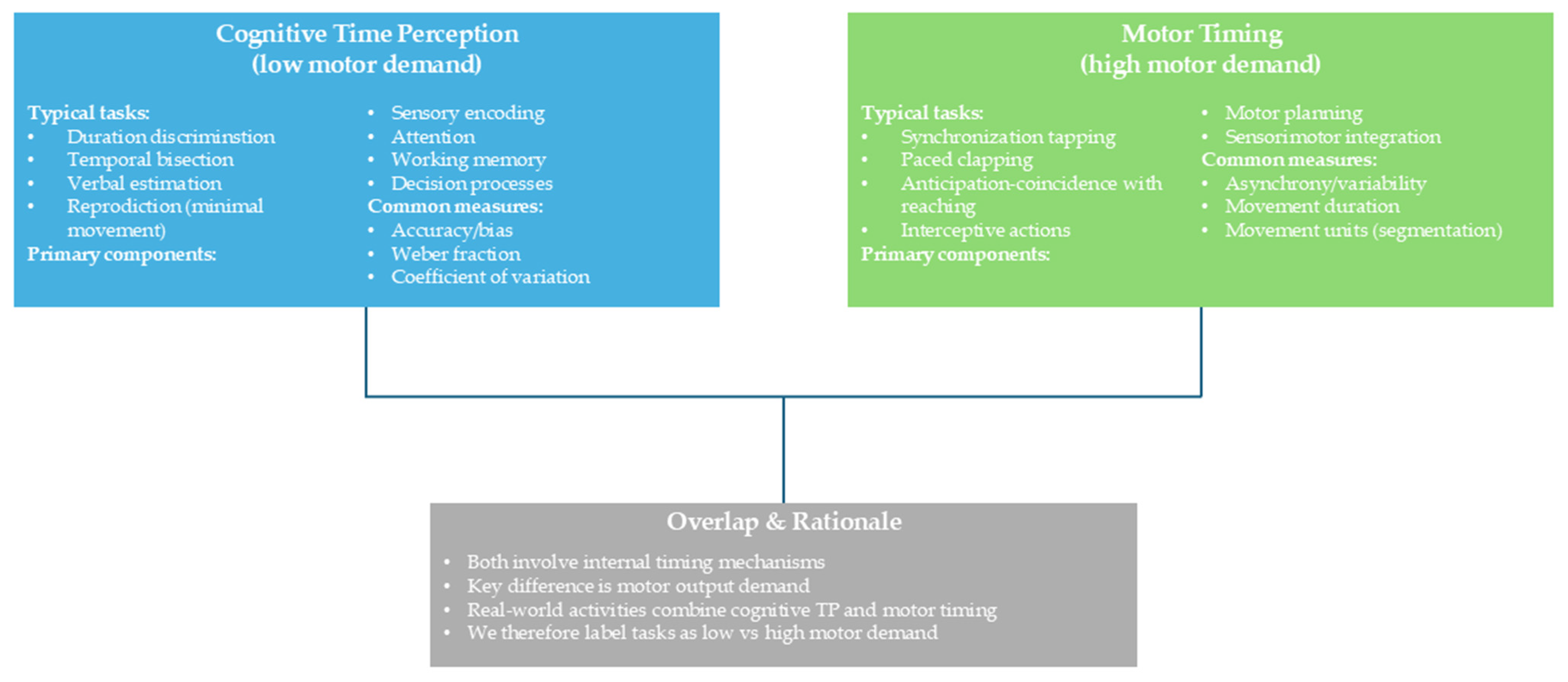
| Database | String |
|---|---|
| Scopus | (“Cerebral palsy” OR “Infant cerebral palsy” OR “Pediatric cerebral palsy” OR “Paediatric Cerebral Palsy” OR “Child* with cerebral Palsy”) AND (“Temporal Processing” OR “Time Processing” OR “Time Perception” OR “Temporal cognition”) AND (“Cognition” OR “Cognitive impairment” OR “Cognitive function” OR “Cognitive development” OR “Neuropsychological impairment” OR “Neuropsychological function” OR “Neuropsychological development” OR “Neuropsychological outcome” OR “Executive function*” OR “Inhibitory control” OR “Memory” OR “Working memory” OR “Attention” OR “Self-regulation” OR “Problem-solving” OR “Decision-making” OR “Learning ability” OR “Memory processing” OR “Processing speed” OR “Intellectual function*” OR “Mental flexibility” OR “Metacognition”) AND (“Motor control” OR “Motor planning” OR “Motor timing” OR “Movement timing” OR “Motor coordination” OR “Temporal motor control”) |
| Embase | (‘cerebral palsy’/exp OR ‘cerebral palsy’ OR ‘infant cerebral palsy’ OR ‘pediatric cerebral palsy’ OR ‘paediatric cerebral palsy’ OR ‘child* with cerebral palsy’) AND (‘temporal processing’/exp OR ‘temporal processing’ OR ‘time processing’ OR ‘time perception’/exp OR ‘time perception’ OR ‘temporal cognition’) AND (‘cognition’/exp OR ‘cognition’ OR ‘cognitive impairment’/exp OR ‘cognitive impairment’ OR ‘cognitive function’/exp OR ‘cognitive function’ OR ‘cognitive development’/exp OR ‘cognitive development’ OR ‘neuropsychological impairment’/exp OR ‘neuropsychological impairment’ OR ‘neuropsychological function’/exp OR ‘neuropsychological function’ OR ‘neuropsychological development’/exp OR ‘neuropsychological development’ OR ‘neuropsychological outcome’ OR ‘executive function*’ OR ‘inhibitory control’/exp OR ‘inhibitory control’ OR ‘memory’/exp OR ‘memory’ OR ‘working memory’/exp OR ‘working memory’ OR ‘attention’/exp OR ‘attention’ OR ‘self-regulation’/exp OR ‘self-regulation’ OR ‘problem-solving’/exp OR ‘problem-solving’ OR ‘decision-making’/exp OR ‘decision-making’ OR ‘learning ability’/exp OR ‘learning ability’ OR ‘memory processing’ OR ‘processing speed’/exp OR ‘processing speed’ OR ‘intellectual function*’ OR ‘mental flexibility’/exp OR ‘mental flexibility’ OR ‘metacognition’/exp OR ‘metacognition’) AND (‘motor control’/exp OR ‘motor control’ OR ‘motor planning’/exp OR ‘motor planning’ OR ‘motor timing’/exp OR ‘motor timing’ OR ‘movement timing’ OR ‘motor coordination’/exp OR ‘motor coordination’ OR ‘temporal motor control’) |
| PMC | (“Cerebral palsy”[All Fields] OR “Infant cerebral palsy”[All Fields] OR “Pediatric cerebral palsy”[All Fields] OR “Paediatric Cerebral Palsy”[All Fields] OR “Child* with cerebral Palsy”[All Fields]) AND (“Temporal Processing”[All Fields] OR “Time Processing”[All Fields] OR “Time Perception”[All Fields] OR “Temporal cognition”[All Fields]) AND (“Cognition”[All Fields] OR “Cognitive impairment”[All Fields] OR “Cognitive function”[All Fields] OR “Cognitive development”[All Fields] OR “Neuropsychological impairment”[All Fields] OR “Neuropsychological function”[All Fields] OR “Neuropsychological development”[All Fields] OR “Neuropsychological outcome”[All Fields] OR “Executive function*”[All Fields] OR “Inhibitory control”[All Fields] OR “Memory”[All Fields] OR “Working memory”[All Fields] OR “Attention”[All Fields] OR “Self-regulation”[All Fields] OR “Problem-solving”[All Fields] OR “Decision-making”[All Fields] OR “Learning ability”[All Fields] OR “Memory processing”[All Fields] OR “Processing speed”[All Fields] OR “Intellectual function*”[All Fields] OR “Mental flexibility”[All Fields] OR “Metacognition”[All Fields]) AND (“Motor control”[All Fields] OR “Motor planning”[All Fields] OR “Motor timing”[All Fields] OR “Movement timing”[All Fields] OR “Motor coordination”[All Fields] OR “Temporal motor control”[All Fields]) |
| Authors | Year | Title | Aim | Experimental Sample | Control Group | Dependent Variable | Use of Functional Scale | Comorbidities | Methods | Results | Limitations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Janeslätt et al. [43] | 2014 | Evaluating intervention using time aids in children with disabilities | To evaluate a complex intervention (time aids) for children (6–11 years) with intellectual/developmental disabilities, focusing on time-processing ability and daily time management. | Mixed sample (N = 47) including ADHD, ASD, mild/moderate ID, spina bifida, CP; assigned to Intervention group (n = 22) or Control group (n = 25). | Yes, Control group (Waiting List design); after 6 months, the control group also received the intervention. | Time-processing ability (KaTid-Child) & managing one’s time (Time-Parent scale). Motor demand: Functional-low. | No GMFCS or MACS usage reported. | Multiple diagnoses in sample (ADHD, ASD, ID, spina bifida, CP). | Randomized Block + Waiting List design; 6-month intervention with time aids; pre-/post-measures of time-processing ability and parent-rated daily time management. | Intervention group improved more than controls in time-processing ability and daily time management; effect sizes were large/medium. After controls received the same intervention, they also improved. | Sample includes multiple diagnoses; no separate analysis for CP only; no standardized CP severity measure; fairly small subgroups. |
| Cabezas & Carriedo [21] | 2020 | Inhibitory control and temporal perception in cerebral palsy | To study whether children/adolescents with CP show deficits in inhibitory control and duration estimation, and to test whether inhibitory difficulties affect time estimation. | CP group (N = 16) with spastic or predominantly spastic CP; mental ages between 5.5–10 yrs. | Yes, Typically developing controls (N = 16), matched by mental age. | Inhibitory control task performance (Stroop-like tasks, etc.) and accuracy in duration estimation tasks. Motor demand: Low. | Yes GMFCS levels I–IV used. | CP can involve various comorbidities; not specified in detail. | Two inhibitory control tasks plus two interval-estimation tasks; compared CP group vs. typically developing controls. | CP group showed lower performance in both inhibition and temporal perception vs. controls; found a relationship between inhibition and time estimation. | Sample size relatively small (N = 16 CP); wide age range; only spastic CP forms included. Nonetheless, they used GMFCS classification, excluding level V. |
| Johansson et al. [37] | 2014 | Timing training in three children with diplegic cerebral palsy: short- and long-term effects on upper-limb movement organization and functioning | To explore effects of a 4-week/12-session synchronized metronome training (SMT) program on motor timing and upper-limb function in three children (diplegic CP). Assess immediate and 6-month outcomes. | Single-case design with three children (ages 12–16) diagnosed with diplegic CP (DCP). | No formal control group. | Motor timing (deviation from metronome beat), upper-limb kinematics, subjective arm/hand functioning. Motor demand: High. | Yes: GMFCS & MACS levels detailed for each child. | DCP is the primary diagnosis; no additional comorbidities described. | Kinematic assessment of a goal-directed upper-limb task (3D motion capture), synchronized metronome training tasks (Interactive Metronome®). Pre-training, post-training (1 week), and follow-up (6 months). | Two of the three children showed notable improvements in movement organization, speed, smoothness, and subjective hand/arm control lasting at 6 months post. One child had minimal changes. | Very small sample (3 children); no control group; individual outcomes; caution generalizing results; but GMFCS & MACS usage is explicitly reported. |
| Olivier et al. [22] | 2015 | Cognitive and motor aspects of a coincidence-timing task in Cerebral Palsy children | To assess how children with CP handle anticipation-coincidence tasks, comparing purely cognitive (verbal timing) and motor (reaching) responses. | CP group (N = 11, ages 6–14); severity rated on a custom 4-level scale (1 = low to 4 = high). Plus 51 typically developing children (6–13 yrs) and 13 healthy adults. | Two groups: 51 typically developing children and 13 healthy adults. | Timing errors (under-/overestimation of an event), measured in verbal vs. motor tasks. Motor demand: Mixed (verbal = Low; reaching = High). | No GMFCS or MACS used; authors mention a custom severity scale. | Primarily CP (motor deficits), no other comorbidities reported. | Participants performed a “coincidence-timing” task triggered by a musical cue. In the “verbal” condition, they responded vocally; in “motor” condition, they reached for a target. Accuracy/variability in timing errors was measured in each condition. | CP children performed similarly to controls in purely verbal timing but had larger and more variable errors in the motor task; they partially compensated for their motor deficits but still showed worse performance than typically developing children or adults. | Small CP sample (N = 11); use of a non-standard 4-point severity scale instead of GMFCS/MACS; results focus on timing performance, do not systematically address functional motor classification. |
| Recommendation | Cabezas & Carriedo, 2020 [21] | Johansson et al., 2014 [37] | Olivier et al., 2015 [22] |
|---|---|---|---|
| (a) Indicate the study’s design with a commonly used term in the title or the abstract. (b) Provide in the abstract an informative and balanced summary of what was carried out and what was found. | Study design mentioned as experimental tasks involving visual and auditory stimuli. | Study described as an intervention exploring motor timing in CP children. | Coincidence-timing task study involving children with cerebral palsy. |
| Explain the scientific background and rationale for the investigation being reported. | Study investigates time perception in children with CP. | Background provided on timing training and motor synchronization in CP. | Rationale focuses on cognitive and motor aspects of timing in CP children. |
| State specific objectives, including any prespecified hypotheses. | To assess time perception in CP vs. typically developing children. The study hypothesized that children with CP would perform worse in temporal estimation and inhibitory control, especially under high interference. It also expected a link between inhibitory control and temporal processing | To evaluate timing ability and retention in CP children. Children with more severe diplegic CP will show improvements in timing ability after 4 weeks of Interactive Metronome. IM training will lead to long-term retention effects in spatio-temporal movement organization. | To dissociate cognitive and motor components of timing tasks. The authors hypothesized that temporal estimation in CP children should not be different with respect to healthy children and adults when simple motor response was involved. Conversely, it was expected that when the coincidence-timing task required more complex movement execution, coincidence-timing was altered with respect to healthy subjects. |
| Present key elements of study design early in the paper. | Study tasks presented via computer using controlled stimulus duration. | Kinematic analysis used to assess timing ability. | Musical sequence and motor response analyzed in CP children. |
| Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection. | Children with CP recruited via clinical records. | Participants recruited from a rehabilitation center in Sweden. | CP children recruited based on ability to perform coincidence-timing. |
| (a) Give the eligibility criteria, and the sources and methods of selection of participants. (b) For matched studies, give matching criteria and number of exposed and unexposed. | Study recruited children with CP and typically developing peers from clinical centers. | Eligibility criteria: diagnosed with diplegic CP, undergoing rehabilitation. | Selection criteria included ability to perform auditory-based timing task. |
| Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable. | Outcome measures: reaction time, temporal perception accuracy. | Measures: kinematic movement trajectories, timing errors. | Coincidence-timing accuracy and motor response times assessed. |
| For each variable of interest, give sources of data and details of methods of assessment (measurement). | Methods included controlled experimental design with standardized stimuli. | Movement tracking and motor timing analysis using optoelectronic system. | Auditory–motor synchronization task measured precision of responses. |
| Describe any efforts to address potential sources of bias. | Efforts included matched control group to compare CP vs. typically developing children. The document does not explicitly describe any strategies to address potential biases in the study. While participant characteristics and measurement methods are mentioned, no specific actions to mitigate selection or measurement bias are reported. Participants were chosen from a specific population which may limit generalizability. | Bias minimized by individualized training protocols and repeated measures. No explicit strategies to address potential bias were discussed. | Addressed by controlling for variability in auditory and motor responses. No strategies for addressing potential bias explicitly discussed. |
| Explain how the study size was arrived at. | Sample size: 16 CP and 16 control participants. No sample size calculation. | Small sample of 3 CP children with detailed movement tracking. No sample size calculation. | Study included 15 CP children, 51 healthy children, and 13 adults. No justification for the small sample size provided. |
| Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why. | Quantitative variables included response times and error rates. | Timing errors analyzed with precision metrics and kinematic models. | Response accuracy and movement execution times assessed quantitatively. |
| (a) Describe all statistical methods, including those used to control for confounding. (b) Describe any methods used to examine subgroups and interactions. (c) Explain how missing data were addressed. (d) If applicable, explain how loss to follow-up was addressed. (e) Describe any sensitivity analyses. | Statistical methods: ANCOVA, correlation and regression analysis between inhibition and timing. | Wilcoxon matched pairs test, effect size calculations. | ANOVA used to compare motor and cognitive timing performance. |
| (a) Report numbers of individuals at each stage of study, e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed. (b) Give reasons for non-participation at each stage. (c) Consider use of a flow diagram. | Participants categorized by CP severity, control-matching was performed. | Participant numbers tracked across intervention stages. | Flow of participants described, including matched controls. |
| (a) Give characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders. (b) Indicate number of participants with missing data for each variable of interest. (c) Cohort study—Summarize follow-up time (e.g., average and total amount). | Demographic data included CP diagnosis details, control group matched. | Participant movement characteristics analyzed quantitatively. | Data on participant demographics, including motor impairment levels. |
| Report numbers of outcome events or summary measures. | Reported outcome measures include reaction time and timing accuracy. | Outcome measures include changes in movement precision. | Timing accuracy and motor responses compared across groups. |
| (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). (b) Report category boundaries when continuous variables were categorized. (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period. | Statistical analysis of unadjusted and adjusted estimates reported. | Precision estimates of movement timing improvements. | Timing errors and confidence intervals analyzed. |
| Report other analyses conducted, e.g., analyses of subgroups and interactions, and sensitivity analyses. | Comparison across subgroups analyzed for variability. | Subgroup analyses for different CP severity levels. | Sensitivity analysis for motor and cognitive components. |
| Summarize key results with reference to study objectives. | Findings summarized regarding inhibitory control and timing. | Results highlight improvements in motor synchronization. | Key results related to motor timing performance summarized. |
| Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias. | Limitations include small sample size and cognitive variability. | Limitations related to small sample and lack of long-term data. No control group. | Study acknowledges bias in measuring motor execution timing. |
| Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence. | Results interpreted within cognitive processing theories. | Findings discussed in relation to neuroplasticity and timing. | Results interpreted within cognitive–motor control models. |
| Discuss the generalizability (external validity) of the study results. | Generalizability limited due to small clinical sample. | Study mainly relevant to rehabilitation contexts. | Limited generalizability to broader CP. |
| Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based. | Funding sources mentioned. | Although the funding sources are mentioned, there is no explicit statement about the potential role of the funders in the study design, data analysis, or interpretation of the results. | The study does not include any statement about funding sources or conflicts of interest, leaving this information unreported |
| Cabezas & Carriedo, 2020 [21] | Johansson et al., 2014 [37] | Olivier et al., 2015 [22] | ||||
|---|---|---|---|---|---|---|
| Item | Response | Comments | Response | Comments | Response | Comments |
| 1. Were the aims/objectives of the study clear? | ● | ● | ● | |||
| 2. Was the study design appropriate for the stated aim(s)? | ● | ● | ● | |||
| 3. Was the sample size justified? | ⸰ | Sample size justification not provided explicitly. | ⸰ | No power analysis or justification for sample size provided | ⸰ | No explicit justification for the small sample size. |
| 4. Was the target/reference population clearly defined? (Is it clear who the research was about?) | ● | ● | ● | |||
| 5. Was the sample frame taken from an appropriate population base so that it closely represented the target/reference population under investigation? | ● | ● | ● | |||
| 6. Was the selection process likely to select subjects/participants that were representative of the target/reference population under investigation? | ⸰ | Bias possible due to selection of participants with specific impairments. | ⸰ | Selection limited by sample size and inclusion of children with severe DCP | ⸰ | Selection may not generalize to all CP children due to limited criteria |
| 7. Were measures undertaken to address and categorise non-responders? | □ | No information on measures for non-responders. | □ | No mention of non-responders or how they were handled | □ | No mention of measures for non-responders |
| 8. Were the risk factor and outcome variables measured appropriate to the aims of the study? | ● | ● | ● | |||
| 9. Were the risk factor and outcome variables measured correctly using instruments/measurements that had been trialled, piloted or published previously? | ● | ● | ● | |||
| 10. Is it clear what was used to determine statistical significance and/or precision estimates? (e.g., p-values, confidence intervals) | ● | ● | ● | |||
| 11. Were the methods (including statistical methods) sufficiently described to enable them to be repeated? | ● | ● | ● | |||
| 12. Were the basic data adequately described? | ● | ● | ● | |||
| 13. Does the response rate raise concerns about non-response bias? | ⸰ | No mention of potential non-response bias. | ⸰ | No evidence of non-response bias mitigation | ⸰ | No explicit discussion of response rate concerns or potential bias |
| 14. If appropriate, was information about non-responders described? | ⸰ | No description of non-responders provided. | ⸰ | No information about non-responders was included. | ⸰ | Non-responders not described in any section of the paper |
| 15. Were the results internally consistent? | ● | ● | ● | |||
| 16. Were the results presented for all the analyses described in the methods? | ● | ● | ● | |||
| 17. Were the authors’ discussions and conclusions justified by the results? | ● | ● | ● | |||
| 18. Were the limitations of the study discussed? | ● | ● | ● | |||
| 19. Were there any funding sources or conflicts of interest that may affect the authors’ interpretation of the results? | ● | ■ | Although the funding sources are mentioned, there is no explicit statement about the potential role of the funders in the study design, data analysis, or interpretation of the results. | □ | Funding sources mentioned but roles not clarified | |
| 20. Was ethical approval or consent of participants attained? | ● | ● | ● | |||
| D1 Risk of Bias Arising from the Randomization Process | D2 Risk of Bias Due to Deviations from the Intended Interventions (Effect of Assignment to Intervention) | D3 Missing Outcome Data | D4 Risk of Bias in Measurement of the Outcome | D5 Risk of Bias in Selection of the Reported Result | Overall |
|---|---|---|---|---|---|
| ⏺ | ⏺ | ⏺ | ⏺ | ⏺ | ⏺ |
| Some concerns | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accogli, G.; Nicolardi, V.; Leucci, M.; Macchitella, L.; Pirani, G.; Oliva, M.C.; Trabacca, A. Time and Mind: A State-of-the-Art Perspective on Time Perception and Cognitive–Motor Interactions in Children and Adolescents with Cerebral Palsy. Children 2025, 12, 1283. https://doi.org/10.3390/children12101283
Accogli G, Nicolardi V, Leucci M, Macchitella L, Pirani G, Oliva MC, Trabacca A. Time and Mind: A State-of-the-Art Perspective on Time Perception and Cognitive–Motor Interactions in Children and Adolescents with Cerebral Palsy. Children. 2025; 12(10):1283. https://doi.org/10.3390/children12101283
Chicago/Turabian StyleAccogli, Giuseppe, Valentina Nicolardi, Mariangela Leucci, Luigi Macchitella, Greta Pirani, Maria Carmela Oliva, and Antonio Trabacca. 2025. "Time and Mind: A State-of-the-Art Perspective on Time Perception and Cognitive–Motor Interactions in Children and Adolescents with Cerebral Palsy" Children 12, no. 10: 1283. https://doi.org/10.3390/children12101283
APA StyleAccogli, G., Nicolardi, V., Leucci, M., Macchitella, L., Pirani, G., Oliva, M. C., & Trabacca, A. (2025). Time and Mind: A State-of-the-Art Perspective on Time Perception and Cognitive–Motor Interactions in Children and Adolescents with Cerebral Palsy. Children, 12(10), 1283. https://doi.org/10.3390/children12101283






