Argonaute2 and Argonaute4 Involved in the Pathogenesis of Kawasaki Disease via mRNA Expression Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Gene Expression Profiling with Microarray (HTA 2.0)
2.3. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, Y.; Zhang, Y.; Li, Y.; Hua, Y.; Zhang, Y. Molecular mechanisms of endothelial dysfunction in Kawasaki-disease-associated vasculitis. Front. Cardiovasc. Med. 2022, 9, 981010. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Lo, M.H.; Cai, X.Y.; Liu, S.F.; Kuo, H.C. Increase expression of CD177 in Kawasaki disease. Pediatr. Rheumatol. Online J. 2019, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Scuccimarri, R. Kawasaki disease. Pediatr. Clin. N. Am. 2012, 59, 425–445. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Diagnosis, Progress, and Treatment Update of Kawasaki Disease. Int. J. Mol. Sci. 2023, 24, 13948. [Google Scholar] [CrossRef]
- Messenger, N.; Messenger, G.; Potts, G. Pustular rash masking Kawasaki disease. Int. J. Dermatol. 2023, 62, e250–e251. [Google Scholar] [CrossRef]
- Jindal, A.K.; Pilania, R.K.; Prithvi, A.; Guleria, S.; Singh, S. Kawasaki disease: Characteristics, diagnosis, and unusual presentations. Expert Rev. Clin. Immunol. 2019, 15, 1089–1104. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, J.; Chen, H.; Zhuge, Y.; Chen, H.; Qian, F.; Zhou, K.; Niu, C.; Wang, F.; Qiu, H.; et al. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis. 2019, 10, 778. [Google Scholar] [CrossRef]
- Tirelli, F.; Marrani, E.; Giani, T.; Cimaz, R. One year in review: Kawasaki disease. Curr. Opin. Rheumatol. 2020, 32, 15–20. [Google Scholar] [CrossRef]
- Balta, S. Endothelial Dysfunction and Inflammatory Markers of Vascular Disease. Curr. Vasc. Pharmacol. 2021, 19, 243–249. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef]
- Caballero-Eraso, C.; Munoz-Hernandez, R.; Asensio Cruz, M.I.; Moreno Luna, R.; Carmona Bernal, C.; López-Campos, J.L.; Stiefel, P.; Sanchez Armengol, A. Relationship between the endothelial dysfunction and the expression of the β1-subunit of BK channels in a non-hypertensive sleep apnea group. PLoS ONE 2019, 14, e0217138. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, X.; Zhi, X.; Meng, D. Epigenetic regulation in diabetic vascular complications. J. Mol. Endocrinol. 2019, 63, R103–R115. [Google Scholar] [CrossRef] [PubMed]
- Koibuchi, H.; Kotani, K.; Minami, T.; Konno, K.; Taniguchi, N. Endothelial dysfunction by flow-mediated dilation assessed ultrasonically in patients with Kawasaki Disease. Minerva Pediatr. 2016, 68, 143–147. [Google Scholar] [PubMed]
- Wang, Y.; Li, T. Advances in understanding Kawasaki disease-related immuno-inflammatory response and vascular endothelial dysfunction. Pediatr. Investig. 2022, 6, 271–279. [Google Scholar] [CrossRef]
- Gao, M.; Yu, T.; Liu, D.; Shi, Y.; Yang, P.; Zhang, J.; Wang, J.; Liu, Y.; Zhang, X. Sepsis plasma-derived exosomal miR-1-3p induces endothelial cell dysfunction by targeting SERP1. Clin. Sci. 2021, 135, 347–365. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, M.; Yu, H.; Wang, L.; Li, X.; Rak, J.; Wang, S.; Zhao, R.C. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct. Target. Ther. 2021, 6, 354. [Google Scholar] [CrossRef]
- Desantis, V.; Potenza, M.A.; Sgarra, L.; Nacci, C.; Scaringella, A.; Cicco, S.; Solimando, A.G.; Vacca, A.; Montagnani, M. microRNAs as Biomarkers of Endothelial Dysfunction and Therapeutic Target in the Pathogenesis of Atrial Fibrillation. Int. J. Mol. Sci. 2023, 24, 5307. [Google Scholar] [CrossRef]
- Routhu, S.K.; Singhal, M.; Jindal, A.K.; Kumar, V.; Yadav, A.K.; Singh, S. Assessment of Endothelial Dysfunction in Acute and Convalescent Phases of Kawasaki Disease Using Automated Edge Detection Software: A Preliminary Study from North India. J. Clin. Rheumatol. 2021, 27, 143–149. [Google Scholar] [CrossRef]
- Loedige, I.; Baranovskii, A.; Mendonsa, S.; Dantsuji, S.; Popitsch, N.; Breimann, L.; Zerna, N.; Cherepanov, V.; Milek, M.; Ameres, S.; et al. mRNA stability and m(6)A are major determinants of subcellular mRNA localization in neurons. Mol. Cell 2023, 83, 2709–2725.e10. [Google Scholar] [CrossRef]
- Suter, B. RNA localization and transport. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 938–951. [Google Scholar] [CrossRef]
- Sonneveld, S.; Verhagen, B.M.P.; Tanenbaum, M.E. Heterogeneity in mRNA Translation. Trends Cell Biol. 2020, 30, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Voronina, A.S.; Pshennikova, E.S. mRNPs: Structure and role in development. Cell Biochem. Funct. 2021, 39, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Del Ry, S.; Cabiati, M.; Bianchi, V.; Randazzo, E.; Peroni, D.; Clerico, A.; Federico, G. C-type natriuretic peptide plasma levels and whole blood mRNA expression show different trends in adolescents with different degree of endothelial dysfunction. Peptides 2020, 124, 170218. [Google Scholar] [CrossRef]
- Orellana, E.A.; Siegal, E.; Gregory, R.I. tRNA dysregulation and disease. Nat. Rev. Genet. 2022, 23, 651–664. [Google Scholar] [CrossRef]
- Otis, J.P.; Mowry, K.L. Hitting the mark: Localization of mRNA and biomolecular condensates in health and disease. Wiley Interdiscip. Rev. RNA 2023, 14, e1807. [Google Scholar] [CrossRef]
- Zhou, D.W.; Wang, K.; Zhang, Y.A.; Ma, K.; Yang, X.C.; Li, Z.Y.; Yu, S.S.; Chen, K.Z.; Qiao, S.L. mRNA therapeutics for disease therapy: Principles, delivery, and clinical translation. J. Mater. Chem. B 2023, 11, 3484–3510. [Google Scholar] [CrossRef]
- Matsuyama, H.; Suzuki, H.I. Systems and Synthetic microRNA Biology: From Biogenesis to Disease Pathogenesis. Int. J. Mol. Sci. 2019, 21, 132. [Google Scholar] [CrossRef]
- Jin, S.; Zhan, J.; Zhou, Y. Argonaute proteins: Structures and their endonuclease activity. Mol. Biol. Rep. 2021, 48, 4837–4849. [Google Scholar] [CrossRef]
- Nakanishi, K. Anatomy of four human Argonaute proteins. Nucleic Acids Res. 2022, 50, 6618–6638. [Google Scholar] [CrossRef]
- Bobadilla Ugarte, P.; Barendse, P.; Swarts, D.C. Argonaute proteins confer immunity in all domains of life. Curr. Opin. Microbiol. 2023, 74, 102313. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2008)–digest version. Circ. J. 2010, 74, 1989–2020. [CrossRef]
- Zhai, L.; Wang, L.; Teng, F.; Zhou, L.; Zhang, W.; Xiao, J.; Liu, Y.; Deng, W. Argonaute and Argonaute-Bound Small RNAs in Stem Cells. Int. J. Mol. Sci. 2016, 17, 208. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Cai, Q.; Qiao, L.; Huang, C.Y.; Wang, S.; Miao, W.; Ha, T.; Wang, Y.; Jin, H. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat. Plants 2021, 7, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Sala, L.; Chandrasekhar, S.; Vidigal, J.A. AGO unchained: Canonical and non-canonical roles of Argonaute proteins in mammals. Front. Biosci. 2020, 25, 1–42. [Google Scholar]
- Johnson, K.C.; Johnson, S.T.; Liu, J.; Chu, Y.; Arana, C.; Han, Y.; Wang, T.; Corey, D.R. Consequences of depleting TNRC6, AGO, and DROSHA proteins on expression of microRNAs. RNA 2023, 29, 1166–1184. [Google Scholar] [CrossRef]
- Liao, J.; Guo, X.; Fan, X.; Zhang, X.; Xu, M. Upregulation of miR-184 and miR-19a-3p induces endothelial dysfunction by targeting AGO2 in Kawasaki disease. Cardiol. Young 2023, 33, 1962–1966. [Google Scholar] [CrossRef]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef]
- Saito, K.; Nakaoka, H.; Takasaki, I.; Hirono, K.; Yamamoto, S.; Kinoshita, K.; Miyao, N.; Ibuki, K.; Ozawa, S.; Watanabe, K.; et al. MicroRNA-93 may control vascular endothelial growth factor A in circulating peripheral blood mononuclear cells in acute Kawasaki disease. Pediatr. Res. 2016, 80, 425–432. [Google Scholar] [CrossRef]
- Wu, R.; Shen, D.; Sohun, H.; Ge, D.; Chen, X.; Wang, X.; Chen, R.; Wu, Y.; Zeng, J.; Rong, X.; et al. miR-186, a serum microRNA, induces endothelial cell apoptosis by targeting SMAD6 in Kawasaki disease. Int. J. Mol. Med. 2018, 41, 1899–1908. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, K.; Hua, Y.; Wu, M.; Liu, L.; Shao, S.; Wang, C. Neurological involvement in Kawasaki disease: A retrospective study. Pediatr. Rheumatol. Online J. 2020, 18, 61. [Google Scholar] [CrossRef] [PubMed]
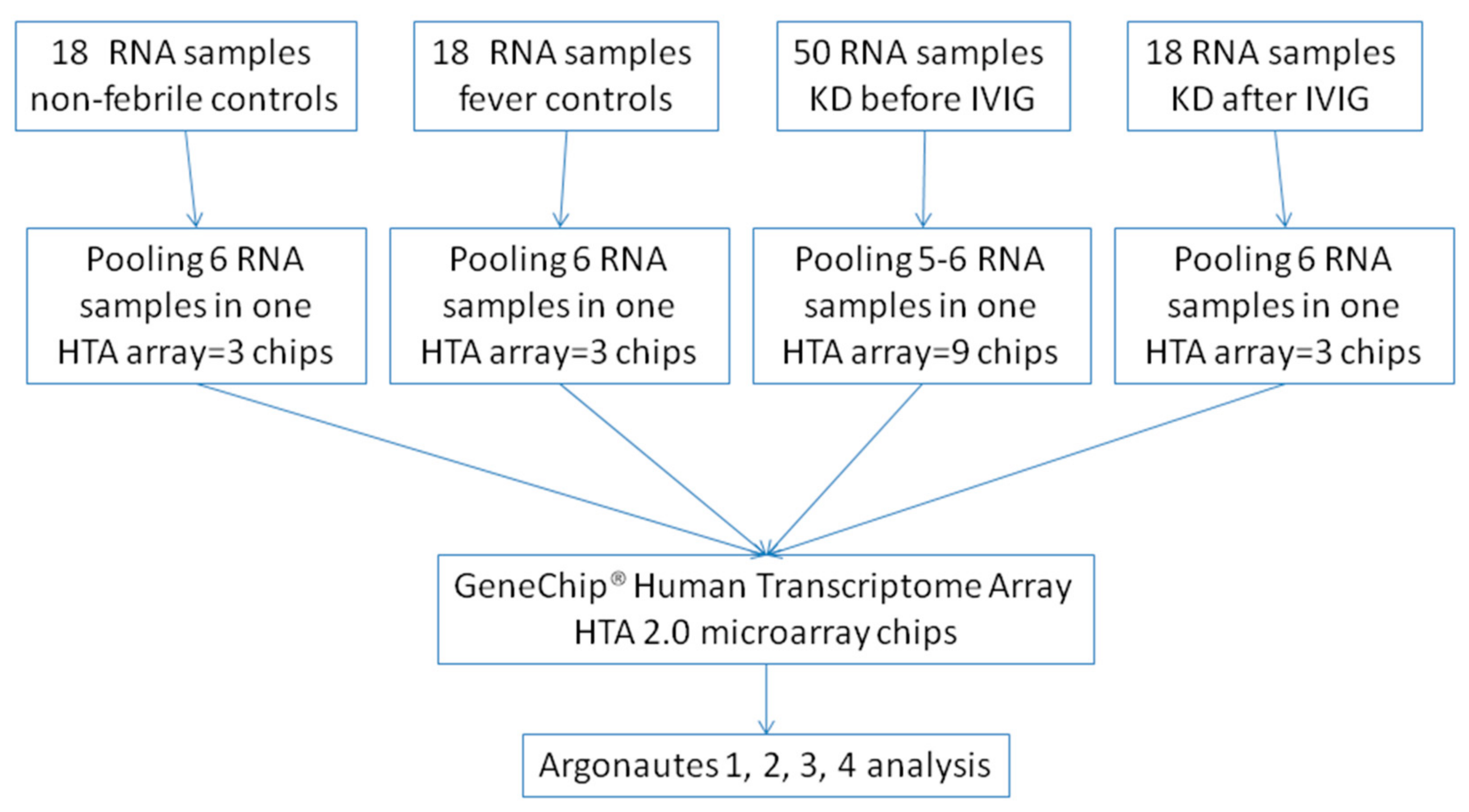
| Non-Febrile Controls (n = 18) | Febrile Controls (n = 18) | KD Before IVIG (n = 50) | KD After IVIG (n = 18) | p Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender, Male, N (%) | 9.0 | (50.0) | 8.0 | (44.4) | 27.0 | (54.0) | 9.0 | (50.0) | 0.918 | ||||
| Age, years | 2.46 | ± | 1.50 | 1.97 | ± | 1.09 | 1.36 | ± | 1.00 | 1.74 | ± | 1.27 | 0.003 ** |
| CRP (mg/L) | N/A | 27.48 | ± | 27.48 | 87.08 | ± | 67.21 | 1.15 | ± | 2.30 | p < 0.001 | ||
| WBC (1000/uL) | 8.98 | ± | 2.18 | 8.86 | ± | 3.17 | 14.96 | ± | 7.40 | 8.67 | ± | 2.06 | p < 0.001 |
| Hemoglobin (g/dL) | 12.67 | ± | 0.60 | 12.09 | ± | 1.14 | 10.97 | ± | 0.93 | 11.91 | ± | 0.84 | p < 0.001 |
| Platelets(1000/uL) | 302.39 | ± | 101.77 | 291.56 | ± | 118.99 | 359.82 | ± | 156.46 | 362.24 | ± | 61.76 | 0.023 * |
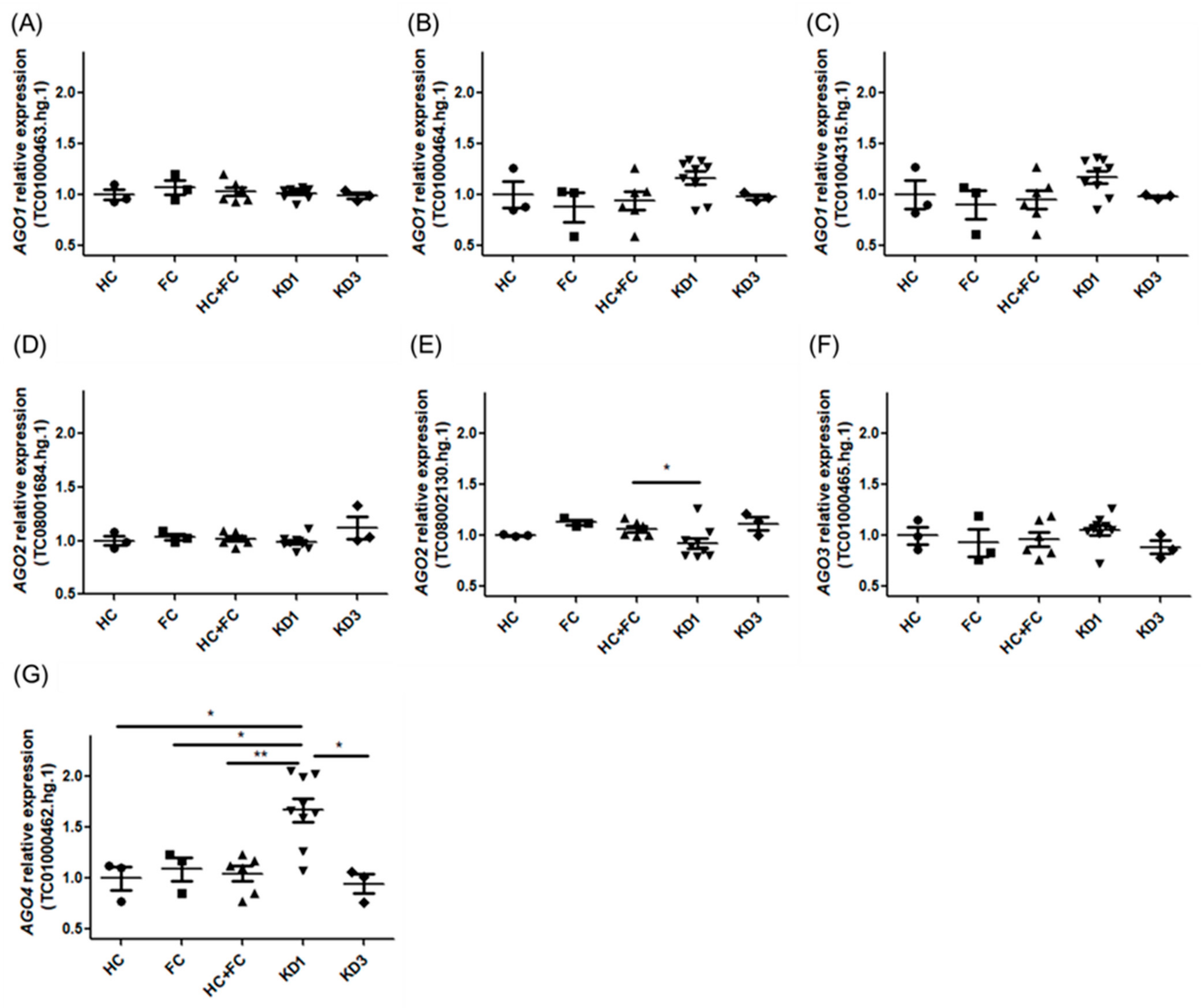
| Gene Symbol | Column ID | HC (n = 3) | FC (n = 3) | KD1 (n = 9) | KD3 (n = 3) | HC + FC (n = 6) |
|---|---|---|---|---|---|---|
| AGO1 | TC01000463.hg.1 | 1.00 ± 0.09 | 1.07 ± 0.12 | 1.02 ± 0.05 | 0.99 ± 0.05 | 1.03 ± 0.10 |
| AGO1 | TC01000464.hg.1 | 1.00 ± 0.23 | 0.88 ± 0.25 | 1.17 ± 0.19 | 0.98 ± 0.04 | 0.94 ± 0.23 |
| AGO1 | TC01004315.hg.1 | 1.00 ± 0.24 | 0.90 ± 0.25 | 1.17 ± 0.18 | 0.98 ± 0.02 | 0.95 ± 0.23 |
| AGO2 | TC08001684.hg.1 | 1.00 ± 0.07 | 1.03 ± 0.05 | 0.98 ± 0.06 | 1.12 ± 0.18 | 1.02 ± 0.06 |
| AGO2 | TC08002130.hg.1 | 1.00 ± 0.01 | 1.13 ± 0.04 | 0.93 ± 0.15 | 1.11 ± 0.11 | 1.06 ± 0.08 |
| AGO3 | TC01000465.hg.1 | 1.00 ± 0.15 | 0.93 ± 0.23 | 1.05 ± 0.15 | 0.89 ± 0.12 | 0.96 ± 0.18 |
| AGO4 | TC01000462.hg.1 | 1.00 ± 0.20 | 1.09 ± 0.21 | 1.67 ± 0.34 | 0.95 ± 0.16 | 1.04 ± 0.19 |
| Gene Symbol | Column ID | Fold-Change (KD1 vs. HC) | p-Value (KD1 vs. HC) | Fold-Change (KD1 vs. FC) | p-Value (KD1 vs. FC) | Fold-Change (KD1 vs. KD3) | p-Value (KD1 vs. KD3) | Fold-Change (KD1 vs. HC + FC) | p-Value (KD1 vs. HC + FC) |
|---|---|---|---|---|---|---|---|---|---|
| AGO1 | TC01000463.hg.1 | 1.02 | 0.644 | 1.30 | 0.405 | 1.19 | 0.518 | 1.30 | 0.814 |
| AGO1 | TC01000464.hg.1 | 1.17 | 0.309 | 0.95 | 0.079 | 1.02 | 0.166 | 1.06 | 0.077 |
| AGO1 | TC01004315.hg.1 | 1.17 | 0.229 | 1.33 | 0.079 | 1.19 | 0.116 | 1.31 | 0.059 |
| AGO2 | TC08001684.hg.1 | 0.98 | 0.926 | 0.95 | 0.166 | 0.88 | 0.052 | 1.05 | 0.346 |
| AGO2 | TC08002130.hg.1 | 0.93 | 0.166 | 0.82 | 0.052 | 0.83 | 0.079 | 0.92 | 0.034 * |
| AGO3 | TC01000465.hg.1 | 1.05 | 0.405 | 1.14 | 0.518 | 1.19 | 0.079 | 1.06 | 0.346 |
| AGO4 | TC01000462.hg.1 | 1.67 | 0.033 * | 1.54 | 0.033 * | 1.76 | 0.013 * | 1.51 | 0.007 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Z.-M.; Chang, H.-C.; Liu, S.-F.; Huang, Y.-H.; Kuo, H.-C. Argonaute2 and Argonaute4 Involved in the Pathogenesis of Kawasaki Disease via mRNA Expression Profiles. Children 2025, 12, 73. https://doi.org/10.3390/children12010073
Lee Z-M, Chang H-C, Liu S-F, Huang Y-H, Kuo H-C. Argonaute2 and Argonaute4 Involved in the Pathogenesis of Kawasaki Disease via mRNA Expression Profiles. Children. 2025; 12(1):73. https://doi.org/10.3390/children12010073
Chicago/Turabian StyleLee, Zon-Min, Hui-Chuan Chang, Shih-Feng Liu, Ying-Hsien Huang, and Ho-Chang Kuo. 2025. "Argonaute2 and Argonaute4 Involved in the Pathogenesis of Kawasaki Disease via mRNA Expression Profiles" Children 12, no. 1: 73. https://doi.org/10.3390/children12010073
APA StyleLee, Z.-M., Chang, H.-C., Liu, S.-F., Huang, Y.-H., & Kuo, H.-C. (2025). Argonaute2 and Argonaute4 Involved in the Pathogenesis of Kawasaki Disease via mRNA Expression Profiles. Children, 12(1), 73. https://doi.org/10.3390/children12010073






