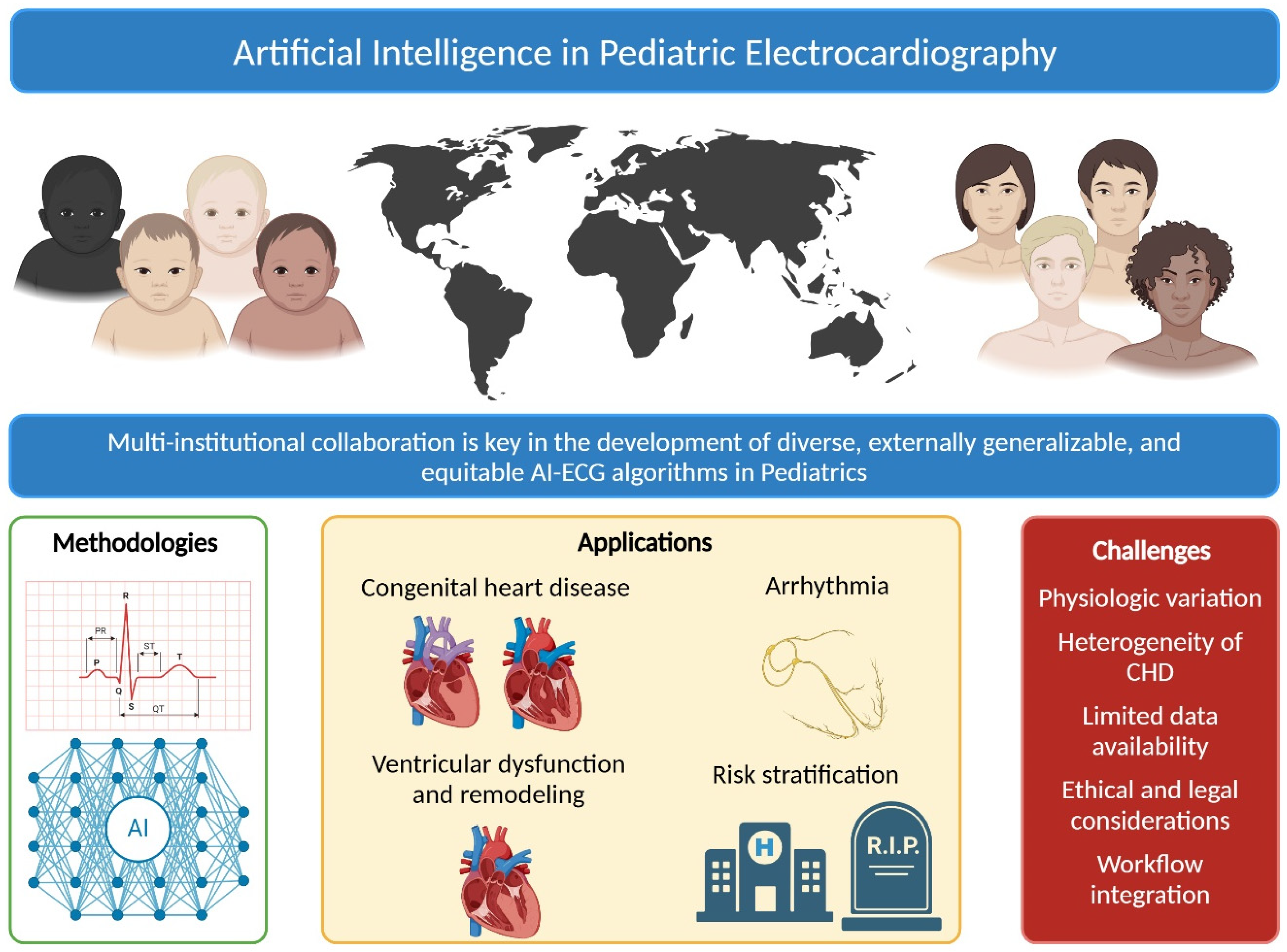
Artificial Intelligence in Pediatric Electrocardiography: A Comprehensive Review
Abstract
1. Introduction
2. Overview of AI Methodologies
3. Systematic Review of AI-ECG in Pediatric Cardiology
3.1. Methods
3.2. Results
3.3. Detection of Congenital Heart Diseases
3.4. Arrhythmia Classification
3.5. Detection of Ventricular Dysfunction
3.6. Risk Stratification
3.7. Other Predictions
3.8. Critical Analysis
4. Challenges in Pediatric ECG Interpretation
4.1. Age-Dependent Physiological Variations
4.2. Heterogeneity of Congenital Heart Disease
4.3. Rarity of Pediatric Heart Disease
4.4. Limited Data Availability and Privacy Concerns
4.5. Ethical and Legal Considerations
4.6. Integration into Clinical Workflow
5. Discussion
Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AUROC | Area Under the Receiver Operating Curve |
| AUPRC | Area Under the Precision Recall Curve |
| CHD | Congenital Heart Disease |
| CNN | Convolutional Neural Network |
| DL | Deep Learning |
| ECG | Electrocardiogram |
| ExAI | Explainable Artificial Intelligence |
| FDA | Food and Drug Administration |
| LSTM | Long Short-Term Memory Networks |
| ML | Machine Learning |
| pECG | Pediatric Electrocardiogram |
| RNN | Recurrent Neural Networks |
| SVM | Support Vector Machines |
References
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.; Anjewierden, S.; Greason, G.; Attia, I.Z.; Lopez-Jimenez, F.; Friedman, P.A.; Noseworthy, P.; Anderson, J.; Kashou, A.; Asirvatham, S.J.; et al. Pediatric sex estimation using AI-enabled ECG analysis: Influence of pubertal development. NPJ Digit. Med. 2024, 7, 176. [Google Scholar] [CrossRef]
- Mayourian, J.; Gearhart, A.; La Cava, W.G.; Vaid, A.; Nadkarni, G.N.; Triedman, J.K.; Powell, A.J.; Wald, R.M.; Valente, A.M.; Geva, T.; et al. Deep Learning-Based Electrocardiogram Analysis Predicts Biventricular Dysfunction and Dilation in Congenital Heart Disease. J. Am. Coll. Cardiol. 2024, 84, 815–828. [Google Scholar] [CrossRef]
- Mayourian, J.; Geggel, R.; La Cava, W.G.; Ghelani, S.J.; Triedman, J.K. Pediatric Electrocardiogram-Based Deep Learning to Predict Secundum Atrial Septal Defects. Pediatr. Cardiol. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Mayourian, J.; La Cava, W.G.; Vaid, A.; Nadkarni, G.N.; Ghelani, S.J.; Mannix, R.; Geva, T.; Dionne, A.; Alexander, M.E.; Duong, S.Q.; et al. Pediatric ECG-Based Deep Learning to Predict Left Ventricular Dysfunction and Remodeling. Circulation 2024, 149, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Ito, S.; Miranda, W.R.; Lopez-Jimenez, F.; Kane, G.C.; Asirvatham, S.J.; Noseworthy, P.A.; Friedman, P.A.; Carter, R.E.; Borlaug, B.A.; et al. Artificial intelligence-enabled ECG for left ventricular diastolic function and filling pressure. NPJ Digit. Med. 2024, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Khan, L.; Noor, I.; Siddique, A.; Shakil, S.; Keen, M.; Zafar, B.; Farooqi, M.; Essam, N.; Khan, M.S.; Siddiqi, T.J.; et al. Artificial intelligence-enhanced electrocardiogram for the diagnosis of cardiac amyloidosis: A systemic review and meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102860. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.M.; Attia, Z.I.; Albert, D.E.; Noseworthy, P.A.; Friedman, P.A.; Ackerman, M.J. Use of Artificial Intelligence and Deep Neural Networks in Evaluation of Patients With Electrocardiographically Concealed Long QT Syndrome From the Surface 12-Lead Electrocardiogram. JAMA Cardiol. 2021, 6, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Suárez, A.B.; Sehrawat, O.; Ackerman, M.J.; Attia, Z.I.; Friedman, P.A.; Noseworthy, P.A.; Maanja, M. Saliency maps provide insights into artificial intelligence-based electrocardiography models for detecting hypertrophic cardiomyopathy. J. Electrocardiol. 2023, 81, 286–291. [Google Scholar] [CrossRef]
- Miura, K.; Yagi, R.; Miyama, H.; Kimura, M.; Kanazawa, H.; Hashimoto, M.; Kobayashi, S.; Nakahara, S.; Ishikawa, T.; Taguchi, I.; et al. Deep learning-based model detects atrial septal defects from electrocardiography: A cross-sectional multicenter hospital-based study. eClinicalMedicine 2023, 63, 102141. [Google Scholar] [CrossRef] [PubMed]
- Noseworthy, P.A.; Attia, Z.I.; Behnken, E.M.; Giblon, R.E.; Bews, K.A.; Liu, S.; Gosse, T.A.; Linn, Z.D.; Deng, Y.; Yin, J.; et al. Artificial intelligence-guided screening for atrial fibrillation using electrocardiogram during sinus rhythm: A prospective non-randomised interventional trial. Lancet 2022, 400, 1206–1212. [Google Scholar] [CrossRef]
- Galloway, C.D.; Valys, A.V.; Shreibati, J.B.; Treiman, D.L.; Petterson, F.L.; Gundotra, V.P.; Albert, D.E.; Attia, Z.I.; Carter, R.E.; Asirvatham, S.J.; et al. Development and Validation of a Deep-Learning Model to Screen for Hyperkalemia From the Electrocardiogram. JAMA Cardiol. 2019, 4, 428–436. [Google Scholar] [CrossRef]
- Naser, J.A.; Lopez-Jimenez, F.; Chang, A.Y.; Baez-Suarez, A.; Attia, Z.I.; Pislaru, S.V.; Pellikka, P.A.; Lin, G.; Kapa, S.; Friedman, P.A.; et al. Artificial Intelligence-Augmented Electrocardiogram in Determining Sex: Correlation with Sex Hormone Levels. Mayo Clin. Proc. 2023, 98, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Amadio, J.M.; Grogan, M.; Muchtar, E.; Lopez-Jimenez, F.; Attia, Z.I.; AbouEzzeddine, O.; Lin, G.; Dasari, S.; Kapa, S.; Borgeson, D.D.; et al. Predictors of mortality by an artificial intelligence enhanced electrocardiogram model for cardiac amyloidosis. ESC Heart Fail. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Lopez-Jimenez, F.; Kapa, S.; Friedman, P.A.; LeBrasseur, N.K.; Klavetter, E.; Mangold, K.E.; Attia, Z.I. Assessing Biological Age: The Potential of ECG Evaluation Using Artificial Intelligence: JACC Family Series. JACC Clin. Electrophysiol. 2024, 10, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Adedinsewo, D.A.; Johnson, P.W.; Douglass, E.J.; Attia, I.Z.; Phillips, S.D.; Goswami, R.M.; Yamani, M.H.; Connolly, H.M.; Rose, C.H.; Sharpe, E.E.; et al. Detecting cardiomyopathies in pregnancy and the postpartum period with an electrocardiogram-based deep learning model. Eur. Heart J. Digit. Health 2021, 2, 586–596. [Google Scholar] [CrossRef]
- Attia, Z.I.; Harmon, D.M.; Dugan, J.; Manka, L.; Lopez-Jimenez, F.; Lerman, A.; Siontis, K.C.; Noseworthy, P.A.; Yao, X.; Klavetter, E.W.; et al. Prospective evaluation of smartwatch-enabled detection of left ventricular dysfunction. Nat. Med. 2022, 28, 2497–2503. [Google Scholar] [CrossRef] [PubMed]
- Sangha, V.; Nargesi, A.A.; Dhingra, L.S.; Khunte, A.; Mortazavi, B.J.; Banina, E.; Adeola, O.; Garg, N.; Brandt, C.A.; Miller, E.J.; et al. Detection of Left Ventricular Systolic Dysfunction From Electrocardiographic Images. Circulation 2023, 148, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Tayal, U.; Verdonschot, J.A.; Hazebroek, M.R.; Howard, J.; Gregson, J.; Newsome, S.; Gulati, A.; Pua, C.J.; Halliday, B.P.; Lota, A.S.; et al. Precision Phenotyping of Dilated Cardiomyopathy Using Multidimensional Data. J. Am. Coll. Cardiol. 2022, 79, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.S.; Thao, V.; Borah, B.J.; Attia, I.Z.; Inojosa, J.M.; Kapa, S.; Carter, R.E.; Friedman, P.A.; Lopez-Jimenez, F.; Yao, X.; et al. Cost Effectiveness of an Electrocardiographic Deep Learning Algorithm to Detect Asymptomatic Left Ventricular Dysfunction. Mayo Clin. Proc. 2021, 96, 1835–1844. [Google Scholar] [CrossRef]
- Yao, X.; Rushlow, D.R.; Inselman, J.W.; McCoy, R.G.; Thacher, T.D.; Behnken, E.M.; Bernard, M.E.; Rosas, S.L.; Akfaly, A.; Misra, A.; et al. Artificial intelligence-enabled electrocardiograms for identification of patients with low ejection fraction: A pragmatic, randomized clinical trial. Nat. Med. 2021, 27, 815–819. [Google Scholar] [CrossRef]
- Roquemen-Echeverri, V.; Jacobs, P.G.; Shalen, E.F.; Schulman, P.M.; Heitner, S.B.; Denfeld, Q.; Wilson, B.; Halvorson, J.; Scott, D.; Londoño-Murillo, T.; et al. External evaluation of a commercial artificial intelligence-augmented digital auscultation platform in valvular heart disease detection using echocardiography as reference standard. Int. J. Cardiol. 2024, 419, 132653. [Google Scholar] [CrossRef]
- Food and Drug Administration. Artificial Intelligence and Machine Learning in Software as a Medical Device. In Software as a Medical Device (SaMD); Food and Drug Administration: Silver Spring, MD, USA, 2024. [Google Scholar]
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.-K.; Li, N.; Keavney, B.D. Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol. 2019, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Zou, Z.; Hay, S.I.; Liu, Y.; Li, S.; Chen, H.; Naghavi, M.; Zimmerman, M.S.; Martin, G.R.; Wilner, L.B.; et al. Global, regional, and national time trends in mortality for congenital heart disease, 1990-2019: An age-period-cohort analysis for the Global Burden of Disease 2019 study. eClinicalMedicine 2022, 43, 101249. [Google Scholar] [CrossRef]
- Van Der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef]
- Dickinson, D.F. The normal ECG in childhood and adolescence. Heart 2005, 91, 1626–1630. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, S.; Zhang, Y.; Chang, Q.; Zhang, Y.; Li, D.; Qiu, J.; Hu, L.; Peng, X.; Du, Y.; et al. Congenital heart disease detection by pediatric electrocardiogram based deep learning integrated with human concepts. Nat. Commun. 2024, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Anjewierden, S.; O’Sullivan, D.; Mangold, K.E.; Greason, G.; Attia, I.Z.; Lopez-Jimenez, F.; Friedman, P.A.; Asirvatham, S.J.; Anderson, J.; Eidem, B.W.; et al. Detection of Right and Left Ventricular Dysfunction in Pediatric Patients Using Artificial Intelligence-Enabled ECGs. J. Am. Heart Assoc. 2024, 13, e035201. [Google Scholar] [CrossRef]
- Lubitz, S.A.; Faranesh, A.Z.; Selvaggi, C.; Atlas, S.J.; McManus, D.D.; Singer, D.E.; Pagoto, S.; McConnell, M.V.; Pantelopoulos, A.; Foulkes, A.S. Detection of Atrial Fibrillation in a Large Population Using Wearable Devices: The Fitbit Heart Study. Circulation 2022, 146, 1415–1424. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Vapnik, V. The Nature of Statistical Learning Theory; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM Sigkdd International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Rusin, C.G.; Acosta, S.I.; Shekerdemian, L.S.; Vu, E.L.; Bavare, A.C.; Myers, R.B.; Patterson, L.W.; Brady, K.M.; Penny, D.J. Prediction of imminent, severe deterioration of children with parallel circulations using real-time processing of physiologic data. J. Thorac. Cardiovasc. Surg. 2016, 152, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Rusin, C.G.; Acosta, S.I.; Vu, E.L.; Ahmed, M.; Brady, K.M.; Penny, D.J. Automated Prediction of Cardiorespiratory Deterioration in Patients With Single Ventricle. J. Am. Coll. Cardiol. 2021, 77, 3184–3192. [Google Scholar] [CrossRef] [PubMed]
- Vu, E.L.; Rusin, C.G.; Penny, D.J.M.; Kibler, K.K.C.; Easley, R.B.; Smith, B.; Andropoulos, D.M.; Brady, K. A Novel Electrocardiogram Algorithm Utilizing ST-Segment Instability for Detection of Cardiopulmonary Arrest in Single Ventricle Physiology: A Retrospective Study. Pediatr. Crit. Care Med. 2017, 18, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Günay, S.; Öztürk, A.; Özerol, H.; Yiğit, Y.; Erenler, A.K. Comparison of emergency medicine specialist, cardiologist, and chat-GPT in electrocardiography assessment. Am. J. Emerg. Med. 2024, 80, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Edenbrandt, L.; Rittner, R. Recognition of lead reversals in pediatric electrocardiograms. Am. J. Cardiol. 1998, 82, 1290–1292. [Google Scholar] [CrossRef]
- Mori, H.; Inai, K.; Sugiyama, H.; Muragaki, Y. Diagnosing Atrial Septal Defect from Electrocardiogram with Deep Learning. Pediatr. Cardiol. 2021, 42, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, M.; Kiuchi, K.; Nishimura, K.; Kusano, K.; Yoshida, A.; Adachi, K.; Hirayama, Y.; Miyazaki, Y.; Fujiwara, R.; Sommer, P.; et al. Accessory pathway analysis using a multimodal deep learning model. Sci. Rep. 2021, 11, 8045. [Google Scholar] [CrossRef]
- Sarkar, P.; Lobmaier, S.; Fabre, B.; González, D.; Mueller, A.; Frasch, M.G.; Antonelli, M.C.; Etemad, A. Detection of maternal and fetal stress from the electrocardiogram with self-supervised representation learning. Sci. Rep. 2021, 11, 24146. [Google Scholar] [CrossRef]
- Siontis, K.C.; Noseworthy, P.A.; Attia, Z.I.; Friedman, P.A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 2021, 18, 465–478. [Google Scholar] [CrossRef]
- de Vries, I.R.; van Laar, J.; van der Hout-van der Jagt, M.B.; Clur, S.B.; Vullings, R. Fetal electrocardiography and artificial intelligence for prenatal detection of congenital heart disease. Acta Obs. Gynecol. Scand. 2023, 102, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Nogimori, Y.; Sato, K.; Takamizawa, K.; Ogawa, Y.; Tanaka, Y.; Shiraga, K.; Masuda, H.; Matsui, H.; Kato, M.; Daimon, M.; et al. Prediction of adverse cardiovascular events in children using artificial intelligence-based electrocardiogram. Int. J. Cardiol. 2024, 406, 132019. [Google Scholar] [CrossRef]
- Rahman, J.; Brankovic, A.; Khanna, S. Machine learning model with output correction: Towards reliable bradycardia detection in neonates. Comput. Biol. Med. 2024, 177, 108658. [Google Scholar] [CrossRef] [PubMed]
- Mayourian, J.; El-Bokl, A.; Lukyanenko, P.; La Cava, W.G.; Geva, T.; Valente, A.M.; Triedman, J.K.; Ghelani, S.J. Electrocardiogram-based deep learning to predict mortality in paediatric and adult congenital heart disease. Eur. Heart J. 2024, ehae651. [Google Scholar] [CrossRef]
- Mayourian, J.; van Boxtel, J.P.; Sleeper, L.A.; Diwanji, V.; Geva, A.; O’leary, E.T.; Triedman, J.K.; Ghelani, S.J.; Wald, R.M.; Valente, A.M.; et al. Electrocardiogram-Based Deep Learning to Predict Mortality in Repaired Tetralogy of Fallot. JACC Clin. Electrophysiol. 2024. [Google Scholar] [CrossRef]
- Rijnbeek, P.R.; Witsenburg, M.; Schrama, E.; Hess, J.; Kors, J.A. New normal limits for the paediatric electrocardiogram. Eur. Heart J. 2001, 22, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Pullan, A.J.; Cheng, L.K.; Nash, M.P.; Ghodrati, A.; MacLeod, R.; Brooks, D.H. The Inverse Problem of Electrocardiography. In Comprehensive Electrocardiology; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Malm, T.; Sjöberg, G.; Nordenstam, F.; Hanséus, K.; Rosenkvist, C.-J.; Liuba, P. Longitudinal ECG changes in tetralogy of Fallot and association with surgical repair. Front. Cardiovasc. Med. 2024, 11, 1349166. [Google Scholar] [CrossRef]
- Mozayan, C.; Levis, J.T. ECG Diagnosis: Dextrocardia. Perm. J. 2019, 23, 18–244. [Google Scholar] [CrossRef]
- Luu, J.; Borisenko, E.; Przekop, V.; Patil, A.; Forrester, J.D.; Choi, J. Practical guide to building machine learning-based clinical prediction models using imbalanced datasets. Trauma. Surg. Acute Care Open 2024, 9, e001222. [Google Scholar] [CrossRef] [PubMed]
- Flach, P.; Kull, M. Precision-Recall-Gain Curves: PR Analysis Done Right. In Advances in Neural Information Processing Systems 28; Cortes, C., Lawrence, N., Lee, D., Sugiyama, M., Garnett, R., Eds.; Massachusetts Institute of Technology (MIT) Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Ignazio, P.; Giorgio, F.; Fabio, R. Designing multi-label classifiers that maximize F measures: State of the art. Pattern Recognit. 2017, 61, 394–404. [Google Scholar]
- Saito, T.; Rehmsmeier, M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS ONE 2015, 10, e0118432. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Milletarì, F.; Xu, D.; Rieke, N.; Hancox, J.; Zhu, W.; Baust, M.; Cheng, Y.; Ourselin, S.; Cardoso, M.J.; et al. Privacy-preserving Federated Brain Tumour Segmentation. Mach. Learn. Med. Imaging 2019, 11861, 133–141. [Google Scholar]
- Sarma, K.V.; Harmon, S.; Sanford, T.; Roth, H.R.; Xu, Z.; Tetreault, J.; Xu, D.; Flores, M.G.; Raman, A.G.; Kulkarni, R.; et al. Federated learning improves site performance in multicenter deep learning without data sharing. J. Am. Med. Inform. Assoc. JAMIA 2021, 28, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Na, L.; Yang, C.; Lo, C.C.; Zhao, F.; Fukuoka, Y.; Aswani, A. Feasibility of Reidentifying Individuals in Large National Physical Activity Data Sets From Which Protected Health Information Has Been Removed With Use of Machine Learning. JAMA Netw. Open 2018, 1, e186040. [Google Scholar] [CrossRef]
- Ghosheh, G.; Li, J.; Zhu, T. A review of Generative Adversarial Networks for Electronic Health Records: Applications, evaluation measures and data sources. arXiv 2022, arXiv:220307018. [Google Scholar]
- Gichoya, J.W.; Banerjee, I.; Bhimireddy, A.R.; Burns, J.L.; Celi, L.A.; Chen, L.-C.; Correa, R.; Dullerud, N.; Ghassemi, M.; Huang, S.-C.; et al. AI recognition of patient race in medical imaging: A modelling study. Lancet Digit. Health 2022, 4, e406–e414. [Google Scholar] [CrossRef]
- Blumenthal, D. A Step toward Interoperability of Health IT. N. Engl. J. Med. 2022, 387, 2201–2203. [Google Scholar] [CrossRef]
- Aiello, M.; Esposito, G.; Pagliari, G.; Borrelli, P.; Brancato, V.; Salvatore, M. How does DICOM support big data management? Investigating its use in medical imaging community. Insights Imaging 2021, 12, 164. [Google Scholar] [CrossRef]
- Mangold, K.E.; Carter, R.E.; Siontis, K.C.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Friedman, P.A.; Attia, Z.I. Unlocking the potential of artificial intelligence in electrocardiogram biometrics: Age-related changes, anomaly detection, and data authenticity in mobile health platforms. Eur. Heart J. Digit. Health 2024, 5, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, A.; Langs, G.; Denk, H.; Zatloukal, K.; Müller, H. Causability and explainability of artificial intelligence in medicine. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2019, 9, e1312. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wong, K.-K.; Zhang, Y. Wireless-powered edge computing with cooperative UAV: Task, time scheduling and trajectory design. IEEE Trans. Wirel. Commun. 2020, 19, 8083–8098. [Google Scholar] [CrossRef]
- Wickramasinghe, N.; Ulapane, N.; Sloane, E.B.; Gehlot, V. Digital Twins for More Precise and Personalized Treatment. Stud. Health Technol. Inf. 2024, 310, 229–233. [Google Scholar]
- Wickramasinghe, N.; Ulapane, N.; Zelcer, J.; Saffery, R. Omics-Based Digital Twins for Personalised Paediatric Healthcare. Stud. Health Technol. Inf. 2024, 318, 180–181. [Google Scholar]
- Salvador, M.; Kong, F.; Peirlinck, M.; Parker, D.W.; Chubb, H.; Dubin, A.M.; Marsden, A.L. Digital twinning of cardiac electrophysiology for congenital heart disease. J. R. Soc. Interface 2024, 21, 20230729. [Google Scholar] [CrossRef]
- de Hond, A.A.H.; Leeuwenberg, A.M.; Hooft, L.; Kant, I.M.J.; Nijman, S.W.J.; van Os, H.J.A.; Aardoom, J.J.; Debray, T.P.A.; Schuit, E.; van Smeden, M.; et al. Guidelines and quality criteria for artificial intelligence-based prediction models in healthcare: A scoping review. NPJ Digit. Med. 2022, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Sander, R.M.; Limon, A. Twelve key challenges in medical machine learning and solutions. Intell.-Based Med. 2022, 6, 100068. [Google Scholar] [CrossRef]

| Description | Primary Author(s) | Year | Title | Model Type | Data Type | n | Outcome Metric | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Detection of limb lead reversal [42] | Edenbrandt L | 1998 | Recognition of lead reversals in pediatric electrocardiograms | ANN | ECG intervals | 1908 patients | Internal AUROC: 0.999 | AI can detect limb lead reversals in pediatric ECGs with high accuracy *. |
| Detection of long QT syndrome [8] | Bos JM | 2021 | Use of Artificial Intelligence and Deep Neural Networks in Evaluation of Patients with Electrocardiographically Concealed Long QT Syndrome from the Surface 12-Lead Electrocardiogram | CNN | 12-lead ECG | 2059 patients in total † | Internal AUROC 0.90 (95% CI, 0.88–0.93), F1 0.84 | Detection of concealed long QT syndrome from ECG data with high accuracy. |
| Detection of atrial septal defects [43] | Mori H | 2021 | Diagnosing Atrial Septal Defect from Electrocardiogram with Deep Learning | CNN and LTSMs | ECG data | 728 patients | Internal AUROC: 0.95, F1 0.81 | Detection of atrial septal defect from ECGs with high accuracy. |
| Multimodal location of accessory pathways [44] | Nishimori M | 2021 | Accessory pathway analysis using a multimodal deep learning model | CNN | 12 lead ECG and chest X-ray images | 294 patients with WPW and 1519 controls | Mean Accuracy: 0.80, F1 0.88 | AI combines ECGs and CXR to locate accessory pathways in WPW syndrome with good accuracy. |
| Detection of maternal/fetal stress [45] | Sarkar P | 2021 | Detection of maternal and fetal stress from the electrocardiogram with self-supervised representation learning | Self-supervised Deep Learning | Maternal -fetus dyads using maternal and maternal abdominal ECGs | Multiple datasets, 210 total | External AUROC: 0.98, specificity 0.982 | AI detects stress levels from maternal and fetal ECGs with high accuracy. |
| Detection of hypertrophic cardiomyopathy [46] | Siontis KC | 2021 | Detection of hypertrophic cardiomyopathy by an artificial intelligence electrocardiogram in children and adolescents | AI ECG analysis | 12-lead ECG data from children and adolescents | 300 patients | Internal AUROC: 0.98, specificity 0.95 | Detection of hypertrophic cardiomyopathy using AI-ECG with high accuracy. |
| Detection of CHD using fetal ECG [47] | de Vries IR | 2023 | Fetal electrocardiography and artificial intelligence for prenatal detection of congenital heart disease | CNN | Fetal (abdominal) ECG data | 122 patients | Internal AUROC: 0.76–0.78 | Fetal AI-ECG can help detect CHD prenatally with fair accuracy. |
| Detection of secundum atrial septal defects [4] | Mayourian J | 2023 | Pediatric ECG-AI to Predict Secundum Atrial Septal Defects | CNN | Pediatric ECG data | 46,261 patients | Internal AUROC: 0.84, AUPRC: 0.46 | AI predicts secundum ASD using ECG data with good accuracy. |
| Prediction of sex across pediatric ages [2] | O’Sullivan D | 2023 | Pediatric sex estimation using AI-enabled ECG analysis: influence of pubertal development | CNN | Pediatric ECG data | 90,133 patients | Internal AUROC: 0.91, specificity 0.83 | Sex estimation based on AI-ECG with a higher discriminatory ability after puberty. |
| Detection of biventricular dysfunction [30] | Anjewierden S & O’Sullivan D | 2023 | Detection of Right and Left Ventricular Dysfunction in Pediatric Patients Using Artificial Intelligence–Enabled ECGs | CNN | Pediatric ECG data | 10,142 ECGs | Internal LVSD: AUROC 0.93, specificity 0.89 RVSD: AUROC 0.90, specificity 0.80 | Detection of LV/RVSD in pediatric patients using ECG data. |
| Detection of CHD [29] | Chen J | 2024 | Congenital heart disease detection by pediatric electrocardiogram based deep learning integrated with human concepts | CNN integrated with wavelet transformations | 9-lead ECG | 65,869 in the training set, 12,000, 7137 and 8121 in internal/external test set. | External AUROC: 0.907–0.917 Specificity: 0.907–0.937 | Integrating human concepts improves CHD detection with high accuracy. |
| Detection of LV dysfunction, dilation, and hypertrophy [5] | Mayourian J | 2024 | Pediatric ECG-Based Deep Learning to Predict Left Ventricular Dysfunction and Remodeling | CNN | 12-lead ECG | 92,377 ECGs in training set, 12,631; 2830 and 5088 in internal/external test set. | LVSD AUROC: 0.94 AUPRC: 0.32 LV dilation AUROC: 0.87 AUPRC: 0.33 LVH AUROC: 0.84 AUPRC: 0.25 | Prediction of left ventricular dysfunction and remodeling with high accuracy. |
| Prediction of biventricular dysfunction in patients with CHD [3] | Mayourian J | 2024 | Deep Learning-Based Electrocardiogram Analysis Predicts Biventricular Dysfunction and Dilation in Congenital Heart Disease | CNN | 12-lead ECG | 8584 ECGs † | External AUROC: LVSD: 0.89 LV dilation: 0.83 RVSD: 0.82 RV dilation: 0.80 | Prediction of biventricular dysfunction and dilation in CHD patients with good accuracy. |
| Prediction of MACE using ECG and BNP [48] | Nogimori Y | 2024 | Prediction of adverse cardiovascular events in children using artificial intelligence-based electrocardiogram | CNN | 12-lead ECG data and BNP levels | 8324 ECGs | AUROC: 0.826 (95% CI, 0.706–0.945), specificity 0.655 | Prediction of MACE using ECG and BNP with good accuracy. |
| Detection of neonatal bradycardia [49] | Rahman J | 2024 | Machine learning model with output correction: Towards reliable bradycardia detection in neonates | RNN, LSTM, CNN | 440 h real time 3-Lead ECG data | 10 patients | Internal AUROC CNN + LSTM: 0.987, AUPRC 0.73 | Improved detection of bradycardia in premature infants, reducing false positive alarms. |
| Prediction of mortality among patients with CHD [50] | Mayourian J | 2024 | Electrocardiogram-based deep learning to predict mortality in paediatric and adult congenital heart disease | CNN | 12-lead ECG | 39,784 patients † | Internal AUROC: 0.79, AUPRC 0.17 | Mortality prediction in pediatric and adult CHD patients with high accuracy. |
| Prediction of mortality in repaired TOF [51] | Mayourian J | 2024 | Electrocardiogram-Based Deep Learning to Predict Mortality in Repaired Tetralogy of Fallot | CNN | 12-lead ECG | 78,578 patients | External AUROC: 0.81, AUPRC 0.21 | Mortality prediction in patients with repaired TOF with good accuracy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leone, D.M.; O’Sullivan, D.; Bravo-Jaimes, K. Artificial Intelligence in Pediatric Electrocardiography: A Comprehensive Review. Children 2025, 12, 25. https://doi.org/10.3390/children12010025
Leone DM, O’Sullivan D, Bravo-Jaimes K. Artificial Intelligence in Pediatric Electrocardiography: A Comprehensive Review. Children. 2025; 12(1):25. https://doi.org/10.3390/children12010025
Chicago/Turabian StyleLeone, David M., Donnchadh O’Sullivan, and Katia Bravo-Jaimes. 2025. "Artificial Intelligence in Pediatric Electrocardiography: A Comprehensive Review" Children 12, no. 1: 25. https://doi.org/10.3390/children12010025
APA StyleLeone, D. M., O’Sullivan, D., & Bravo-Jaimes, K. (2025). Artificial Intelligence in Pediatric Electrocardiography: A Comprehensive Review. Children, 12(1), 25. https://doi.org/10.3390/children12010025






