Unveiling the Respiratory Muscle Strength in Duchenne Muscular Dystrophy: The Impact of Nutrition and Thoracic Deformities, Beyond Spirometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Protocol
2.2. Measurements
2.3. Orthopedic Assessment
2.4. Ethical Approval
2.5. Statistical Analysis
3. Results
3.1. Study Population
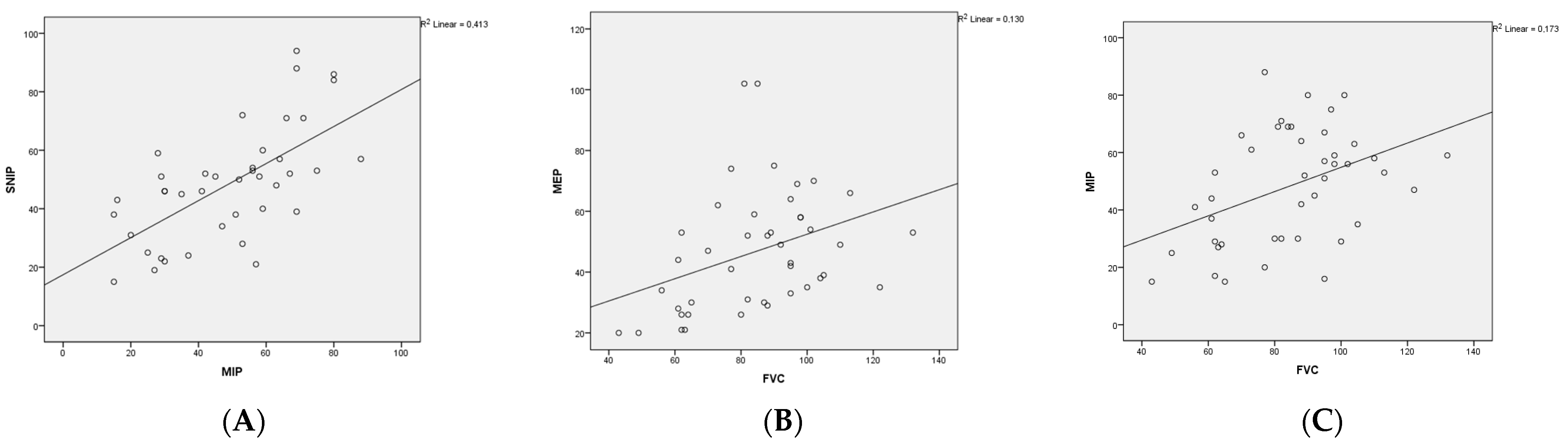
3.2. Respiratory Assessments
3.3. Effect of Kyphoscoliosis, Chest Deformity and BMIp Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wasilewska, E.; Sobierajska-Rek, A.; Malgorzewicz, S.; Solinski, M.; Jassem, E. Benefits of Telemonitoring of Pulmonary Function-3-Month Follow-Up of Home Electronic Spirometry in Patients with Duchenne Muscular Dystrophy. J. Clin. Med. 2022, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Leon-Astudillo, C.; Okorie, C.U.A.; McCown, M.Y.; Dy, F.J.; Puranik, S.; Prero, M.; ElMallah, M.K.; Treat, L.; Gross, J.E. ATS Core Curriculum 2022. Pediatric Pulmonary Medicine: Updates in pediatric neuromuscular disease. Pediatr. Pulmonol. 2023, 58, 1866–1874. [Google Scholar] [CrossRef]
- Finder, J.D.; Birnkrant, D.; Carl, J.; Farber, H.J.; Gozal, D.; Iannaccone, S.T.; Kovesi, T.; Kravitz, R.M.; Panitch, H.; Schramm, C.; et al. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am. J. Respir. Crit. Care Med. 2004, 170, 456–465. [Google Scholar] [CrossRef]
- Gauld, L.M.; Boynton, A.; Betts, G.A.; Johnston, H. Spirometry is affected by intelligence and behavior in Duchenne muscular dystrophy. Pediatr. Pulmonol. 2005, 40, 408–413. [Google Scholar] [CrossRef]
- Choi, W.H.; Shin, M.J.; Jang, M.H.; Lee, J.S.; Kim, S.Y.; Kim, H.Y.; Hong, Y.; Kim, C.; Shin, Y.B. Maximal Inspiratory Pressure and Maximal Expiratory Pressure in Healthy Korean Children. Ann. Rehabil. Med. 2017, 41, 299–305. [Google Scholar] [CrossRef]
- Nicot, F.; Hart, N.; Forin, V.; Boule, M.; Clement, A.; Polkey, M.I.; Lofaso, F.; Fauroux, B. Respiratory muscle testing: A valuable tool for children with neuromuscular disorders. Am. J. Respir. Crit. Care Med. 2006, 174, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Chiang, J.; Qian, H.; Amin, R. Maximal static respiratory and sniff pressures in healthy children. A systematic review and meta-analysis. Ann. Am. Thorac. Soc. 2019, 16, 478–487. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.E.; Cripe, L.; Hadjiyannakis, S.; Olson, A.K.; et al. Diagnosis and management of duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Khirani, S.; Ramirez, A.; Aubertin, G.; Boule, M.; Chemouny, C.; Forin, V.; Fauroux, B. Respiratory muscle decline in duchenne muscular dystrophy. Pediatr. Pulmonol. 2014, 49, 473–481. [Google Scholar] [CrossRef]
- Laveneziana, P.; Albuquerque, A.; Aliverti, A.; Babb, T.; Barreiro, E.; Dres, M.; Dube, B.P.; Fauroux, B.; Gea, J.; Guenette, J.A.; et al. Ers statement on respiratory muscle testing at rest and during exercise. Eur. Respir. J. 2019, 53, 1801214. [Google Scholar] [CrossRef]
- Neve, V.; Cuisset, J.M.; Edme, J.L.; Carpentier, A.; Howsam, M.; Leclerc, O.; Matran, R. Sniff nasal inspiratory pressure in the longitudinal assessment of young duchenne muscular dystrophy children. Eur. Respir. J. 2013, 42, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mei, Q.Q.; Xin, J.; Zhang, H.Y.; Wu, S.W.; Liu, C.F. The assessment of sniff nasal inspiratory pressure in patients with duchenne muscular dystrophy in china. Brain Dev. 2018, 40, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Fauroux, B.; Aubertin, G.; Cohen, E.; Clement, A.; Lofaso, F. Sniff nasal inspiratory pressure in children with muscular, chest wall or lung disease. Eur. Respir. J. 2009, 33, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Pennati, F.; Arrigoni, F.; LoMauro, A.; Gandossini, S.; Russo, A.; D’Angelo, M.G.; Aliverti, A. Diaphragm involvement in duchenne muscular dystrophy (dmd): An mri study. J. Magn. Reson. Imaging 2020, 51, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.H.; Choi, W.A.; Kim, D.H.; Kang, S.W. Postural vital capacity difference with aging in duchenne muscular dystrophy. Muscle Nerve 2015, 52, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Pandit, C.; Kennedy, B.; Waters, K.; Young, H.; Jones, K.; Fitzgerald, D.A. Can postural changes in spirometry in children with duchenne muscular dystrophy predict sleep hypoventilation? Paediatr. Respir. Rev. 2023, 49, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Lee, J.W.; Suh, M.R.; Choi, W.A.; Kang, S.W.; Oh, H.J. Correlation of serum creatine kinase level with pulmonary function in duchenne muscular dystrophy. Ann. Rehabil. Med. 2017, 41, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Rodillo, E.; Noble-Jamieson, C.M.; Aber, V.; Heckmatt, J.Z.; Muntoni, F.; Dubowitz, V. Respiratory muscle training in duchenne muscular dystrophy. Arch. Dis. Child. 1989, 64, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Lott, D.J.; Taivassalo, T.; Senesac, C.R.; Willcocks, R.J.; Harrington, A.M.; Zilke, K.; Cunkle, H.; Powers, C.; Finanger, E.L.; Rooney, W.D.; et al. Walking activity in a large cohort of boys with duchenne muscular dystrophy. Muscle Nerve 2021, 63, 192–198. [Google Scholar] [CrossRef]
- Torres, L.A.; Martinez, F.E.; Manco, J.C. Correlation between standing height, sitting height, and arm span as an index of pulmonary function in 6-10-year-old children. Pediatr. Pulmonol. 2003, 36, 202–208. [Google Scholar] [CrossRef]
- Neyzi, O.; Furman, A.; Bundak, R.; Gunoz, H.; Darendeliler, F.; Baş, F. Growth references for Turkish children aged 6 to 18 years. Acta Pædiatrica 2006, 95, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 cdc growth charts for the united states: Methods and development. Vital Health Stat. 2002, 246, 1–190. [Google Scholar]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Quanjer, P.H.; Tammeling, G.J.; Cotes, J.E.; Pedersen, O.F.; Peslin, R.; Yernault, J.C. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, european community for steel and coal. Official statement of the european respiratory society. Eur. Respir. J. Suppl. 1993, 16, 5–40. [Google Scholar] [CrossRef] [PubMed]
- LoMauro, A.; Romei, M.; Gandossini, S.; Pascuzzo, R.; Vantini, S.; D’Angelo, M.G.; Aliverti, A. Evolution of respiratory function in duchenne muscular dystrophy from childhood to adulthood. Eur. Respir. J. 2018, 51, 1701418. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; Valentine, R.E. Clinical measurement of the thoracic kyphosis. A study of the intra-rater reliability in subjects with and without shoulder pain. BMC Musculoskelet. Disord. 2010, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Whitelaw, W.A. The assessment of maximal respiratory mouth pressures in adults. Respir. Care. 2009, 54, 1348–1359. [Google Scholar] [PubMed]
- Fitting, J.W.; Paillex, R.; Hirt, L.; Aebischer, P.; Schluep, M. Sniff nasal pressure: A sensitive respiratory test to assess progression of amyotrophic lateral sclerosis. Ann. Neurol. 1999, 46, 887–893. [Google Scholar] [CrossRef]
- Janssens, J.P.; Adler, D.; Iancu Ferfoglia, R.; Poncet, A.; Genton Graf, L.; Leuchter, I.; Escher Imhof, M.; Heritier Barras, A.C. Assessing inspiratory muscle strength for early detection of respiratory failure in motor neuron disease: Should we use mip, snip, or both? Respiration 2019, 98, 114–124. [Google Scholar] [CrossRef]
- Kaslow, J.A.; Soslow, J.H.; Burnette, W.B.; Raucci, F.J.; Hills, T.J.; Ibach, M.G.; Hebblethwaite, R.C.; Arps, K.M.; Sokolow, A.G. Improving access and guideline adherence in pulmonary care in patients with duchenne muscular dystrophy. Respir. Care. 2022, 67, 347–352. [Google Scholar] [CrossRef]
- Levine, H.; Goldfarb, I.; Katz, J.; Carmeli, M.; Shochat, T.; Mussaffi, H.; Aharoni, S.; Prais, D.; Nevo, Y. Pulmonary function tests for evaluating the severity of duchenne muscular dystrophy disease. Acta Paediatr. 2023, 112, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Sobierajska-Rek, A.; Wasilewska, E.; Sledzinska, K.; Jablonska-Brudlo, J.; Malgorzewicz, S.; Wasilewski, A.; Szalewska, D. The association between the respiratory system and upper limb strength in males with duchenne muscular dystrophy: A new field for intervention? Int. J. Environ. Res. Public Health 2022, 19, 15675. [Google Scholar] [CrossRef] [PubMed]
- Inkley, S.R.; Oldenburg, F.C.; Vignos, P.J., Jr. Pulmonary function in duchenne muscular dystrophy related to stage of disease. Am. J. Med. 1974, 56, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Fromageot, C.; Lofaso, F.; Annane, D.; Falaize, L.; Lejaille, M.; Clair, B.; Gajdos, P.; Raphael, J.C. Supine fall in lung volumes in the assessment of diaphragmatic weakness in neuromuscular disorders. Arch. Phys. Med. Rehabil. 2001, 82, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, S.; Khirani, S.; Dabaj, I.; Cavassa, E.; Amaddeo, A.; Arroyo, J.O.; Desguerre, I.; Richard, P.; Cutrera, R.; Ferreiro, A.; et al. Diaphragmatic dysfunction in sepn1-related myopathy. Neuromuscul. Disord. 2017, 27, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kang, S.W.; Lee, S.C.; Choi, W.A.; Kim, D.H. How respiratory muscle strength correlates with cough capacity in patients with respiratory muscle weakness. Yonsei Med. J. 2010, 51, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Poussel, M.; Kaminsky, P.; Renaud, P.; Laroppe, J.; Pruna, L.; Chenuel, B. Supine changes in lung function correlate with chronic respiratory failure in myotonic dystrophy patients. Respir. Physiol. Neurobiol. 2014, 193, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Fayssoil, A.; Chaffaut, C.; Prigent, H.; Laforet, P.; Clair, B.; Orlikowski, D.; Ogna, A.; Chevret, S.; Meng, P.; Annane, D.; et al. Nutritional status, swallowing disorders, and respiratory prognosis in adult duchenne muscular dystrophy patients. Pediatr. Pulmonol. 2021, 56, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Willig, T.N.; Carlier, L.; Legrand, M.; Riviere, H.; Navarro, J. Nutritional assessment in duchenne muscular dystrophy. Dev. Med. Child. Neurol. 1993, 35, 1074–1082. [Google Scholar] [CrossRef]
- Lee, J.W.; Oh, H.J.; Choi, W.A.; Kim, D.J.; Kang, S.W. Relationship between eating and digestive symptoms and respiratory function in advanced duchenne muscular dystrophy patients. J. Neuromuscul. Dis. 2020, 7, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.; Carey, K.; Ho, G.; Mallitt, K.A.; Widger, J.; Farrar, M. The relationship of body habitus and respiratory function in duchenne muscular dystrophy. Respir. Med. 2016, 119, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Inal-Ince, D.; Savci, S.; Arikan, H.; Saglam, M.; Vardar-Yagli, N.; Bosnak-Guclu, M.; Dogru, D. Effects of scoliosis on respiratory muscle strength in patients with neuromuscular disorders. Spine J. 2009, 9, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Ramappa, M. Can ‘sniff nasal inspiratory pressure’ determine severity of scoliosis in paediatric population? Arch. Orthop. Trauma. Surg. 2009, 129, 1461–1464. [Google Scholar] [CrossRef]
- Allen, S.M.; Hunt, B.; Green, M. Fall in vital capacity with posture. Br. J. Dis. Chest. 1985, 79, 267–271. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n:44) | Ambulatory Patients (n:25) | Non-Ambulatory Patients (n:19) | p Value | |
|---|---|---|---|---|
| Age (years), (mean ± std) | 10.8 ± 2.9 | 9.2 ± 2.1 | 13 ± 2.3 | 0.000 * |
| Male, n (%) | 42 (95.5) | 23 (92) | 19 (100) | 0.212 |
| Body-mass index percentile (BMIp) (median) (25–75 percentile) | 52.5 (12.9–86.4) | 49.6 (27–81.5) | 55.5 (0.9–84) | 0.499 |
| Age at diagnosis (years), (median) (25–75 percentile) | 3.5 (1.6–5) | 4 (1.8–5) | 3 (1.5–5) | 0.426 |
| Consanguineous marriage, n (%) | 11 (25) | 5 (20) | 6 (31.6) | 0.385 |
| Family history of DMD patients, n (%) | 11 (25) | 8 (32) | 3 (15.8) | 0.224 |
| Kyphosis, n (%) | 13 (29.5) | 11 (44) | 2 (10.5) | 0.017 * |
| Scoliosis, n (%) | 12 (27.3) | 2 (8) | 10 (52.6) | 0.001 * |
| COBB angle, °, (median) (25–75 percentile) | 6 (2–11) | 5 (0–7.7) | 10 (4–16) | 0.005 * |
| Chest deformity, n (%) | 12 (27.3) | 4 (16) | 8 (42.1) | 0.057 |
| Other orthopedic problems (limb contractures, pes equinovarus etc…) n (%) | 25 (56.8) | 9 (36) | 16 (84.2) | 0.002 * |
| Cardiological problems, n (%) | 6 (13.6) | 0 (0) | 6 (31.6) | 0.003 * |
| Venous blood gas pCO2 (mmHg) (mean ± std) | 37.7 ± 4.1 | 36.8 ± 3.1 | 39.0 ± 5.1 | 0.450 |
| Steroid treatment, (years), (median) (25–75 percentile) | 4 (2–6) | 3 (1.5–5) | 6 (4–11.1) | 0.005 * |
| Ambulatory Patients (n:25) | Non-Ambulatory Patients (n:19) | |||||
|---|---|---|---|---|---|---|
| Upright Position | Supine Position | p Value | Upright Position | Supine Position | p Value | |
| FVC (%) (mean ± std) | 92.3 ± 17.4 | 87.3 ± 19.2 | 0.000 * | 75.7 ± 18.9 | 71.1 ± 19.0 | 0.021 * |
| FEV1 (%) (mean ± std) | 98.2 ± 17.0 | 91.8 ± 18.9 | 0.000 * | 83.8 ± 18.5 | 77.6 ± 18.8 | 0.002 * |
| FEV1/FVC (%) (mean ± std) | 103.3 ± 4.2 | 102.4 ± 4.8 | 0.330 | 107.0 ± 6.1 | 106.4 ± 6.0 | 0.457 |
| FEF2575 (%) (mean ± std) | 100.8 ± 14.3 | 92.8 ± 16.3 | 0.001 * | 97.1 ± 25.9 | 88.3 ± 26.1 | 0.040 * |
| MIP (cm H2O) (mean ± std) | 51.0 ± 20.2 | NA | NA | 44.5 ± 19.3 | NA | NA |
| SNIP (cm H2O) (mean ± std) | 52.6 ± 15.7 | NA | NA | 42.1 ± 23.3 | NA | NA |
| MEP (cm H2O) (median, 25–75p) | 49 (35–67.5) | NA | NA | 39 (26–53.2) | NA | NA |
| Postural FVC Difference < 7.5% (n:27) | Postural FVC Difference > 7.5% (n:17) | p Value | |
|---|---|---|---|
| MIP (cm H2O) (mean ± std) | 52.38 ± 19.79 | 43.81 ± 18.13 | 0.168 |
| SNIP (cm H2O) (mean ± std) | 53.40 ± 19 | 41.93 ± 17.54 | 0.049 * |
| MEP (cm H2O) (median, 25–75p) | 47 (31–59) | 38 (28.50–53.75) | 0.407 |
| FVC (%) (mean ± std) | 85.07 ± 17.51 | 85.35 ± 23.50 | 0.838 |
| FEV1 (%) (mean ± std) | 94 ± 15.73 | 91.53 ± 21.39 | 0.665 |
| FEV1/FVC (%) (mean ± std) | 104.96 ± 5.71 | 104.88 ± 5.04 | 0.946 |
| FEF2575 (%) (mean ± std) | 98.80 ± 19.48 | 103.17 ± 16.35 | 0.449 |
| BMIp Status | Kyphoscoliosis and Low BMIp Association | |||||
|---|---|---|---|---|---|---|
| Low BMI Group (n:28) | Normal BMI Group (n:16) | p value | Group with Kyphoscoliosis and Low BMI (n:16) | Group without Both Kyphoscoliosis and Low BMI (n:30) | p Value | |
| MIP (mean ± std) | 39.6 ± 18.6 | 61.7 ± 14.0 | 0.000 * | 37.1 ± 16.4 | 53.7 ± 19.3 | 0.010 * |
| MEP (median, 25–75p) | 35 (26–49.7) | 54 (47–64) | 0.001 * | 36.5 (26–44.5) | 52 (33–65) | 0.010 * |
| SNIP (mean ± std) | 40.9 ± 14.3 | 61.7 ± 21.7 | 0.001 * | 38.5 ± 12.8 | 53.7 ± 20.7 | 0.018 * |
| FVC (%) (mean ± std) | 84.1 ± 22.4 | 85.1 ± 14.9 | 0.854 | 85.3 ± 27.3 | 85.1 ± 15.6 | 0.960 |
| FEV1 (%) (mean ± std) | 91.0 ± 21.5 | 92.0 ± 14.0 | 0.867 | 92.5 ± 26.7 | 91.7 ± 14.5 | 0.801 |
| FEV1/FVC (%) (mean ± std) | 105.4 ± 4.7 | 104.5 ± 6.6 | 0.627 | 105.1 ± 4.1 | 104.8 ± 5.9 | 0.859 |
| FEF2575 (%) (mean ± std) | 95.2 ± 17.1 | 104.1 ± 22.8 | 0.159 | 95.2 ± 20.3 | 101.1 ± 19.9 | 0.743 |
| COBB angle, °, (median) (25–75p) | 5.5 (1.2–8) | 9 (4–12) | 0.107 | 11 (6–16) | 3 (1–6.5) | 0.000 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuksel Kalyoncu, M.; Gokdemir, Y.; Yilmaz Yegit, C.; Yanaz, M.; Gulieva, A.; Selcuk, M.; Karabulut, Ş.; Metin Çakar, N.; Ergenekon, P.; Erdem Eralp, E.; et al. Unveiling the Respiratory Muscle Strength in Duchenne Muscular Dystrophy: The Impact of Nutrition and Thoracic Deformities, Beyond Spirometry. Children 2024, 11, 994. https://doi.org/10.3390/children11080994
Yuksel Kalyoncu M, Gokdemir Y, Yilmaz Yegit C, Yanaz M, Gulieva A, Selcuk M, Karabulut Ş, Metin Çakar N, Ergenekon P, Erdem Eralp E, et al. Unveiling the Respiratory Muscle Strength in Duchenne Muscular Dystrophy: The Impact of Nutrition and Thoracic Deformities, Beyond Spirometry. Children. 2024; 11(8):994. https://doi.org/10.3390/children11080994
Chicago/Turabian StyleYuksel Kalyoncu, Mine, Yasemin Gokdemir, Cansu Yilmaz Yegit, Muruvvet Yanaz, Aynur Gulieva, Merve Selcuk, Şeyda Karabulut, Neval Metin Çakar, Pinar Ergenekon, Ela Erdem Eralp, and et al. 2024. "Unveiling the Respiratory Muscle Strength in Duchenne Muscular Dystrophy: The Impact of Nutrition and Thoracic Deformities, Beyond Spirometry" Children 11, no. 8: 994. https://doi.org/10.3390/children11080994
APA StyleYuksel Kalyoncu, M., Gokdemir, Y., Yilmaz Yegit, C., Yanaz, M., Gulieva, A., Selcuk, M., Karabulut, Ş., Metin Çakar, N., Ergenekon, P., Erdem Eralp, E., Öztürk, G., Unver, O., Turkdogan, D., Sahbat, Y., Akgülle, A. H., Karakoç, F., & Karadag, B. (2024). Unveiling the Respiratory Muscle Strength in Duchenne Muscular Dystrophy: The Impact of Nutrition and Thoracic Deformities, Beyond Spirometry. Children, 11(8), 994. https://doi.org/10.3390/children11080994








