Nutritional Support Best Practices in Pediatric Blood and Marrow Transplant Patients: An Integrative Review
Abstract
1. Introduction
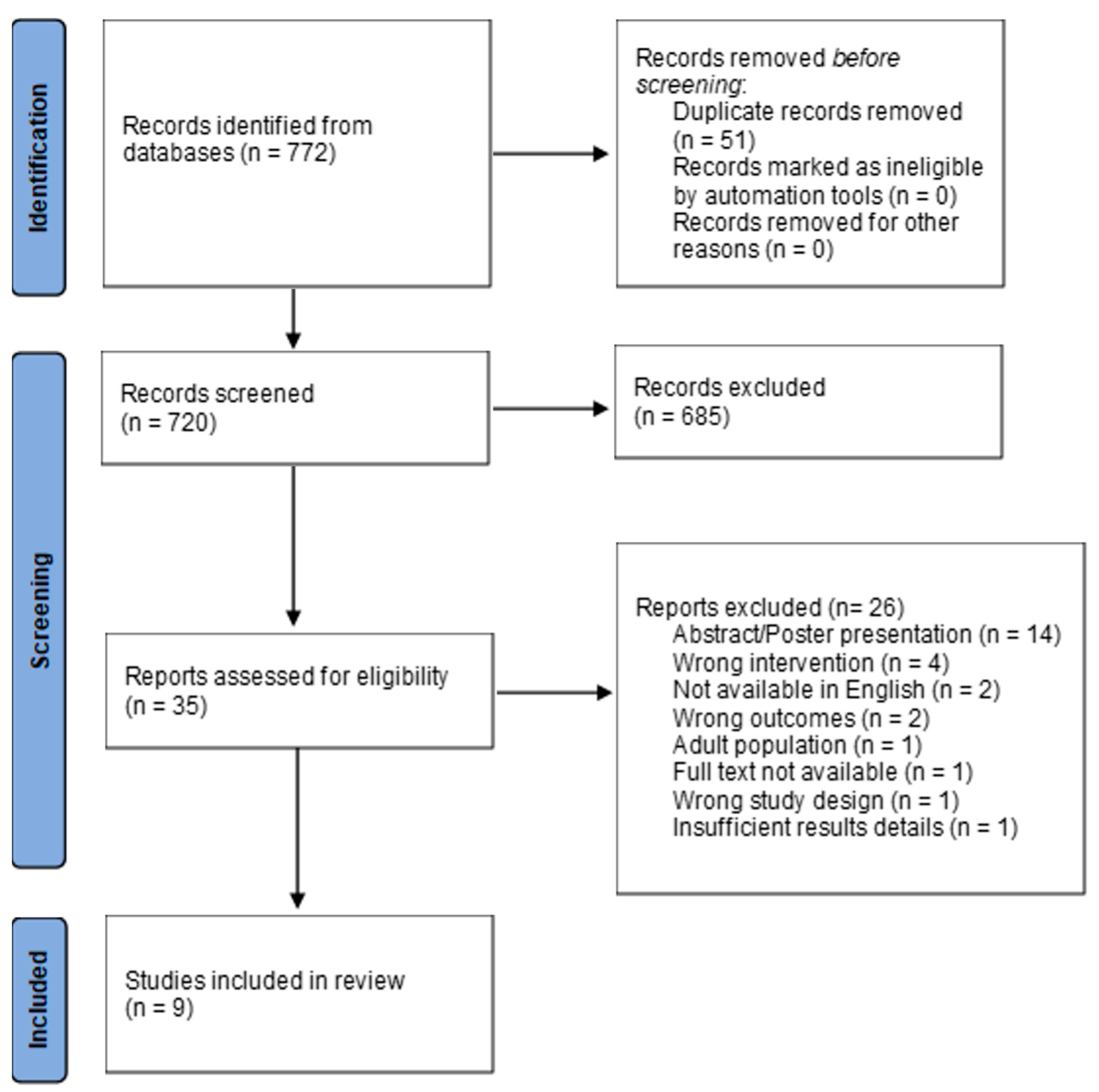
2. Methods
3. Results
3.1. Intervention Threshold
3.2. Energy Requirements
3.3. Length of Stay (LOS)
3.4. Engraftment
3.5. Acute Gastrointestinal (GI) GVHD
3.6. Infections
3.7. Veno-Occlusive Disease (VOD)
3.8. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mehta, N.M.; Corkins, M.R.; Lyman, B.; Malone, A.; Goday, P.S.; Carney, L.; Monczka, J.L.; Plogsted, S.W.; Schwenk, W.F. Defining Pediatric Malnutrition. J. Parenter. Enter. Nutr. 2013, 37, 460–481. [Google Scholar] [CrossRef] [PubMed]
- Larson-Nath, C.; Goday, P. Malnutrition in Children with Chronic Disease. Nutr. Clin. Pract. 2019, 34, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, B.; Yan, X.; Cai, J.; Wang, Y. Comprehensive evaluation of nutritional status before and after hematopoietic stem cell transplantation in 170 patients with hematological diseases. Chin. J. Cancer Res. 2016, 28, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Bargetzi, A.; Zueger, N.; Bargetzi, M.; Medinger, M.; Bounoure, L.; Gomes, F.; Stanga, Z.; Mueller, B.; Schuetz, P. Revisiting nutritional support for allogeneic hematologic stem cell transplantation—A systematic review. Bone Marrow Transplant. 2017, 52, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Brinksma, A.; Huizinga, G.; Sulkers, E.; Kamps, W.; Roodbol, P.; Tissing, W. Malnutrition in childhood cancer patients: A review on its prevalence and possible causes. Crit. Rev. Oncol./Hematol. 2012, 83, 249–275. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; White, M.; Davies, P.S.W. Body composition of children with cancer. Am. J. Clin. Nutr. 2010, 92, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.; Hopkins, M.; Arbuckle, L.; Williams, N.; Forsythe, L.; Bujkiewicz, S.; Pizer, B.; Estlin, E.; Picton, S. Nutritional problems in children treated for medulloblastoma: Implications for enteral nutrition support. Pediatr. Blood Cancer 2009, 53, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Barron, M.A.; Pencharz, P.B. Nutritional issues in infants with cancer. Pediatr. Blood Cancer 2007, 49, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Schiavetti, A.; Fornari, C.; Bonci, E.; Clerico, A.; Guidi, R. Nutritional Status in Childhood Malignancies. Nutr. Cancer 2002, 44, 153–155. [Google Scholar] [CrossRef]
- Lange, B.J.; Gerbing, R.B.; Feusner, J.; Skolnik, J.; Sacks, N.; Smith, F.O.; Alonzo, T.A. Mortality in Overweight and Underweight Children with Acute Myeloid Leukemia. J. Am. Med. Assoc. 2005, 293, 203–211. [Google Scholar] [CrossRef]
- Pedretti, L.; Massa, S.; Leardini, D.; Muratore, E.; Rahman, S.; Pession, A.; Esposito, S.; Masetti, R. Role of Nutrition in Pediatric Patients with Cancer. Nutrients 2023, 15, 710. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.K.; Neogi, S.; Lal, A.; Higa, A.; Fung, E. Nutritional Deficiencies Are Common in Patients with Transfusion-Dependent Thalassemia and Associated with Iron Overload. J. Food Nutr. Res. 2018, 6, 674–681. [Google Scholar] [CrossRef]
- Zemrani, B.; Yap, J.K.; Van Dort, B.; Evans, V.; Bartle, J.; Shandley, D.; Smart, J.; Bines, J.E.; Cole, T. Nutritional challenges in children with primary immunodeficiencies undergoing hematopoietic stem cell transplant. Clin. Nutr. 2020, 39, 2832–2841. [Google Scholar] [CrossRef] [PubMed]
- Yesilipek, M.A. Hematopoetic stem cell transplantation in children. Turk. Arch. Pediatr. 2014, 49, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.C. Importance of nutrition in pediatric oncology. Indian J. Cancer 2015, 52, 176–178. [Google Scholar] [CrossRef]
- Koç, N.; Gündüz, M.; Tavil, B.; Azik, M.F.; Coşkun, Z.; Yardımcı, H.; Uçkan, D.; Tunç, B. Beneficial Effect of the Nutritional Support in Children Who Underwent Hematopoietic Stem Cell Transplant. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2017, 15, 458. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, C.G.; Garofolo, A.; Leite, H.P. Sarcopenia in children and adolescents submitted to hematopoietic stem cell transplantation. Hematol. Transfus. Cell Ther. 2024. [Google Scholar] [CrossRef] [PubMed]
- Goates, S.; Du, K.; Braunschweig, C.A.; Arensberg, M.B. Economic Burden of Disease-Associated Malnutrition at the State Level. PLoS ONE 2016, 11, e0161833. [Google Scholar] [CrossRef] [PubMed]
- Corkins, M.R. Why Is Diagnosing Pediatric Malnutrition Important? Nutr. Clin. Pract. 2017, 32, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Freijer, K.; van Puffelen, E.; Joosten, K.F.; Hulst, J.M.; Koopmanschap, M.A. The costs of disease related malnutrition in hospitalized children. Clin. Nutr. ESPEN 2018, 23, 228–233. [Google Scholar] [CrossRef]
- Gupta, H.; Pant, G.; Verma, N. Malnutrition in childhood cancer patients: Magnitude, key indicators and outcome. Pediatr. Hematol. Oncol. J. 2022, 7, 155–160. [Google Scholar] [CrossRef]
- McMillen, K.K.; Coghlin-Dickson, T.; Adintori, P.A. Optimization of nutrition support practices early after hematopoietic cell transplantation. Bone Marrow Transplant. 2020, 56, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, Z.; Demir, H.; Karhan, A.N.; Saltik Temizel, I.N.; Özen, H. Evaluation of non-infectious complications in children receiving parenteral nutrition. Ir. J. Med. Sci. 2023, 192, 2735–2739. [Google Scholar] [CrossRef] [PubMed]
- Azarnoush, S.; Bruno, B.; Beghin, L.; Guimber, D.; Nelken, B.; Yakoub-Agha, I.; Seguy, D. Enteral nutrition: A first option for nutritional support of children following allo-SCT? Bone Marrow Transplant. 2012, 47, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Ralls, M.W.; Miyasaka, E.; Teitelbaum, D.H. Intestinal Microbial Diversity and Perioperative Complications. J. Parenter. Enter. Nutr. 2014, 38, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.D.; Ladas, E.J. The role of nutrition in pediatric oncology. Expert Rev. Anticancer Ther. 2020, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.; Carney, L.N.; Corkins, M.R.; Monczka, J.; Smith, E.; Smith, S.E.; Spear, B.A.; White, J.V. Consensus Statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition. Nutr. Clin. Pract. 2015, 30, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Corkins, M.R.; Griggs, K.C.; Groh-Wargo, S.; Han-Markey, T.L.; Helms, R.A.; Muir, L.V.; Szeszycki, E.E. Standards for Nutrition Support. Nutr. Clin. Pract. 2013, 28, 263–276. [Google Scholar] [CrossRef]
- Bechtold, M.L.; Brown, P.M.; Escuro, A.; Grenda, B.; Johnston, T.; Kozeniecki, M.; Limketkai, B.N.; Nelson, K.K.; Powers, J.; Ronan, A.; et al. When is enteral nutrition indicated? J. Parenter. Enter. Nutr. 2022, 46, 1470–1496. [Google Scholar] [CrossRef]
- Fabozzi, F.; Trovato, C.M.; Diamanti, A.; Mastronuzzi, A.; Zecca, M.; Tripodi, S.I.; Masetti, R.; Leardini, D.; Muratore, E.; Barat, V.; et al. Management of Nutritional Needs in Pediatric Oncology: A Consensus Statement. Cancers 2022, 14, 3378. [Google Scholar] [CrossRef]
- Mehta, N.M.; Skillman, H.E.; Irving, S.Y.; Coss-Bu, J.A.; Vermilyea, S.; Farrington, E.A.; McKeever, L.; Hall, A.M.; Goday, P.S.; Braunschweig, C. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. J. Parenter. Enter. Nutr. 2017, 41, 706–742. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.Y. Enteral Nutrition in Pediatric Patients. Pediatr. Gastroenterol. Hepatol. Nutr. 2018, 21, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.; Dearholt, S.L.; Bissett, K.; Ascenzi, J.; Whalen, M. Johns Hopkins Evidence-Based Practice for Nurses and Healthcare Professionals: Model and Guidelines; Sigma Theta Tau International Honor Society of Nursing: Indianapolis, IN, USA, 2022. [Google Scholar]
- CDC Clinical Growth Charts. Available online: https://www.cdc.gov/growthcharts/clinical_charts.htm (accessed on 1 February 2024).
- Soussi, M.A.; Besbes, H.; Mellouli, F.; Drira, C.; Lazreg, O.; Belghith, A.; Zouari, B.; Zaouali, S.; Bejaoui, M.; Razgallah Khrouf, M. Parenteral Nutrition Complications in Children Undergoing Bone Marrow Transplantation. J. Pediatr. Hematol./Oncol. 2019, 41, e473–e477. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Needle, J.J.; Hirani, S.P. Early outcomes of gastrostomy feeding in paediatric allogenic bone marrow transplantation: A retrospective cohort study. Clin. Nutr. ESPEN 2019, 31, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kairiene, I.; Vaisvilas, M.; Vasciunaite, A.; Tubutyte, G.; Nedzelskiene, I.; Pasauliene, R.; Muleviciene, A.; Rascon, J. Impact of percutaneous endoscopic gastrostomy on pediatric bone marrow transplantation outcomes: Retrospectice single-center cohort study. J. Parenter. Enter. Nutr. 2023, 47, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, C.G.; Daudt, L.E.; Jochims, A.M.K.; Paz, A.; Mello, E.D.d. Nutritional aspects in allogeneic hematopoietic stem cell transplantation in children and adolescents in a tertiary hospital. Nutr. Hosp. 2019, 36, 20–24. [Google Scholar] [CrossRef]
- Zama, D.; Muratore, E.; Biagi, E.; Forchielli, M.L.; Rondelli, R.; Candela, M.; Prete, A.; Pession, A.; Masetti, R. Enteral nutrition protects children undergoing allogeneic hematopoietic stem cell transplantation from blood stream infections. Nutr. J. 2020, 19, 29. [Google Scholar] [CrossRef]
- Evans, J.; Green, D.; Gibson, F.; O’Connor, G.; Lanigan, J. Complications and outcomes of gastrostomy versus nasogastric tube feeding in paediatric allogeneic bone marrow transplant: A prospective cohort study. Clin. Nutr. ESPEN 2023, 55, 58–70. [Google Scholar] [CrossRef]
- Alsalamah, S.; Alramyan, R.; Alakel, R.; Altheyeb, F.; Alrashed, R.; Masuadi, E.; Alyousif, G.; Bin Sunaid, F.F.; Alsultan, A.; Essa, M.F. The outcome and complications of total parenteral nutrition in pediatric hematopoietic stem cell transplantation. Pediatr. Transplant. 2022, 26, e14198. [Google Scholar] [CrossRef]
- Mellgren, K.; Nicolajsen, T.; Christoforaki, T.P.; Juan, S.M.; Mårtensson, T.; Toporski, J.; Casswall, T.H.; Gustafsson, B. A retrospective case–control study of gastrostomy use in children undergoing hematopoietic cell transplantation. Pediatr. Transplant. 2023, 27, e14520. [Google Scholar] [CrossRef] [PubMed]
- Joffe, L.; Ladas, E.J. Nutrition during childhood cancer treatment: Current understanding and a path for future research. Lancet Child Adolesc. Health 2020, 4, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Ifversen, M.; Meisel, R.; Sedlacek, P.; Kalwak, K.; Sisinni, L.; Hutt, D.; Lehrnbecher, T.; Balduzzi, A.; Diesch, T.; Jarisch, A.; et al. Supportive Care during Pediatric Hematopoietic Stem Cell Transplantation: Prevention of Infections. A Report from Workshops on Supportive Care of the Paediatric Diseases Working Party (PDWP) of the European Society for Blood and Marrow Transplantation (EBMT). Front. Pediatr. 2021, 9, 705179. [Google Scholar] [CrossRef] [PubMed]
- Casirati, A.; Salcedo, I.; Cereda, E.; Chabannon, C.; Ruggeri, A.; Kuball, J.; Clout, R.; Mooyaart, J.E.; Kenyon, M.; Caccialanza, R.; et al. The European Society for Blood and Marrow Transplantation (EBMT) roadmap and perspectives to improve nutritional care in patients undergoing hematopoietic stem cell transplantation on behalf of the Cellular Therapy and Immunobiology Working Party (CTIWP) and the Nurses Group (NG) of the EBMT. Bone Marrow Transplant. 2023, 58, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, F.; Bruno, B.; Alarcón Fuentes, M.; De Berranger, E.; Guimber, D.; Behal, H.; Gandemer, V.; Spiegel, A.; Sirvent, A.; Yakoub-Agha, I.; et al. Better early outcome with enteral rather than parenteral nutrition in children undergoing MAC allo-SCT. Clin. Nutr. 2018, 37, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.E.; Parrott, F.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Canter, R.R.; McLennan, B.K.; Tan, J.C.; Bear, D.E.; Segaran, E.; et al. A multicentre, randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of early nutritional support via the parenteral versus the enteral route in critically ill patients (CALORIES). Health Technol. Assess. 2016, 20, 1–144. [Google Scholar] [CrossRef]
- Kvammen, J.A.; Thomassen, R.A.; Buechner, J.; Sitsabesan, A.; Bentsen, B.S.; Bechensteen, A.G.; Henriksen, C. Impact of Allogeneic Hematopoietic Stem Cell Transplantation on Nutritional Status and Intake in Children. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Zama, D.; Bossù, G.; Leardini, D.; Muratore, E.; Biagi, E.; Prete, A.; Pession, A.; Masetti, R. Insights into the role of intestinal microbiota in hematopoietic stem-cell transplantation. Ther. Adv. Hematol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Zama, D.; Gori, D.; Muratore, E.; Leardini, D.; Rallo, F.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A.; Masetti, R. Enteral versus Parenteral Nutrition as Nutritional Support after Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Transplant. Cell. Therapy. 2021, 27, 180.e1–180.e8. [Google Scholar] [CrossRef]
- Murphy, J.D.; Cooke, K.R.; Symons, H.J.; VanGraafeiland, B. Enteral nutrition optimization program for children undergoing blood & marrow transplantation: A quality improvement project. J. Pediatr. Nurs. 2024, 74, 61–68. [Google Scholar] [CrossRef]
- Wang, Y.M.; Taggart, C.B.; Huber, J.F.; Davies, S.M.; Smith, D.F.; Hogenesch, J.B.; Dandoy, C.E. Daytime-restricted parenteral feeding is associated with earlier oral intake in children following stem cell transplant. J. Clin. Investig. 2023, 133, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kerby, E.H.; Li, Y.; Getz, K.D.; Smith, E.C.; Smith, L.T.; Bunin, N.J.; Seif, A.E. Nutritional risk factors predict severe acute graft-versus-host disease and early mortality in pediatric allogeneic hematopoietic stem cell transplantation. Pediatr. Blood Cancer 2018, 65, e26853. [Google Scholar] [CrossRef] [PubMed]
- Loeffen, E.A.H.; Brinksma, A.; Miedema, K.G.E.; de Bock, G.H.; Tissing, W.J.E. Clinical implications of malnutrition in childhood cancer patients--infections and mortality. Support. Care Cancer 2015, 23, 143. [Google Scholar] [CrossRef] [PubMed]
- Daloğlu, H.; Uygun, V.; Öztürkmen, S.; Yalçın, K.; Karasu, G.; Yeşilipek, A. Pre-transplantation vitamin D deficiency increases acute graft-versus-host disease after hematopoietic stem cell transplantation in thalassemia major patients. Clin. Transplant. 2023, 37, e14874. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gil, A.; Carrillo-Cruz, E.; Marrero-Cepeda, C.; Rodríguez, G.; Pérez-Simón, J.A. Effect of Vitamin D on Graft-versus-Host Disease. Biomedicines 2022, 10, 987. [Google Scholar] [CrossRef] [PubMed]
- Lazarow, H.; Singer, R.; Compher, C.; Gilmar, C.; Kucharczuk, C.R.; Mangan, P.; Salam, K.; Cunningham, K.; Stadtmauer, E.A.; Landsburg, D.J. Effect of malnutrition-driven nutritional support protocol on clinical outcomes in autologous stem cell transplantation patients. Support. Care Cancer 2021, 29, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Hirose, E.Y.; de Molla, V.C.; Gonçalves, M.V.; Pereira, A.D.; Szor, R.S.; da Fonseca, A.R.B.M.; Fatobene, G.; Serpa, M.G.; Xavier, E.M.; Tucunduva, L.; et al. The impact of pretransplant malnutrition on allogeneic hematopoietic stem cell transplantation outcomes. Clin. Nutr. ESPEN 2019, 33, 213–219. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
| Author and Date | Evidence Type | Sample, Sample Size, Setting | Intervention | Findings That Help Answer the EBP Question | Observable Measures | Limitations | Evidence Level, Quality |
|---|---|---|---|---|---|---|---|
| Alsalamah et al., 2022 [42] | Retrospective cohort study | Children <14 undergoing BMT at a hospital in Saudi Arabia
| Review of nutritional support (PN, NG-tube, oral) use and complications | PN used most over NG and oral, associated with increased VOD, aGVHD, and mucositis. Reduced-intensity conditioning patients less likely to use TPN by 70% | Nutritional support length of time by type, incidence of mucositis, VOD, aGVHD, infection. Measurement interval periods not defined. | Retrospective design. Small sample size. Unclear if PN groups also had EN. | Level III, Quality B |
| Evans et al., 2023 [41] | Prospective cohort study | Children 1–9 undergoing allogeneic BMT at an English hospital
| Prophylactic G-tube placement vs NG-tube. Included some PN use | Both groups experienced at least 1 complication, majority minor. No statistically significant differences between groups on measured outcomes. | Nutritional support means and length of time, incidence of tube complications, infections, weight, height, MUAC, caloric and nutrient intake, OS, non-relapse mortality, GVHD. Measured weekly from Day −7 to day +42, then monthly until day +180. | Small sample size. G-tubes primarily in non-malignancies. PN added if unable to meet energy caloric requirements (first-line PN use excluded) but usage of PN not reported. | Level III, Quality B |
| Evans et al., 2019 [37] | Retrospective cohort study | Children <18 undergoing BMT after reduced-intensity or myeloablative conditioning at an English hospital
| Prophylactically placed g-tube vs without (PN, NG-tube, oral, and combinations) with G-tube subgroup of EN only vs EN with PN | Decreased length of stay in EN only subgroup compared to EN-PN. No difference in LOS, OS at day 100, incidence of aGVHD, infection rates between g-tube and no G-tube and EN alone vs EN-PN | Changes in nutritional status, and post-transplantation outcomes. Measurement interval periods not defined, but included admission, discharge, and Day +100 | Absence of randomization and control group. Retrospective design with missing data. Excluded cord blood patients. Large number of immunodeficiency patients. | Level III, Quality B |
| Kairiene et al., 2023 [38] | Retrospective cohort study | Children <18 admitted for allogeneic BMT at Lithuanian hospital
| Prophylactically placed g-tube vs without feeding tubes. Included some PN | Improved transplant-related mortality and 5-yr OS with PEG | MRD status, time of G-tube use, changes in nutritional status, caloric intake, time to engraftment, aGVHD, 5-year event-free survival, OS. | Retrospective over 12 yrs.; practice change during that time. PN added if unable to meet energy caloric requirements; usage not reported. More marrow vs peripheral blood in G-tube group. Some loss of data. | Level II, Quality B |
| Lewandowski et al., 2019 [39] | Retrospective cohort study | Children 0–19 admitted for BMT in a Brazilian hospital
| Review of nutritional status, support used, and complications | No variation in LOS among nutritional diagnoses. Higher rate of PN than EN in all patients. A total of 89% of patients had at least one infection, 21% had GVHD. | Nutritional support means, LOS, time to engraftment, incidence of GVHD, GI symptoms, infection rate, caloric intake, weight gain/loss. Measured at admission, day 0, +14, +21, and +28 | Not originally published in English, possible translation errors. BMT-related outcomes were not delineated by type of nutritional support received. No control. | Level III, Quality C |
| Mellgren et al., 2023 [43] | Retrospective cohort study | Children <18 yrs. admitted for allogeneic BMT at three Swedish hospitals
| Prophylactically placed G-tube vs NG-tube, Included some PN | NG-tube group lost more weight. G-tube group had earlier neutrophil engraftment but more fevers/infections. No differences in aGVHD, OS, non-relapse mortality, relapse. | Nutritional support means and time usage, non-relapse mortality, relapse, incidence of GVHD, infection, time to engraftment, height, weight. Measured at time of BMT, +90, +180, and +365 | Multi-center with 1 center with universal G-tube placement prophylactically. A total of 43 patients deceased or lost to follow-up. PN use not equal between groups. | Level III, Quality B |
| Soussi et al., 2019 [36] | Retrospective cohort study | Children 2–17 undergoing allogeneic BMT at a hospital in Tunisia, receiving PN
| Review of PN use and complications | Total of 136 observed complications. Infections were the most common complications (32%), then electrolyte disorders (28%). Risk correlated with duration of PN exposure. GVHD in 29%. | Incidence of infections, electrolytes/metabolic imbalances, hepatobiliary complications, mucositis, GVHD, death. Measured from date of PN initiation to completion | Overestimation of PN complications due to difficulty to confirm association of PN use with cause. Reports difficulty with collecting biological parameters reliably. No control. | Level III, Quality C |
| Zama et al., 2020 [40] | Retrospective and prospective cohort study | Children 2–20 undergoing myeloablative or non-myeloablative conditioning for allogeneic BMT admitted to an Italian hospital
| Comparison between group receiving EN at least 7 days vs. primarily PN | The rate of bloodstream infections was higher in PN group. Trend towards lower rate of severe and steroid-resistant aGVHD in EN group. Longer time to platelet recovery in EN group. No difference in neutrophil engraftment, days of GCSF, LOS, mucositis, aGVHD. | Nutritional status, weight loss, and clinical outcomes including neutrophil and platelet engraftment, mucositis, aGVHD, VOD, and blood stream infection occurrences. Measured on admission, weekly until Day +35, discharge, +60, and +90 | Mix of patients who had only PN and those who received PN with EN for up to six days in one group, and those who received PN with EN for more than seven days or EN only in 2nd group. Small sample size. | Level III, Quality B |
| Zemrani et al., 2019 [13] | Retrospective cohort study | Children <20 with primary immunodeficiencies (PID) undergoing reduced-intensity or myeloablative conditioning allogeneic BMT at an Australian hospital
| Review of nutritional status, support used, and complications | A total of 33% of patients met criteria for malnutrition prior to BMT. EN was initially used for most. A total of 77% required PN. Exclusive EN was associated with shorter LOS. PN associated with hyperglycemia and hyper-triglyceridemia. | Nutritional support means, weight, height, albumin/electrolytes, neutrophil and platelet engraftment date, mucositis, acute and chronic GVHD, infection rate, VOD, LOS, OS. Measured at start, Day +30, +90, +180, and +365 | BMT-related outcome measures were not delineated by nutritional support received or prep intensity. No control/comparison group. Results are not generalizable to non-PID BMT patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, J.D.; Symons, H.J.; Cooke, K.R. Nutritional Support Best Practices in Pediatric Blood and Marrow Transplant Patients: An Integrative Review. Children 2024, 11, 637. https://doi.org/10.3390/children11060637
Murphy JD, Symons HJ, Cooke KR. Nutritional Support Best Practices in Pediatric Blood and Marrow Transplant Patients: An Integrative Review. Children. 2024; 11(6):637. https://doi.org/10.3390/children11060637
Chicago/Turabian StyleMurphy, Jessica D., Heather J. Symons, and Kenneth R. Cooke. 2024. "Nutritional Support Best Practices in Pediatric Blood and Marrow Transplant Patients: An Integrative Review" Children 11, no. 6: 637. https://doi.org/10.3390/children11060637
APA StyleMurphy, J. D., Symons, H. J., & Cooke, K. R. (2024). Nutritional Support Best Practices in Pediatric Blood and Marrow Transplant Patients: An Integrative Review. Children, 11(6), 637. https://doi.org/10.3390/children11060637






