Kidney Measurement and Glomerular Filtration Rate Evolution in Children with Polycystic Kidney Disease
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Outcomes and Definitions
2.3. Variables of Interest
2.4. Statistical Analysis
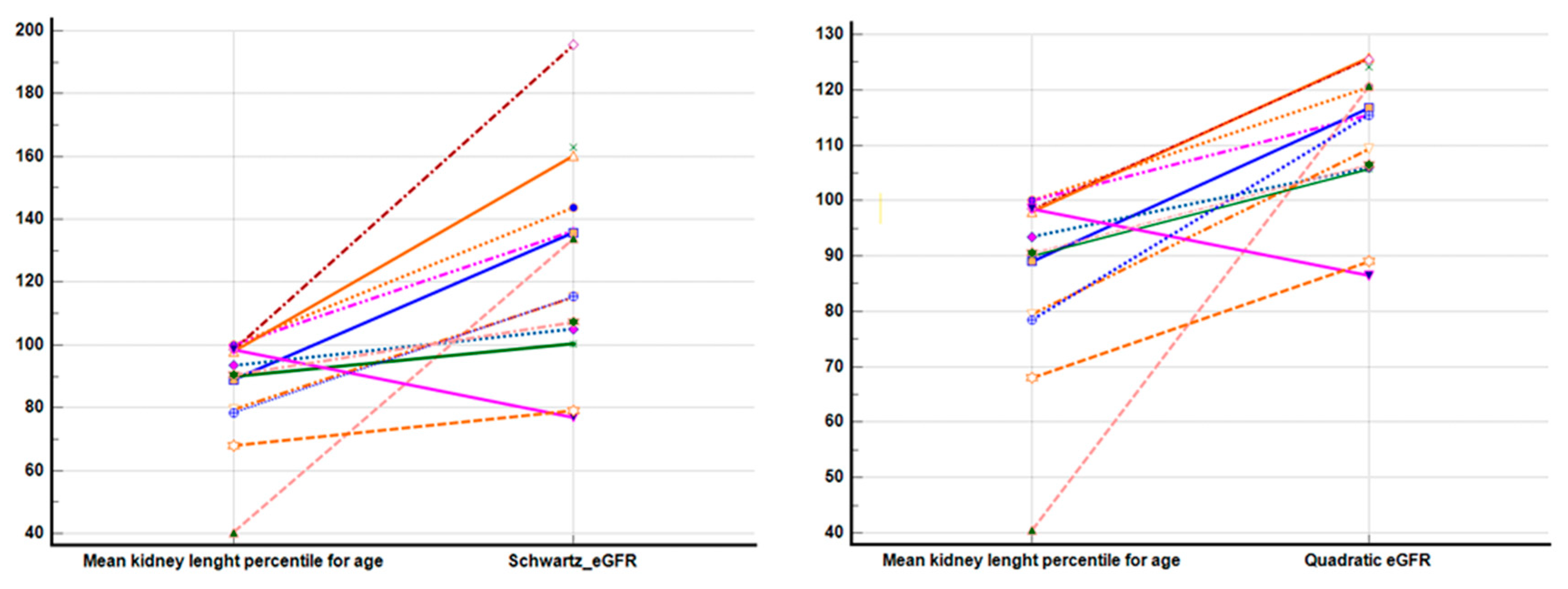
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalgaard, O.Z. Bilateral polycystic disease of the kidneys; a follow-up of two hundred and eighty-four patients and their families. Acta Med. Scand. Suppl. 1957, 328, 1–255. [Google Scholar]
- Willey, C.J.; Blais, J.D.; Hall, A.K.; Krasa, H.B.; Makin, A.J.; Czerwiec, F.S. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol. Dial. Transplant. 2017, 32, 1356–1363. [Google Scholar] [CrossRef]
- Lanktree, M.B.; Haghighi, A.; Guiard, E.; Iliuta, I.-A.; Song, X.; Harris, P.C.; Paterson, A.D.; Pei, Y. Prevalence estimates of polycystic kidney and liver disease by population sequencing. J. Am. Soc. Nephrol. 2018, 29, 2593–2600. [Google Scholar] [CrossRef]
- Solazzo, A.; Testa, F.; Giovanella, S.; Busutti, M.; Furci, L.; Carrera, P.; Ferrari, M.; Ligabue, G.; Mori, G.; Leonelli, M.; et al. The prevalence of autosomal dominant polycystic kidney disease (ADPKD): A meta-analysis of European literature and prevalence evaluation in the Italian province of Modena suggest that ADPKD is a rare and underdiagnosed condition. PLoS ONE 2018, 13, e0190430. [Google Scholar] [CrossRef]
- Pretorius, D.H.; Lee, M.E.; Manco-Johnson, M.L.; Weingast, G.R.; Sedman, A.B.; Gabow, P.A. Diagnosis of autosomal dominant polycystic kidney disease in utero and in the young infant. J. Ultrasound Med. 1987, 6, 249–255. [Google Scholar] [CrossRef][Green Version]
- MacDermot, K.D.; Saggar-Malik, A.K.; Economides, D.L.; Jeffery, S. Prenatal diagnosis of autosomal dominant polycystic kidney disease (PKD1) presenting in utero and prognosis for very early onset disease. J. Med. Genet. 1998, 35, 13–16. [Google Scholar] [CrossRef][Green Version]
- Helal, I.; Reed, B.; McFann, K.; Yan, X.-D.; Fick-Brosnahan, G.M.; Cadnapaphornchai, M.; Schrier, R.W. Glomerular hyperfiltration and renal progression in children with autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2439–2443. [Google Scholar] [CrossRef]
- Wong, H.; Vivian, L.; Weiler, G.; Filler, G. Patients with autosomal dominant polycystic kidney disease hyperfiltrate early in their disease. Am. J. Kidney Dis. 2004, 43, 624–628. [Google Scholar] [CrossRef]
- Chapman, A.B.; Johnson, A.M.; Gabow, P.A.; Schrier, R.W. Overt proteinuria and microalbuminuria in autosomal-dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1994, 5, 1349–1354. [Google Scholar] [CrossRef]
- Kubo, S.; Nakajima, M.; Fukuda, K.; Nobayashi, M.; Sakaki, T.; Aoki, K.; Hirao, Y.; Yoshioka, A. A 4-year-old girl with autosomal dominant polycystic kidney disease complicated by a ruptured intracranial aneurysm. Eur. J. Pediatr. 2004, 163, 675–677. [Google Scholar] [CrossRef]
- Nishiura, J.L.; Neves, R.F.; Eloi, S.R.; Cintra, S.M.; Ajzen, S.A.; Heilberg, I.P. Evaluation of nephrolithiasis in autosomal dominant polycystic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, B.; Alzubaidi, M.; Velez, J.C.Q. Evaluation and Management of Gross Hematuria in Autosomal Dominant Polycystic Kidney Disease: A Point of Care Guide for Practicing Internists. Am. J. Med. Sci. 2018, 356, 177–180. [Google Scholar] [CrossRef]
- Idrizi, A.; Barbullushi, M.; Koroshi, A.; Dibra, M.; Bolleku, E.; Bajrami, V.; Xhaferri, X.; Thereska, N. Urinary tract infections in polycystic kidney disease. Med. Arch. 2011, 65, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Higashihara, E.; Horie, S.; Muto, S.; Mochizuki, T.; Nishio, S.; Nutahara, K. Renal disease progression in autosomal dominant polycystic kidney disease. Clin. Exp. Nephrol. 2012, 16, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.J.; Chapman, A.B.; Torres, V.E. Volume progression in autosomal dominant polycystic kidney disease: The major factor determining clinical outcomes. Clin. J. Am. Soc. Nephrol. 2006, 1, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Meijer, E.; Rook, M.; Tent, H.; Navis, G.; van der Jagt, E.J.; de Jong, P.E.; Gansevoort, R.T. Early renal abnormalities in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.; Winyard, P.; Marlais, M.; Cuthell, O.; Harris, T.; Chong, J.; Sayer, J.; Gale, D.P.; Moore, L.; Turner, K.; et al. Clinical practice guideline monitoring children and young people with, or at risk of developing autosomal dominant polycystic kidney disease (ADPKD). BMC Nephrol. 2019, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.R.; Moore, B.S.; Luo, J.Z.; Sartori, G.; Fang, B.; Jacobs, S.; Abdalla, Y.; Taher, M.; Carey, D.J.; Triffo, W.J.; et al. Exome Sequencing of a Clinical Population for Autosomal Dominant Polycystic Kidney Disease. JAMA 2022, 328, 2412–2421. [Google Scholar] [CrossRef] [PubMed]
- Cadnapaphornchai, M.A. Autosomal dominant polycystic kidney disease in children. Curr. Opin. Pediatr. 2015, 27, 193–200. [Google Scholar] [CrossRef]
- Barua, M.; Cil, O.; Paterson, A.D.; Wang, K.; He, N.; Dicks, E.; Parfrey, P.; Pei, Y. Family history of renal disease severity predicts the mutated gene in ADPKD. J. Am. Soc. Nephrol. 2009, 20, 1833–1838. [Google Scholar] [CrossRef]
- Audrézet, M.P.; Cornec-Le Gall, E.; Chen, J.M.; Redon, S.; Quéré, I.; Creff, J.; Bénech, C.; Maestri, S.; Le Meur, Y.; Férec, C. Autosomal dominant polycystic kidney disease: Comprehensive mutation analysis of PKD1 and PKD2 in 700 unrelated patients. Hum. Mutat. 2012, 33, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.M.; Wuyts, B.; Stove, V.; Walle, J.V.; Delanghe, J.R. Compensating for the influence of total serum protein in the Schwartz formula. Clin. Chem. Lab. Med. 2012, 50, 1597–1600. [Google Scholar] [CrossRef]
- Gao, A.; Cachat, F.; Faouzi, M.; Bardy, D.; Mosig, D.; Meyrat, B.J.; Girardin, E.; Chehade, H. Comparison of the glomerular filtration rate in children by the new revised Schwartz formula and a new generalized formula. Kidney Int. 2013, 83, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef]
- Brion, L.P.; Fleischman, A.R.; McCarton, C.; Schwartz, G.J. A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: Noninvasive assessment of body composition and growth. J. Pediatr. 1986, 109, 699. [Google Scholar] [CrossRef]
- Piepsz, A.; Tondeur, M.; Ham, H. Revisiting normal 51Crethylenediaminetetraacetic acid clearance values in children. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Cachat, F.; Combescure, C.; Cauderay, M.; Girardin, E.; Chehade, H. A systematic review of glomerular hyperfltration assessment and defnition in the medical literature. Clin. J. Am. Soc. Nephrol. 2015, 10, 382–389. [Google Scholar] [CrossRef]
- Blake, G.M.; Gardiner, N.; Gnanasegaran, G.; Sabina, D. Reference ranges for 51Cr-EDTA measurements of glomerular fltration rate in children. Nucl. Med. Commun. 2005, 26, 983–987. [Google Scholar] [CrossRef]
- Obrycki, Ł.; Sarnecki, J.; Lichosik, M.; Sopińska, M.; Placzyńska, M.; Stańczyk, M.; Mirecka, J.; Wasilewska, A.; Michalski, M.; Lewandowska, W.; et al. Kidney length normative values—New percentiles by age and body surface area in Central European children and adolescents. Pediatr. Nephrol. 2023, 38, 1187–1193. [Google Scholar] [CrossRef]
- Obrycki, Ł.; Sarnecki, J.; Lichosik, M.; Sopińska, M.; Placzyńska, M.; Stańczyk, M.; Mirecka, J.; Wasilewska, A.; Michalski, M.; Lewandowska, W.; et al. Kidney length normative values in children aged 0–19 years—A multicenter study. Pediatr. Nephrol. 2022, 37, 1075–1085. [Google Scholar] [CrossRef]
- Kasap Demir, B.; Mutlubaş, F.; Soyaltın, E.; Alparslan, C.; Arya, M.; Alaygut, D.; Arslansoyu Çamlar, S.; Berdeli, A.; Yavaşcan, Ö. Demographic and clinical characteristics of children with autosomal dominant polycystic kidney disease: A single center experience. Turk. J. Med. Sci. 2021, 51, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Fencl, F.; Janda, J.; Bláhová, K.; Hríbal, Z.; Stekrová, J.; Puchmajerová, A.; Seeman, T. Genotype-phenotype correlation in children with autosomal dominant polycystic kidney disease. Pediatr. Nephrol. 2009, 24, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Gabow, P.A.; Kimberling, W.J.; Strain, J.D.; Manco-Johnson, M.L.; Johnson, A.M. Utility of ultrasonography in the diagnosis of autosomal dominant polycystic kidney disease in children. J. Am. Soc. Nephrol. 1997, 8, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Cochat, P.; Rognant, N.; Ranchin, B.; Hadj-Aissa, A.; Dubourg, L. Which creatinine and cystatin C equations can be reliably used in children? Clin. J. Am. Soc. Nephrol. 2011, 6, 552–560. [Google Scholar] [CrossRef]
- Pottel, H.; Mottaghy, F.M.; Zaman, Z.; Martens, F. On the relationship between glomerular filtration rate and serum creatinine in children. Pediatr. Nephrol. 2010, 25, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Pottel, H.; Adebayo, O.C.; Nkoy, A.B.; Delanaye, P. Glomerular hyperfiltration: Part 1—Defining the threshold—Is the sky the limit? Pediatr. Nephrol. 2023, 38, 2523–2527. [Google Scholar] [CrossRef]
- Chen, E.W.; Chong, J.; Valluru, M.K.; Durkie, M.; Simms, R.J.; Harris, P.C.; Ong, A.C. Combining genotype with height-adjusted kidney length predicts rapid progression of ADPKD. Nephrol. Dial. Transplant. 2024, gfad270. [Google Scholar] [CrossRef]
| Age (years) | Glomerular Hyperfiltration Thresholds |
|---|---|
| ≤0.10 | 69.6 |
| 0.10–0.30 | 89.7 |
| 0.30–0.66 | 98.9 |
| 0.66–1.00 | 116.5 |
| 1.00–1.50 | 126.4 |
| 1.50–2.00 | 130 |
| >2.00 | 135 |
| Parameter | PKD1 N = 6 | PKD2 N = 6 | NA N = 4 | Total N = 16 | p Value |
|---|---|---|---|---|---|
| Sex—female | 4 (66.7%) | 5 (83.3%) | 3 (75%) | 12 (75%) | 0.711 1 |
| Age years A + SD | 5.59 (4.71) | 13 (5.13) | 8.5 (5.32) | 9.09 (5.74) | 0.07 2 |
| Intrauterine renal cysts | 2 (33.3%) | - | - | 2 (12.5%) | 0.808 1 |
| Age at diagnosis A + SD | 5.59 (4.71) | 13 (5.13) | 8.5 (5.32) | 9.09 (5.74) | 0.07 2 |
| Family history | 4 (66.7%) | 4 (66.7%) | 1 (25%) | 9 (56.2%) | 0.226 1 |
| Patients with cysts on both kidneys | 4 (66.7%) | 3 (50%) | 2 (50%) | 9 (56.2%) | 0.803 1 |
| Maximal cyst diameter cm M + IQR | 0.65 (0.3–0.8) | 1.3 (0.4–2) | 1.1 (0.8–1.6) | 0.75 (0.5–1.6) | 0.306 3 |
| Parenchymal index cm, M + IQR | 0.7 (0.45–0.72) | 0.725 (0.7–0.8) | 0.9 (0.87–0.95) | 0.75 (0.7–0.83) | 0.01 3 |
| Average kidneys length cm M + IQR | 9.57 (6.88–10.25) | 11.27 (10.95–11.62) | 10.17 (9.77–11.18) | 10.27 (9.2–11.27) | 0.14 3 |
| Percentiles per age M + IQR | 88.75 (57–100) | 90 (75.87–92.5) | 96 (91.25–99.25) | 90.5 (78.75–98.5) | 0.503 3 |
| Percentiles per height M + IQR | 86.75 (44.5–100) | 91 (83–98.87) | 97 (93–100) | 94 (80–100) | 0.558 3 |
| Percentiles per BSA M + IQR | 77.5 (49–100) | 96.5 (81.87–98.5) | 96.5 (77.5–98.75) | 96.5 (61.62–99.87) | 0.892 3 |
| Parameter | PKD1 N = 6 | PKD2 N = 6 | NA N = 4 | Total N = 16 | p Value |
|---|---|---|---|---|---|
| Serum creatinine at diagnostic Jaffe unadjusted μmol/L A + SD | 30.5 (11.82) | 46.66 (18.44) | 44.5 (21.04) | 40.06 (17.54) | 0.249 1 |
| Serum creatinine at diagnostic Jaffe adjusted to proteins μmol/L A + SD | 38.54 (9.37) | 46.27 (18.44) | 44.11 (21.04) | 42.83 (15.61) | 0.71 1 |
| Serum creatinine at diagnostic enzymatic equivalent μmol/L A + SD | 39.67 (9.61) | 47.6 (18.9) | 45.38 (21.56) | 44.07 (16) | 0.71 1 |
| eGFR mL/min/1.73 sm A + SD (N = 14—patients older than 2 years) (Quadratic) | 117.81 (7.07) | 111.06 (13.68) | 107.42 (15.24) | 111.95 (12.43) | 0.521 1 |
| eGFR mL/min/1.73 sm A + SD (N = 14—patients older than 2 years) (Schwartz) | 136.42 (18.48) | 126.76 (43.59) | 115.42 (30.59) | 126.28 (33.07) | 0.702 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stroescu, R.; Gafencu, M.; Steflea, R.M.; Chisavu, F. Kidney Measurement and Glomerular Filtration Rate Evolution in Children with Polycystic Kidney Disease. Children 2024, 11, 575. https://doi.org/10.3390/children11050575
Stroescu R, Gafencu M, Steflea RM, Chisavu F. Kidney Measurement and Glomerular Filtration Rate Evolution in Children with Polycystic Kidney Disease. Children. 2024; 11(5):575. https://doi.org/10.3390/children11050575
Chicago/Turabian StyleStroescu, Ramona, Mihai Gafencu, Ruxandra Maria Steflea, and Flavia Chisavu. 2024. "Kidney Measurement and Glomerular Filtration Rate Evolution in Children with Polycystic Kidney Disease" Children 11, no. 5: 575. https://doi.org/10.3390/children11050575
APA StyleStroescu, R., Gafencu, M., Steflea, R. M., & Chisavu, F. (2024). Kidney Measurement and Glomerular Filtration Rate Evolution in Children with Polycystic Kidney Disease. Children, 11(5), 575. https://doi.org/10.3390/children11050575









