Progressive Quadriparesis of a Toddler with a Posterior Cranial Fossa Arachnoid Cyst (AC): Illustrative Case Report and Narrative Literature Review
Abstract
1. Introduction and Clinical Significance
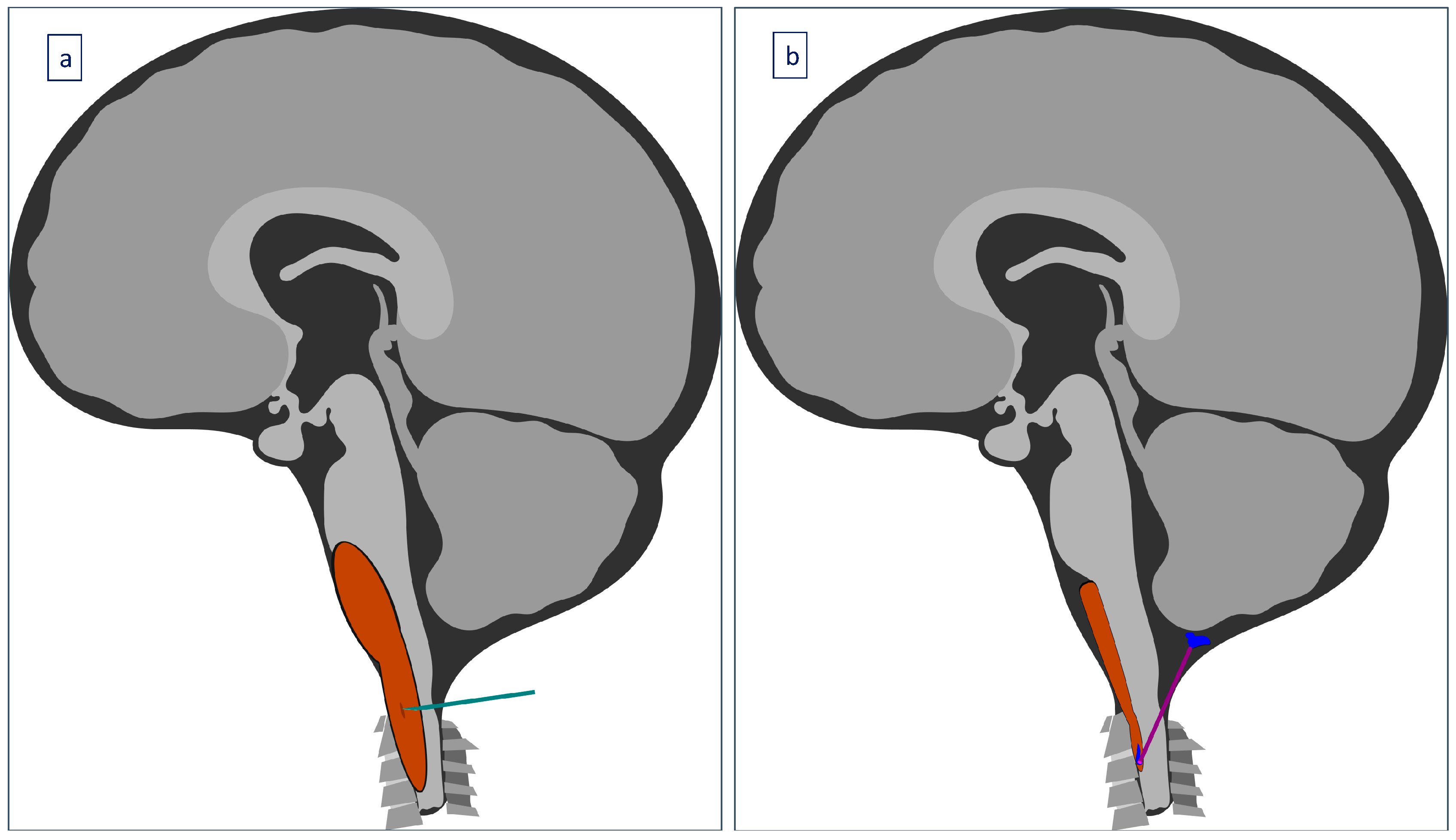
2. Case Presentation
3. Results
4. Discussion
4.1. Epidemiology
4.2. Etiology
4.3. Pathophysiology
4.4. Clinical Presentation
4.5. Assessment and Diagnosis
4.6. Management
4.7. Complications
4.8. Outcome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Öcal, E. Understanding intracranial arachnoid cysts: A review of etiology, pathogenesis, and epidemiology. Childs. Nerv. Syst. 2023, 39, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Dwarakanath, S.; Suri, A.; Mahapatra, A.K.; Mehta, V.S.; Gaikwad, S.; Sarkar, C. Endoscopic assisted excision of a retroclival arachnoid cyst presenting as hysterical breathlessness. Childs. Nerv. Syst. 2006, 22, 424–427. [Google Scholar] [CrossRef]
- Zada, G.; Krieger, M.D.; McNatt, S.A.; Bowen, I.; McComb, J.G. Pathogenesis and treatment of intracranial arachnoid cysts in pediatric patients younger than 2 years of age. Neurosurg. Focus 2007, 22, 1–5. [Google Scholar] [CrossRef]
- Kwiatkowska, K.; Dębicka, M.; Maryniak, A.; Kwiatkowski, S. Cognitive Impairment in Children with Arachnoid Cyst of Sylvian Fissure: Does it Justify the Neurosurgical Treatment? J. Neurol. Surg. A Cent. Eur. Neurosurg. 2020, 81, 362–367. [Google Scholar] [CrossRef]
- Erdeve, S.S.; Ocal, G.; Berberoglu, M.; Siklar, Z.; Hacihamdioglu, B.; Evliyaoglu, O.; Fitoz, S. The endocrine spectrum of intracranial cysts in childhood and review of the literature. J. Pediatr. Endocrinol. Metab. 2011, 24, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Eidlitz-Markus, T.; Zeharia, A.; Haimi Cohen, Y.; Konen, O. Characteristics and Management of Arachnoid Cyst in the Pediatric Headache Clinic Setting. Headache 2014, 54, 1583–1590. [Google Scholar] [CrossRef]
- Muhlestein, W.E.; Maher, C.O. Incidental Intracranial Cysts in Children. Pediatr. Clin. N. Am. 2021, 68, 775–782. [Google Scholar] [CrossRef]
- Akutagawa, K.; Tamura, G.; Tsurubuchi, T.; Ishikawa, E.; Matsumura, A.; Inagaki, T. Quadrigeminal arachnoid cyst with perinatal encephalocele. Childs. Nerv. Syst. 2020, 36, 1393–1397. [Google Scholar] [CrossRef]
- Ahmed, A.K.; Cohen, A.R. Intracranial arachnoid cysts. Childs. Nerv. Syst. 2023, 39, 2771–2778. [Google Scholar] [CrossRef] [PubMed]
- Beltagy, M.A.E.; Enayet, A.E.R. Surgical indications in pediatric arachnoid cysts. Childs. Nerv. Syst. 2023, 39, 87–92. [Google Scholar] [CrossRef]
- Olaya, J.E.; Ghostine, M.; Rowe, M.; Zouros, A. Endoscopic fenestration of a cerebellopontine angle arachnoid cyst resulting in complete recovery from sensorineural hearing loss and facial nerve palsy. J. Neurosurg. Pediatr. 2011, 7, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, J.; Frantzias, J.; Lavrador, J.P.; Bodi, I.; Zebian, B. Posterior fossa arachnoid cyst causing torticollis and gastro-oesophageal reflux in an infant. Childs. Nerv. Syst. 2018, 34, 2519–2523. [Google Scholar] [CrossRef]
- Cuny, M.L.; Pallone, M.; Piana, H.; Boddaert, N.; Sainte-Rose, C.; Vaivre-Douret, L.; Piolino, P.; Puget, S. Neuropsychological improvement after posterior fossa arachnoid cyst drainage. Childs. Nerv. Syst. 2017, 33, 135–141. [Google Scholar] [CrossRef]
- Talreja, R.; Fonseca, L.D.; Chikkannaiah, M.; Kumar, G. Intracranial Arachnoid Cyst in Children: Clinical Presentation and Risk Factors for Surgical Intervention. Pediatr. Neurosurg. 2024, 59, 55–65. [Google Scholar] [CrossRef]
- Peraud, A.; Schuler-Ortoli, M.; Schaal, M.; Reister, F.; Ehrhardt, H.; Friebe-Hoffmann, U. Staged neurosurgical approach for giant and progressive neonatal arachnoid cysts: A case series and review of the literature. Childs. Nerv. Syst. 2024, 40, 1997–2007. [Google Scholar] [CrossRef]
- Marin-Sanabria, E.A.; Yamamoto, H.; Nagashima, T.; Kohmura, E. Evaluation of the management of arachnoid cyst of the posterior fossa in pediatric population: Experience over 27 years. Childs. Nerv. Syst. 2007, 23, 535–542. [Google Scholar] [CrossRef]
- Chan, J.; Huang, C.; Liu, Y.; Lin, C.; Huang, J. Chronic Subdural Hematoma Associated with Arachnoid Cyst in Young Adults: A Case Report. Kaohsiung J. Med. Scie. 2008, 24, 41–44. [Google Scholar] [CrossRef]
- Prasad, S.; Avery, R.A.; De Alba Campomanes, A.; Sutton, L.N.; Liu, G.T. Symptomatic Increased Intracranial Pressure Due to Arachnoid Cysts. Pediatr. Neurol. 2011, 44, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Yahal, O.; Katorza, E.; Zvi, E.; Berkenstadt, M.; Hoffman, C.; Achiron, R.; Bar-Yosef, O. Prenatal diagnosis of arachnoid cysts: MRI features and neurodevelopmental outcome. Eur. J. Radiol. 2019, 113, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Bryden, A.; Majors, N.; Puri, V.; Moriarty, T. A Rare Case of Spontaneous Arachnoid Cyst Rupture Presenting as Right Hemiplegia and Expressive Aphasia in a Pediatric Patient. Children 2021, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Eom, S.; Shim, K.-W.; Kim, D.-S. Neurocognitive and psychological profiles in pediatric arachnoid cyst. Childs. Nerv. Syst. 2009, 25, 1071–1076. [Google Scholar] [CrossRef]
- Al-Holou, W.N.; Yew, A.Y.; Boomsaad, Z.E.; Garton, H.J.L.; Muraszko, K.M.; Maher, C.O. Prevalence and natural history of arachnoid cysts in children: Clinical article. J. Neurosurg. Pediatr. 2010, 5, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, J.W.; Phi, J.H.; Kim, S.-K.; Cho, B.-K.; Wang, K.-C. Enlarging arachnoid cyst: A false alarm for infants. Childs. Nerv. Syst. 2012, 28, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Smedley, A.; Sparrow, O.; Mathad, N.; Waters, R.; Chakraborty, A.; Tsitouras, V. Natural History of Intracranial Arachnoid Cysts. World Neurosurg. 2019, 126, e1315–e1320. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, J.Y.; Phi, J.H.; Cho, B.-K.; Shin, M.-S.; Kim, S.-K. Neurocognitive profile in children with arachnoid cysts before and after surgical intervention. Childs. Nerv. Syst. 2019, 35, 517–522. [Google Scholar] [CrossRef]
- Sandvik, U.; Adolfsson, T.; Jacobson, D.N.; Tedroff, K. Cognition in Children with Arachnoid Cysts. J. Clin. Med. 2020, 9, 850. [Google Scholar] [CrossRef]
- El Damaty, A.; Issa, M.; Paggetti, F.; Seitz, A.; Unterberg, A. Intracranial arachnoid cysts: What is the appropriate surgical technique? A retrospective comparative study with 61 pediatric patients. World Neurosurg. X 2023, 19, 100195. [Google Scholar] [CrossRef]
- White, M.L.; Das, J.M. Arachnoid Cysts; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Karaaslan, B.; Doğan, E.; Börcek, A.Ö. Management of Neonatal Facial Paralysis due to Cerebellopontine Angle Arachnoid Cyst: A Case Report. Pediatr. Neurosurg. 2019, 54, 253–257. [Google Scholar] [CrossRef] [PubMed]
- El-Ghandour, N.M.F. Endoscopic treatment of intracranial cysts in infants: Personal experience and review of literature. Childs. Nerv. Syst. 2021, 37, 3447–3453. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Yamamoto, T.; Horinaka, N.; Maeda, M. Arachnoid Cyst Is a Risk Factor for Chronic Subdural Hematoma in Juveniles: Twelve Cases of Chronic Subdural Hematoma Associated with Arachnoid Cyst. J. Neurotrauma 2002, 19, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Cinalli, G.; Spennato, P.; Columbano, L.; Ruggiero, C.; Aliberti, F.; Trischitta, V.; Buonocore, M.C.; Cianciulli, E. Neuroendoscopic treatment of arachnoid cysts of the quadrigeminal cistern: A series of 14 cases: Clinical article. J. Neurosurg. Pediatr. 2010, 6, 489–497. [Google Scholar] [CrossRef]
- Olsson, S.E.; Ulualp, S.O.; Kou, Y.-F. Tinnitus Triggered by a Cerebellopontine Arachnoid Cyst in an Adolescent. Am. J. Case Rep. 2022, 23, e938294. [Google Scholar] [CrossRef] [PubMed]
- Di Perna, G.; Piatelli, G.; Rossi, A.; Consales, A.; Fiaschi, P.; Castaldo, M.; Pavanello, M. Coexisting Retrocerebellar Arachnoid Cyst and Chiari Type 1 Malformation: 3 Pediatric Cases of Surgical Management Tailored to the Pathogenic Mechanism and Systematic Review of the Literature. World Neurosurg. 2021, 148, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Jafrani, R.; Raskin, J.; Kaufman, A.; Lam, S. Intracranial arachnoid cysts: Pediatric neurosurgery update. Surg. Neurol. Int. 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Gosalakkal, J.A. Intracranial arachnoid cysts in children: A review of pathogenesis, clinical features, and management. Pediatr. Neurol. 2002, 26, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulos, D.; Roth, J.; Beni-Adani, L.; Constantini, S. Interpeduncular arachnoid cysts in infants and children: Insight into the entity based on a case series with long-term follow-up. Childs. Nerv. Syst. 2011, 27, 429–438. [Google Scholar] [CrossRef]
- De Keersmaecker, B.; Ramaekers, P.; Claus, F.; Witters, I.; Ortibus, E.; Naulaers, G.; Van Calenbergh, F.; De Catte, L. Outcome of 12 antenatally diagnosed fetal arachnoid cysts: Case series and review of the literature. Eur. J. Paediatr. Neurol. 2015, 19, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-J.; Glushakova, O.; Mondello, S.; Van, K.; Hayes, R.L.; Lyeth, B.G. Acute Temporal Profiles of Serum Levels of UCH-L1 and GFAP and Relationships to Neuronal and Astroglial Pathology following Traumatic Brain Injury in Rats. J. Neurotrauma 2015, 32, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.K.; Oh, T.; Han, K.J.; Chang, D.; Sun, P.P. A Case of Torticollis in an 8-Month-Old Infant Caused by Posterior Fossa Arachnoid Cyst: An Important Entity for Differential Diagnosis. Pediatr. Rep. 2021, 13, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Sengul, G.; Tuzun, Y.; Cakir, M.; Duman, S.; Colak, A.; Kadioglu, H.H.; Aydin, I.H. Neuroendoscopic Approach to Quadrigeminal Cistern Arachnoid Cysts. Eurasian J. Med. 2012, 44, 18–21. [Google Scholar] [CrossRef]
- Grossman, T.B.; Uribe-Cardenas, R.; Radwanski, R.E.; Souweidane, M.M.; Hoffman, C.E. Arachnoid cysts: Using prenatal imaging and need for pediatric neurosurgical intervention to better understand their natural history and prognosis. J. Matern. Fetal Neonatal Med. 2022, 35, 4728–4733. [Google Scholar] [CrossRef] [PubMed]
- Melikian, G.; Arutiunov, N.V.; Melnikov, A.V. Unusual Intraventricular Herniation of the Suprasellar Arachnoid Cyst and its Successful Endoscopic Management. Minim. Invasive Neurosurg. 2003, 46, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Kundishora, K. Epigenomic dysregulation correlates with arachnoid cyst formation and neurodevelopmental symptoms. Nat. Med. 2023, 29, 541–542. [Google Scholar] [CrossRef] [PubMed]
- Kirmizigoz, S.; Dogan, A.; Kayhan, S.; Sarialtin, S.Y.; Tehli, O. Comparison of Surgical Techniques for Intracranial Arachnoid Cysts: A Volumetric Analysis. Turk. Neurosurg. 2023, 33, 1038–1046. [Google Scholar] [CrossRef]
- Bond, A.E.; Zada, G.; Bowen, I.; McComb, J.G.; Krieger, M.D. Spinal arachnoid cysts in the pediatric population: Report of 31 cases and a review of the literature. J. Neurosurg. Pediatr. 2012, 9, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Anderson, R.C.E.; Feldstein, N.A.; Brockmeyer, D.L. Expansion of arachnoid cysts in children: Report of two cases and review of the literature. J. Neurosurg. Pediatr. 2005, 102, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, D.I.; McComb, J.G.; Krieger, M.D. Chemical analysis of fluid obtained from intracranial arachnoid cysts in pediatric patients. J. Neurosurg. Pediatr. 2005, 103, 427–432. [Google Scholar] [CrossRef]
- Shim, K.-W.; Lee, Y.-H.; Park, E.-K.; Park, Y.-S.; Choi, J.-U.; Kim, D.-S. Treatment option for arachnoid cysts. Childs. Nerv. Syst. 2009, 25, 1459–1466. [Google Scholar] [CrossRef]
- Almousa, A.S.; Alotaibi, S.N.; Al Wadany, M.M.; Al Wadany, F.M.; Alharbi, A.S. Spontaneous Rupture of Arachnoid Cyst in a Child: A Rare Case Report. Cureus 2023, 15, e33652. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Mei, W.-Z.; Chen, Y.; Chen, J.-W.; Lin, Z.-X. Analysis on clinical characteristics of intracranial Arachnoid Cysts in 488 pediatric cases. Int. J. Clin. Exp. Med. 2015, 8, 18343–18350. [Google Scholar] [PubMed]
- Rechtman, E.; Puget, S.; Saitovitch, A.; Lemaitre, H.; Fillion, L.; Tacchella, J.M.; Boisgontier, J.; Cuny, M.L.; Boddaert, N.; Zilbovicius, M. Posterior Fossa Arachnoid Cyst in a Pediatric Population is Associated with Social Perception and Rest Cerebral Blood Flow Abnormalities. Cerebellum 2020, 19, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; You, C.; Han, G.; Liu, C.; Wang, C.; Wang, J. Individual surgical treatment of intracranial arachnoid cyst in pediatric patients. Neurol. India 2013, 61, 400. [Google Scholar] [CrossRef]
- Helland, C.A.; Wester, K. A population-based study of intracranial arachnoid cysts: Clinical and neuroimaging outcomes following surgical cyst decompression in children. J. Neurosurg. Pediatr. 2006, 105, 385–390. [Google Scholar] [CrossRef]
- Rabiei, K.; Högfeldt, M.J.; Doria-Medina, R.; Tisell, M. Surgery for intracranial arachnoid cysts in children—A prospective long-term study. Childs. Nerv. Syst. 2016, 32, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Li, Y.; Zhu, F.; Zang, D.; Zhao, C.; Li, C.; Tong, D.; Zhang, H.; Chen, Q. Children with Intracranial Arachnoid Cysts: Classification and Treatment. Medicine 2015, 94, e1749. [Google Scholar] [CrossRef]
- Yadav, Y.; Parihar, V.; Sinha, M.; Jain, N. Endoscopic treatment of the suprasellar arachnoid cyst. Neurol. India. 2010, 58, 280. [Google Scholar] [CrossRef]
- Thomas, B.P.; Pearson, M.M.; Wushensky, C.A. Active spontaneous decompression of a suprasellar-prepontine arachnoid cyst detected with routine magnetic resonance imaging: Case report. J. Neurosurg. Pediatr. 2009, 3, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Oberbauer, R.W.; Haase, J.; Pucher, R. Arachnoid cysts in children: A European co-operative study. Childs. Nerv. Syst. 1992, 8, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lee, J.Y.; Phi, J.H.; Kim, S.-K.; Wang, K.-C. Stricter indications are recommended for fenestration surgery in intracranial arachnoid cysts of children. Childs. Nerv. Syst. 2015, 31, 77–86. [Google Scholar] [CrossRef]
- De Simone, M.; Fontanella, M.M.; Choucha, A.; Schaller, K.; Machi, P.; Lanzino, G.; Bijlenga, P.; Kurz, F.T.; Lövblad, K.O.; De Maria, L. Current and Future Applications of Arterial Spin Labeling MRI in Cerebral Arteriovenous Malformations. Biomedicines 2024, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- De Simone, M.; Iaconetta, G.; Palermo, G.; Fiorindi, A.; Schaller, K.; De Maria, L. Clustering Functional Magnetic Resonance Imaging Time Series in Glioblastoma Characterization: A Review of the Evolution, Applications, and Potentials. Brain Sci. 2024, 14, 296. [Google Scholar] [CrossRef] [PubMed]
- Magnéli, S.; Cesarini, K.G.; Grabowska, A.; Rostami, E. Cystoventricular Drainage of Intracranial Arachnoid Cysts in Adults. World Neurosurg. 2021, 152, e297–e301. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, D.H.; Vogel, T.D.; Baker, A.A.; Patel, N.B.; Ackerman, L.L.; Smith, J.L.; Boaz, J.C. Cyst-ventricle stent as primary or salvage treatment for posterior fossa arachnoid cysts. J. Neurosurg. Pediatr. 2011, 7, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Schmutzer-Sondergeld, M.; Gencer, A.; Niedermeyer, S.; Quach, S.; Stoecklein, V.M.; Teske, N.; Schichor, C.; Terpolilli, N.A.; Kunz, M.; Thon, N. Evaluation of surgical treatment strategies and outcome for cerebral arachnoid cysts in children and adults. Acta Neurochir. 2024, 166, 39. [Google Scholar] [CrossRef] [PubMed]
- Soleman, J.; Kozyrev, D.A.; Constantini, S.; Roth, J. Surgical treatment and outcome of posterior fossa arachnoid cysts in infants. J. Neurosurg. Pediatr. 2021, 28, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Gallieni, M.; Samii, A.; Di Rocco, C.; Samii, M. Surgical management of cerebellopontine angle arachnoid cysts associated with hearing deficit in pediatric patients. J. Neurosurg. Pediatr. 2018, 21, 119–123. [Google Scholar] [CrossRef] [PubMed]
- De Simone, M.; Choucha, A.; Dannhoff, G.; Kong, D.-S.; Zoia, C.; Iaconetta, G. Treating Trigeminal Schwannoma through a Transorbital Approach: A Systematic Review. J. Clin. Med. 2024, 13, 3701. [Google Scholar] [CrossRef]
- Fuentes, A.M.; Yun, J.J.; Jane, J.A. Nontraumatic symptomatic de novo arachnoid cyst in an adolescent patient treated with cystoperitoneal shunting: Illustrative case. J. Neurosurg. Case Lessons 2024, 7, CASE23584. [Google Scholar] [CrossRef]
- Ono, K.; Mukae, N.; Nishimura, A.; Arimura, K.; Mizoguchi, M.; Yoshimoto, K.; Iihara, K. Impaired visual acuity as an only symptom of shunt malfunction, long time after initial cyst-peritoneal shunting for arachnoid cyst: A case report. Surg. Neurol. Int. 2022, 13, 68. [Google Scholar] [CrossRef]
- Srinivasan, U.S.; Lawrence, R. Posterior fossa arachnoid cysts in adults: Surgical strategy: Case series. Asian J. Neurosurg. 2015, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Boody, B.; Lucasti, C.J.; Schroeder, G.D.; Heller, J.E.; Vaccaro, A.R. Extradural Arachnoid Cyst Excision. Clin. Spine Surg. 2019, 32, E403–E406. [Google Scholar] [CrossRef] [PubMed]
- Afana, H.B.; Kananeh, S.F.M.; Duraisamy, R.; Farah, A.; Figueiredo, N. Long-Term Recurrent Intramedullary Arachnoid Cyst: Case Report and Literature Review. Asian J. Neurosurg. 2023, 18, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Morioka, T.; Suzuki, S.O.; Takahata, Y.; Mizoguchi, M. Congenital interdural arachnoid cyst of the tentorium cerebelli. Childs. Nerv. Syst. 2020, 36, 1071–1074. [Google Scholar] [CrossRef]
- Joshi, V.P.; Valsangkar, A.; Nivargi, S.; Vora, N.; Dekhne, A.; Agrawal, A. Giant posterior fossa arachnoid cyst causing tonsillar herniation and cervical syringomyelia. J. Craniovertebr. Junction Spine 2013, 4, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Amelot, A.; Beccaria, K.; Blauwblomme, T.; Bourgeois, M.; Paternoster, G.; Cuny, M.L.; Zerah, M.; Sainte-Rose, C.; Puget, S. Microsurgical, endoscopic, and shunt management of pediatric temporosylvian arachnoid cysts: A comparative study. J. Neurosurg. Pediatr. 2019, 23, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Linares Torres, J.; Ros López, B.; Iglesias Moroño, S.; Ibáñez Botella, G.; Ros Sanjuán, Á.; Arráez Sánchez, M.Á. Neuroendoscopic treatment of arachnoid cysts in the paediatric population. Series results for 20 patients. Neurocirugía 2020, 31, 165–172. [Google Scholar] [CrossRef]
- Mudreac, A.; Behbahani, M.; Chiu, R.G.; Patil, S.N.; Reddy, A.K.; Khalid, S.I.; Mehta, A.I. Pediatric cerebral cysts: Comparison of early complications following fenestration versus shunting procedures. Neurol. Res. 2021, 43, 736–743. [Google Scholar] [CrossRef]





| Localization (Frequency %) | References |
|---|---|
| Supratentorial (42–84%) | |
| [2,7,29,31,32,35,36,37,38,39,40] |
| [2,17,29,31,32,37,38,39,40] |
| [2,37,40] |
| [2,37,40] |
| [37] |
| [21,29,32,34,36,38,39,41] |
| Infratentorial (12–46%) | |
| [2,7,29,32,33,34,37,39,40] |
| [2,37,40] |
| [29] |
| [2,7,34] |
| [2,34,37] |
| [35,37] |
| [28] |
| Symptoms or Syndromes (Frequency %) | References |
|---|---|
| Headaches (26–60%) | [5,7,17,25,31,35,47,48,51,52,53,54,55] |
| Intracranial hypertension symptomatology (vomiting, visual disturbances, bradycardia, hypertension) (14–49%) | [2,3,6,7,18,24,29,31,35,36,37,39,48,54,56,57] |
| Hydrocephalus (18%) | [2,5,7,17,24,29,32,35,36,37,39,47,56,57,58] |
| Local mass-effect neurological deficits (6–32%) | [2,3,5,7,24,28,29,31,32,35,53,56,59] |
| Craniomegaly/macrocephaly (5–71%) | [2,3,6,17,29,31,35,36,39,51,53,54,57,60] |
| Endocrine disorders (5–7%) | [2,5,7,35,36,37,39,57,60] |
| Seizures (11–26%) | [2,3,5,6,17,25,29,31,35,36,39,47,48,53,55,56,60] |
| Developmental delay and cognitive deficits (5–33%) | [2,3,5,6,7,25,29,31,35,36,39,47,53,54,55,57,60] |
| Gait disturbance (10–33%) | [36,53,55] |
| Cerebellopontine angle syndrome with tinnitus, hearing loss, facial palsies, nystagmus, and vertigo (7–12%) | [29,35,39,52] |
| Speech disorders—aphasia (2%) | [2,59] |
| Spinal cord compression | [28,36] |
| Trigeminal neuralgia | [6] |
Other less frequently reported symptoms:
| [6,28,35] [6,28,35] [36,39,57] |
Spinal arachnoid cysts:
| [46] |
| Type of Malformation | References |
|---|---|
| Intra-axial cystic tumors such as pilocytic astrocytomas or hemangioblastomas | [35,36] |
| Mega cisterna magna | [34,35,38] |
| Dermoid and epidermoid cysts | [2,29,35] |
| Non-neoplastic cysts (neuroglial, neurenteric, porencephalic) | [35,38] |
| Blake’s pouch cysts | [34,38] |
| Dandy–Walker malformation | [34,36,38] |
| Choroid plexus cysts | [38,42] |
| Neurocysticercosis | [35] |
| Cavum veli interpositi | [42] |
| Craniopharyngioma | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassilopoulos, T.; Miliaraki, M.; Tsitsipanis, C.; Ntotsikas, K.; Chochlidakis, N.; Karabetsos, D.; Moustakis, N.; Theofanopoulos, A.; Lazarioti, S.; Papastergiou, V.; et al. Progressive Quadriparesis of a Toddler with a Posterior Cranial Fossa Arachnoid Cyst (AC): Illustrative Case Report and Narrative Literature Review. Children 2024, 11, 1463. https://doi.org/10.3390/children11121463
Vassilopoulos T, Miliaraki M, Tsitsipanis C, Ntotsikas K, Chochlidakis N, Karabetsos D, Moustakis N, Theofanopoulos A, Lazarioti S, Papastergiou V, et al. Progressive Quadriparesis of a Toddler with a Posterior Cranial Fossa Arachnoid Cyst (AC): Illustrative Case Report and Narrative Literature Review. Children. 2024; 11(12):1463. https://doi.org/10.3390/children11121463
Chicago/Turabian StyleVassilopoulos, Thanos, Marianna Miliaraki, Christos Tsitsipanis, Konstantinos Ntotsikas, Nikolaos Chochlidakis, Dimitrios Karabetsos, Nikolaos Moustakis, Athanasios Theofanopoulos, Sofia Lazarioti, Vasilios Papastergiou, and et al. 2024. "Progressive Quadriparesis of a Toddler with a Posterior Cranial Fossa Arachnoid Cyst (AC): Illustrative Case Report and Narrative Literature Review" Children 11, no. 12: 1463. https://doi.org/10.3390/children11121463
APA StyleVassilopoulos, T., Miliaraki, M., Tsitsipanis, C., Ntotsikas, K., Chochlidakis, N., Karabetsos, D., Moustakis, N., Theofanopoulos, A., Lazarioti, S., Papastergiou, V., Kritikou, G., & Yannopoulos, A. (2024). Progressive Quadriparesis of a Toddler with a Posterior Cranial Fossa Arachnoid Cyst (AC): Illustrative Case Report and Narrative Literature Review. Children, 11(12), 1463. https://doi.org/10.3390/children11121463






