Mitrofanoff Appendicovesicostomy in Robotic Paediatric Surgery—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Eligibility Criteria
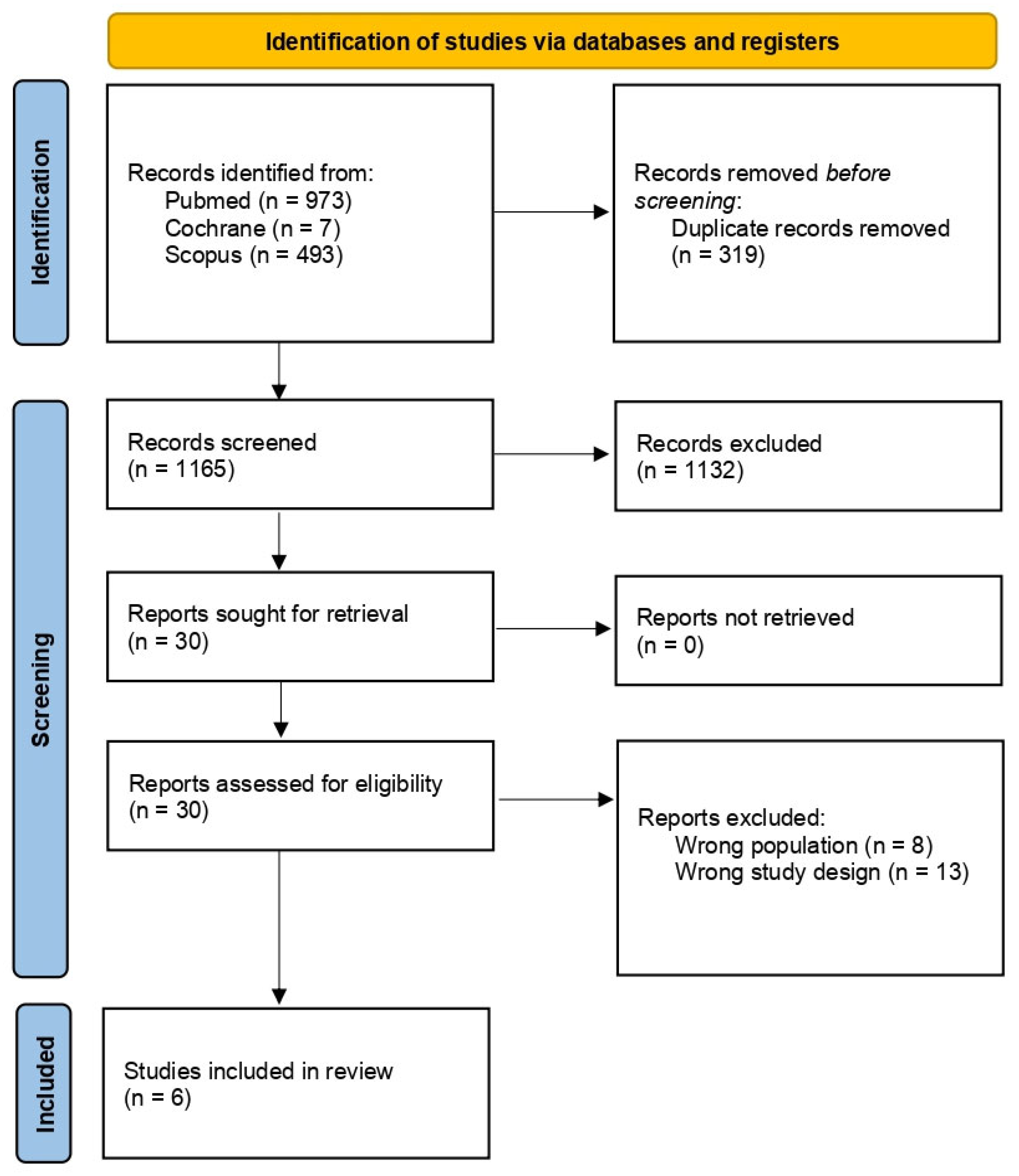
2.1.1. Identification
2.1.2. Screening and Eligibility
2.2. Data Extraction
2.3. Methodological Quality Appraisal
2.4. Statistics
3. Results
3.1. Study Characteristics
3.2. Robotic-Assisted Laparoscopic Augmentation Ileocystoplasty and Mitrofanoff Procedure
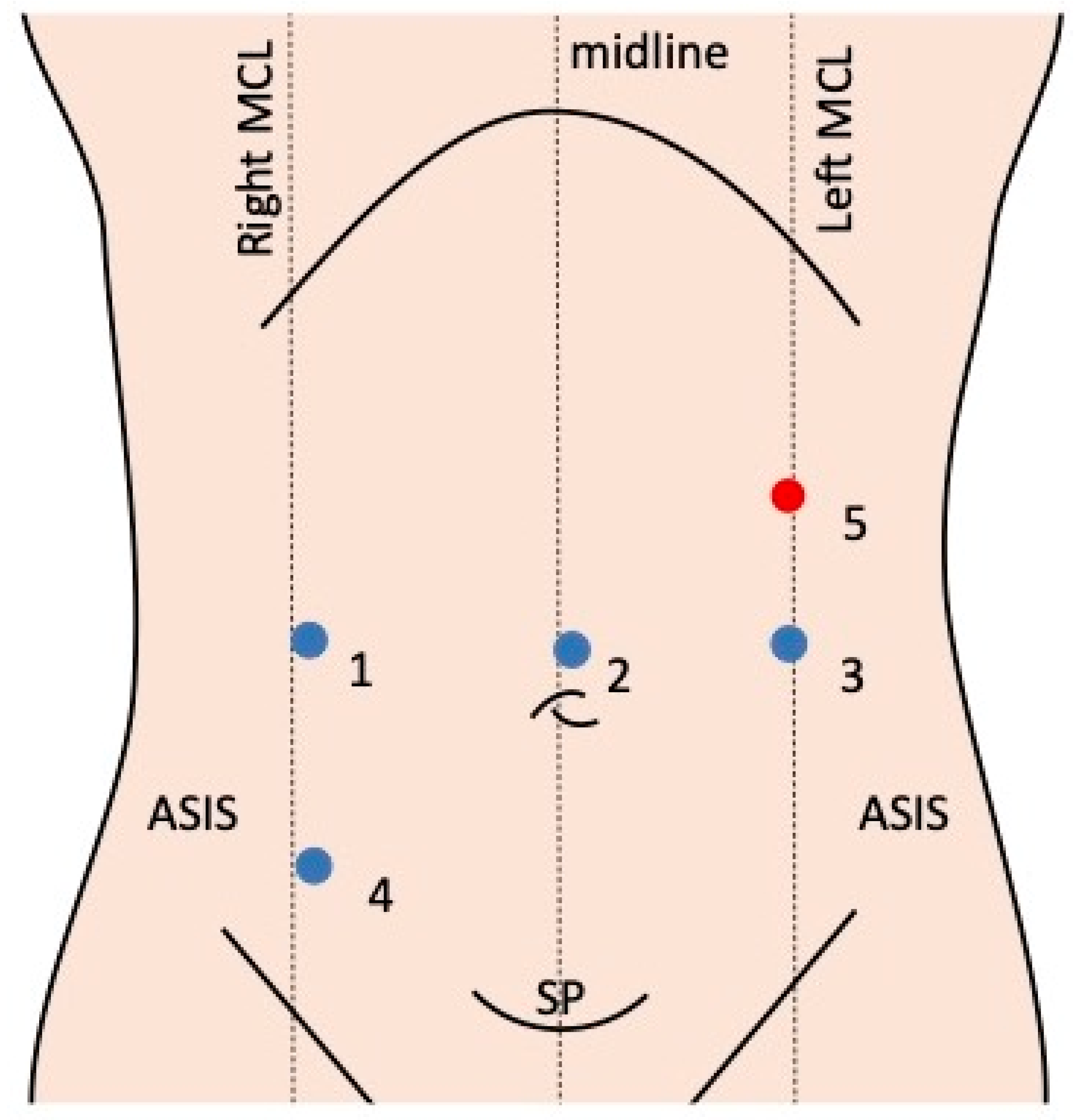
3.3. Robotic Systems and Port Placement
3.4. Surgical Technique
3.5. Methodological Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duhoky, R.; Claxton, H.; Piozzi, G.N.; Khan, J.S. Robotic Approach to Paediatric Gastrointestinal Diseases: A Systematic Review. Children 2024, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Gander, R.; Asensio, M.; Fatou Royo, G.; López, M. Pediatric laparoscopic mitrofanoff procedure-preliminary results of a simplified rechnique. J. Pediatr. Urol. 2022, 18, 112.e1–112.e7. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, M.-K.; Malone, P.S. Educational article: The Mitrofanoff procedure. J. Pediatr. Urol. 2010, 6, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Cerulo, M.; Lepore, B.; Coppola, V.; D’Auria, D.; Esposito, G.; Carulli, R.; Del Conte, F.; Escolino, M. Robotic-assisted pyeloplasty in children: A systematic review of the literature. J. Robot. Surg. 2023, 17, 1239–1246. [Google Scholar] [CrossRef]
- Bagrodia, A.; Gargollo, P. Robot-assisted bladder neck reconstruction, bladder neck sling, and appendicovesicostomy in children: Description of technique and initial results. J. Endourol. 2011, 25, 1299–1305. [Google Scholar] [CrossRef]
- Liard, A.; Seguier-Lipszyc, E.; Mathiot, A.; Mitrofanoff, P. The Mitrofanoff procedure: 20 years later. J. Urol. 2001, 165, 2394–2398. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Passerotti, C.C.; Penna, F.J.; Retik, A.B.; Peters, C.A. Robotic assisted laparoscopic Mitrofanoff appendicovesicostomy: Preliminary experience in a pediatric population. J. Urol. 2009, 182, 1528–1534. [Google Scholar] [CrossRef]
- Famakinwa, O.J.; Rosen, A.M.; Gundeti, M.S. Robot-assisted laparoscopic Mitrofanoff appendicovesicostomy technique and outcomes of extravesical and intravesical approaches. Eur. Urol. 2013, 64, 831–836. [Google Scholar] [CrossRef]
- Adamic, B.; Kirkire, L.; Andolfi, C.; Labbate, C.; Aizen, J.; Gundeti, M. Robot-assisted laparoscopic augmentation ileocystoplasty and Mitrofanoff appendicovesicostomy in children: Step-by-step and modifications to UChicago technique. BJUI Compass 2020, 1, 32–40. [Google Scholar] [CrossRef]
- Pedraza, R.; Weiser, A.; Franco, I. Laparoscopic appendicovesicostomy (Mitrofanoff procedure) in a child using the da Vinci robotic system. J. Urol. 2004, 171, 1652–1653. [Google Scholar] [CrossRef]
- Wille, M.A.; Zagaja, G.P.; Shalhav, A.L.; Gundeti, M.S. Continence Outcomes in Patients Undergoing Robotic Assisted Laparoscopic Mitrofanoff Appendicovesicostomy. J. Urol. 2010, 185, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Gundeti, M.S.; Acharya, S.S.; Zagaja, G.P.; Shalhav, A.L. Paediatric robotic-assisted laparoscopic augmentation ileocystoplasty and Mitrofanoff appendicovesicostomy (RALIMA): Feasibility of and initial experience with the University of Chicago technique. BJU Int. 2010, 107, 962–969. [Google Scholar] [CrossRef]
- Harris, C.F.; Cooper, C.S.; Hutcheson, J.C.; Snyder, H.M., 3rd. Appendicovesicostomy: The mitrofanoff procedure-a 15-year perspective. J. Urol. 2000, 163, 1922–1926. [Google Scholar] [CrossRef]
- Kaefer, M.; Retik, A.B. The Mitrofanoff principle in continent urinary reconstruction. Urol. Clin. North. Am. 1997, 24, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.C.; Dietrich, M.S.; Trusler, L.; DeMarco, R.T.; Pope, J.C.t.; Brock, J.W., 3rd; Adams, M.C. Continent catheterizable channels and the timing of their complications. J. Urol. 2006, 176, 1816–1820; discussion 1820. [Google Scholar] [CrossRef]
- Leslie, B.; Lorenzo, A.J.; Moore, K.; Farhat, W.A.; Bagli, D.J.; Pippi Salle, J.L. Long-term followup and time to event outcome analysis of continent catheterizable channels. J. Urol. 2011, 185, 2298–2302. [Google Scholar] [CrossRef]
- Bonadona, V.; Bonaiti, B.; Olschwang, S.; Grandjouan, S.; Huiart, L.; Longy, M.; Guimbaud, R.; Buecher, B.; Bignon, Y.J.; Caron, O.; et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011, 305, 2304–2310. [Google Scholar] [CrossRef]
- Strand, W.R.; McDougall, E.M.; Leach, F.S.; Allen, T.D.; Pearle, M.S. Laparoscopic creation of a catheterizable cutaneous ureterovesicostomy. Urology 1997, 49, 272–275. [Google Scholar] [CrossRef]
- Chung, S.Y.; Meldrum, K.; Docimo, S.G. Laparoscopic assisted reconstructive surgery: A 7-year experience. J. Urol. 2004, 171, 372–375. [Google Scholar] [CrossRef]
- Chavez, M.R.; Moore, C.; Matthews, L.R.; Danner, O.; Nguyen, J.; Childs, E.; Udobi, K. Cecal volvulus with gangrene following Mitrofanoff procedure. Urol. Case Rep. 2018, 21, 21–23. [Google Scholar] [CrossRef]
- Abdulfattah, S.; Eftekharzadeh, S.; Ai, E.; Aghababian, A.; Overland, M.; Mittal, S.; Srinivasan, A.K.; Shukla, A.R. Is robot-assisted appendicovesicostomy equivalent to the current gold standard open procedure? A comparative analysis. J. Pediatr. Urol. 2024. [Google Scholar] [CrossRef]
| Intraoperative | Postoperative |
|---|---|
| Enhanced precision and dexterity: Robotic instruments provide surgeons with greater precision and dexterity compared to straight laparoscopic instruments, optimising the most delicate and intricate procedures. | Minimally invasive: Robotic surgery is minimally invasive, resulting in smaller incisions, less pain, and faster recovery for paediatric patients. |
| Improved visualisation: High-definition 3D visualisation systems provide surgeons with a magnified and detailed view of the surgical field, enhancing the accuracy and reducing the risk of complications. | Shorter hospital stays: Patients undergoing robotic surgery typically experience shorter hospital stays, allowing for a quicker return to normal activities. |
| Reduced blood loss: Robotic surgery often results in less blood loss compared to open surgery, leading to a reduced need for blood transfusions. | Less postoperative pain: Smaller incisions and less tissue trauma contribute to reduced postoperative pain, leading to lower analgesic requirements. |
| Ergonomic benefits for the surgeon: Robotic surgery can reduce surgeon fatigue and discomfort, leading to improved surgical outcomes. | Improved cosmetic outcomes: Smaller incisions and less tissue manipulation result in better cosmetic outcomes, which can be particularly important for paediatric patients. |
| Potential for less scarring: Minimally invasive techniques can lead to less scarring, which can be a significant benefit for children. |
| Author, Year | Country | Study Design | Sample Size, n | Time Period | Median Age (Range), Years | Disease | Procedure | Robotic Platform | Weight | OT, Min | LOS, Days | POC | Reinterventions | Follow-Up, Months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wille, 2010 [11] | USA | RSC | 8 * | February 2008–April 2010 | 9 (7–12) | Neurogenic bladder | Robotic-assisted laparoscopic Mitrofanoff procedure | da Vinci | NA | NA | NA | 50% | 37.5% | 16.5 (3–29) |
| Nguyen, 2009 [7] | USA | RSC | 6 * | NA | 8.5 (4.3–11.7) | Bladder dysfunction of various aetiologies | Robotic-assisted laparoscopic Mitrofanoff procedure | da Vinci | NA | 442 ± 181 | 23 ± 2 | 33.3% | 0% | 21 (6–42.4) |
| Bagrodia, 2011 [5] | USA | RSC | 2 * | April 2010–August 2010 | 6.5 (5–8) | Myelomeningocele (spina bifida) | Robotic-assisted laparoscopic Mitrofanoff procedure | da Vinci | NA | 360 ± 356 | 1.8 ± 1.9 | 0% | 0% | NS |
| Gundeti, 2010 [13] | USA | PSC | 4 * | February–November 2008 | 10 (7–11) | Neurogenic bladder secondary to spina bifida | Robotic-assisted laparoscopic augmentation ileocystoplasty and Mitrofanoff procedure | S/Original da Vinci | 23 ± 51 | 660 ± 480 | 7 ± 5 | 75% | 75% | 18 (14–22) |
| Famakinwa, 2013 [8] | USA | RSC | 13 * | 2008–2013 (month not specified) | 9 (7–11) | Bladder dysfunction of various aetiologies | Robotic-assisted laparoscopic Mitrofanoff procedure ± augmentation ileocistoplasty | NA | NA | NA | NA | 38.4% | 23% | 24.7 (2.3–43.2) |
| Adamic, 2020 [9] | USA | RSC | 10 * | 2008–2017 (month not specified) | 10 (7.4–11.8) | Bladder dysfunction of various aetiologies | Robotic-assisted laparoscopic augmentation ileocystoplasty and Mitrofanoff procedure | da Vinci | 23.4 ± 65.7 | 480 ± 923 | 4 ± 8 | 91.6% | 91.6% | 91.9% (1.5–129.4) |
| Study | Selection | Compara-Bility | Outcomes | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of the Exposure | Outcome Not Present at the Start of the Study | Assessment of the Outcome | Follow-Up Length | Adequacy of the Follow-Up of the Cohorts | |||
| Wille, 2010 [11] | * | * | * | * | * | * | * | 6 | |
| Nguyen, 2009 [7] | * | * | * | ** | * | * | 6 | ||
| Bagrodia, 2011 [5] | * | * | * | 3 | |||||
| Gundeti, 2010 [13] | * | * | * | * | * | * | * | 6 | |
| Famakinwa, 2013 [8] | * | * | * | * | * | 5 | |||
| Adamic, 2020 [9] | * | * | * | * | * | * | 6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronconi Di Giuseppe, D.; Claxton, H.; Duhoky, R.; Piozzi, G.N.; Khan, J.S. Mitrofanoff Appendicovesicostomy in Robotic Paediatric Surgery—A Systematic Review. Children 2024, 11, 1442. https://doi.org/10.3390/children11121442
Ronconi Di Giuseppe D, Claxton H, Duhoky R, Piozzi GN, Khan JS. Mitrofanoff Appendicovesicostomy in Robotic Paediatric Surgery—A Systematic Review. Children. 2024; 11(12):1442. https://doi.org/10.3390/children11121442
Chicago/Turabian StyleRonconi Di Giuseppe, Diana, Harry Claxton, Rauand Duhoky, Guglielmo Niccolò Piozzi, and Jim S. Khan. 2024. "Mitrofanoff Appendicovesicostomy in Robotic Paediatric Surgery—A Systematic Review" Children 11, no. 12: 1442. https://doi.org/10.3390/children11121442
APA StyleRonconi Di Giuseppe, D., Claxton, H., Duhoky, R., Piozzi, G. N., & Khan, J. S. (2024). Mitrofanoff Appendicovesicostomy in Robotic Paediatric Surgery—A Systematic Review. Children, 11(12), 1442. https://doi.org/10.3390/children11121442






