Investigation of the Etiology of Molar Incisor Hypomineralization in Children Residing in Konya Province and Surrounding Areas, Türkiye
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Sample
2.2. Ethical Consideration
2.3. Intraoral Examination
2.4. Etiological Evaluation
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
- -
- It has been observed that prenatal factors such as maternal diseases experienced in the last 3 months of pregnancy and perinatal factors such as premature birth and birth complications are not associated with MIH.
- -
- The duration of breastfeeding and fluoride, calcium, and vitamin supplements taken in the first 4 years of life have no association with MIH.
- -
- It has been determined that frequent diarrhea and fever attacks in the first 4 years of life, febrile convulsions, asthma, pneumonia, and lower respiratory tract diseases have been observed to be associated with MIH.
- -
- It has been observed that childhood diseases with rashes, kidney diseases, and urinary tract infections are not associated with MIH.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seow, W.K. Clinical diagnosis of enamel defects: Pitfalls and practical guidelines. Int. Dent. J. 1997, 47, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Ten Cate’s Oral Histology-E-Book: Development, Structure, and Function; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Suckling, G.; Nelson, D.; Patel, M. Macroscopic and scanning electron microscopic appearance and hardness values of developmental defects in human permanent tooth enamel. Adv. Dent. Res. 1989, 3, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Leal, S.C.; Takeshita, E.M. Pediatric Restorative Dentistry; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Weerheijm, K.L.; Mejàre, I. Molar incisor hypomineralization: A questionnaire inventory of its occurrence in member countries of the European Academy of Paediatric Dentistry (EAPD). Int. J. Paediatr. Dent. 2003, 13, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Weerheijm, K.L.; Duggal, M.; Mejàre, I.; Papagiannoulis, L.; Koch, G.; Martens, L.C.; Hallonsten, A.-L. Judgement criteria for Molar Incisor Hypomincralisation (MIH) in epidemiologic studies: A summary of the European meeting on MIH held in Athens, 2003. Eur. J. Paediatr. Dent. 2003, 4, 110–114. [Google Scholar]
- de Aguiar Grossi, J.; Cabral, R.N.; Leal, S.C. Caries experience in children with and without molar-incisor Hypomineralisation: A case-control study. Caries Res. 2017, 51, 419–424. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Ki, Y.; Chu, V. Molar incisor hypomineralization in Hong Kong Chinese children. Int. J. Paediatr. Dent. 2008, 18, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Soviero, V.; Haubek, D.; Trindade, C.; Da Matta, T.; Poulsen, S. Prevalence and distribution of demarcated opacities and their sequelae in permanent 1st molars and incisors in 7 to 13-year-old Brazilian children. Acta Odontol. Scand. 2009, 67, 170–175. [Google Scholar] [CrossRef]
- Zhao, D.; Dong, B.; Yu, D.; Ren, Q.; Sun, Y. The prevalence of molar incisor hypomineralization: Evidence from 70 studies. Int. J. Paediatr. Dent. 2018, 28, 170–179. [Google Scholar] [CrossRef]
- Mahoney, E.K.; Morrison, D.G. The prevalence of molar-incisor hypomineralisation (MIH) in Wainuiomata children. N. Z. Dent. J. 2009, 105, 121–127. [Google Scholar]
- Onat, H.; Tosun, G. Molar incisor hypomineralization. J. Pediatr. Dent. 2013, 1, 53. [Google Scholar] [CrossRef]
- Silva, M.J.; Scurrah, K.J.; Craig, J.M.; Manton, D.J.; Kilpatrick, N. Etiology of molar incisor hypomineralization—A systematic review. Community Dent. Oral Epidemiol. 2016, 44, 342–353. [Google Scholar] [CrossRef]
- Bodrumlu, E.H.; Avşar, A. Büyük azı kesici diş hipomineralizasyonu: Etiyolojisi ve kliniği. Acta Odontol. Scand. 2015, 32, 90–97. [Google Scholar] [CrossRef]
- Sönmez, H.; Yıldırım, G.; Bezgin, T. Putative factors associated with molar incisor hypomineralisation: An epidemiological study. Eur. Arch. Paediatr. Dent. 2013, 14, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Da Costa-Silva, C.M.; Jeremias, F.; de Souza, J.F.; De Cássia Loiola Cordeiro, R.; Santos-Pinto, L.; Cilense Zuanon, A.C. Molar incisor hypomineralization: Prevalence, severity and clinical consequences in Brazilian children. Int. J. Paediatr. Dent. 2010, 20, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Tapias-Ledesma, M.A.; Jiménez, R.; Lamas, F.; González, A.; Carrasco, P.; De Miguel, A.G. Factors associated with first molar dental enamel defects: A multivariate epidemiological approach. J. Dent. Child. 2003, 70, 215–220. [Google Scholar]
- Laisi, S.; Ess, A.; Sahlberg, C.; Arvio, P.; Lukinmaa, P.-L.; Alaluusua, S. Amoxicillin may cause molar incisor hypomineralization. J. Dent. Res. 2009, 88, 132–136. [Google Scholar] [CrossRef]
- Kühnisch, J.; Thiering, E.; Heitmüller, D.; Tiesler, C.M.; Grallert, H.; Heinrich-Weltzien, R.; Hickel, R.; Heinrich, J.; GINI-10 Plus Study Group; LISA-10 Plus Study Group. Genome-wide association study (GWAS) for molar–incisor hypomineralization (MIH). Clin. Oral Investig. 2014, 18, 677–682. [Google Scholar] [CrossRef]
- Ghanim, A.; Morgan, M.; Marino, R.; Bailey, D.; Manton, D. Molar incisor hypomineralisation: Prevalence and defect characteristics in Iraqi children. Int. J. Paediatr. Dent. 2011, 21, 413–421. [Google Scholar] [CrossRef]
- Giuca, M.R.; Cappe, M.; Carli, E.; Lardani, L.; Pasini, M. Investigation of clinical characteristics and etiological factors in children with molar incisor hypomineralization. Int. J. Dent. 2018, 2018, 7584736. [Google Scholar] [CrossRef]
- Avery, J.K.; Steele, P.F.; Avery, N. Oral Development and Histology; Thieme: New York, NY, USA, 2002. [Google Scholar]
- Caruso, S.; Bernardi, S.; Pasini, M.; Giuca, M.R.; Docimo, R.; Continenza, M.; Gatto, R. The process of mineralisation in the development of human tooth. Eur. J. Paediatr. Dent. 2016, 17, 322–326. [Google Scholar]
- Pitiphat, W.; Luangchaichaweng, S.; Pungchanchaikul, P.; Angwaravong, O.; Chansamak, N. Factors associated with molar incisor hypomineralization in T hai children. Eur. J. Oral Sci. 2014, 122, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Jälevik, B.; Klingberg, G.; Barregård, L.; Norén, J.G. The prevalence of demarcated opacities in permanent first molars in a group of Swedish children. Acta Odontol. Scand. 2001, 59, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Whatling, R.; Fearne, J.M. Molar incisor hypomineralization: A study of aetiological factors in a group of UK children. Int. J. Paediatr. Dent. 2008, 18, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, A.; Manton, D.; Bailey, D.; Marino, R.; Morgan, M. Risk factors in the occurrence of molar–incisor hypomineralization amongst a group of Iraqi children. Int. J. Paediatr. Dent. 2013, 23, 197–206. [Google Scholar] [CrossRef]
- Allazzam, S.M.; Alaki, S.M.; El Meligy, O.A.S. Molar incisor hypomineralization, prevalence, and etiology. Int. J. Dent. 2014, 2014, 234508. [Google Scholar] [CrossRef]
- Bukhari, S.T.; Alhasan, H.A.; Qari, M.T.; Sabbagh, H.J.; Farsi, N.M. Prevalence and risk factors of molar incisor hypomineralization in the Middle East: A systematic review and meta-analysis. J. Taibah Univ. Med. Sci. 2023, 18, 696–710. [Google Scholar] [CrossRef]
- Arrow, P. Risk factors in the occurrence of enamel defects of the first permanent molars among schoolchildren in Western Australia. Community Dent. Oral Epidemiol. 2009, 37, 405–415. [Google Scholar] [CrossRef]
- Brogårdh Roth, S.; Matsson, L.; Klingberg, G. Molar-incisor hypomineralization and oral hygiene in 10-to-12-yr-old Swedish children born preterm. Eur. J. Oral Sci. 2011, 119, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Koruyucu, M.; Özel, S.; Tuna, E.B. Prevalence and etiology of molar-incisor hypomineralization (MIH) in the city of Istanbul. J. Dent. Sci. 2018, 13, 318–328. [Google Scholar] [CrossRef]
- Fatturi, A.L.; Wambier, L.M.; Chibinski, A.C.; Assunção, L.R.d.S.; Brancher, J.A.; Reis, A.; Souza, J.F. A systematic review and meta analysis of systemic exposure associated with molar incisor hypomineralization. Community Dent. Oral Epidemiol. 2019, 47, 407–415. [Google Scholar] [CrossRef]
- Alaluusua, S. Aetiology of molar-incisor hypomineralisation: A systematic review. Eur. Arch. Paediatr. Dent. 2010, 11, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Lygidakis, N.; Garot, E.; Somani, C.; Taylor, G.; Rouas, P.; Wong, F. Best clinical practice guidance for clinicians dealing with children presenting with molar-incisor-hypomineralisation (MIH): An updated European Academy of Paediatric Dentistry policy document. Eur. Arch. Paediatr. Dent. 2022, 23, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Garot, E.; Rouas, P.; Somani, C.; Taylor, G.; Wong, F.; Lygidakis, N. An update of the aetiological factors involved in molar incisor hypomineralisation (MIH): A systematic review and meta-analysis. Eur. Arch. Paediatr. Dent. 2022, 23, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Cutrera, R.; Baraldi, E.; Indinnimeo, L.; Del Giudice, M.M.; Piacentini, G.; Scaglione, F.; Ullmann, N.; Moschino, L.; Galdo, F.; Duse, M. Management of acute respiratory diseases in the pediatric population: The role of oral corticosteroids. Ital. J. Pediatr. 2017, 43, 31. [Google Scholar] [CrossRef] [PubMed]
- Wogelius, P.; Viuff, J.H.; Haubek, D. Use of asthma drugs and prevalence of molar incisor hypomineralization. Int. J. Paediatr. Dent. 2020, 30, 734–740. [Google Scholar] [CrossRef]
- Hoffmann, U.; Neumann, C.; Bauer, C.-P.; Berdel, D.; von Berg, A.; Koletzko, S.; Garcia-Godoy, F.; Hickel, R.; Heinrich, J. Respiratory diseases are associated with molar-incisor hypomineralizations. Swiss Dent. J. SSO–Sci. Clin. Top. 2014, 124, 286–293. [Google Scholar]
- Beentjes, V.; Weerheijm, K.; Groen, H. Factors involved in the aetiology of molar-incisor hypomineralisation (MIH). Eur. J. Paediatr. Dent. 2002, 3, 9–13. [Google Scholar]
- Kuscu, O.O.; Caglar, E.; Sandalli, N. The prevalence and aetiology of molar-incisor hypomineralisation in a group of children in Istanbul. Eur. J. Paediatr. Dent. 2008, 9, 139–144. [Google Scholar]
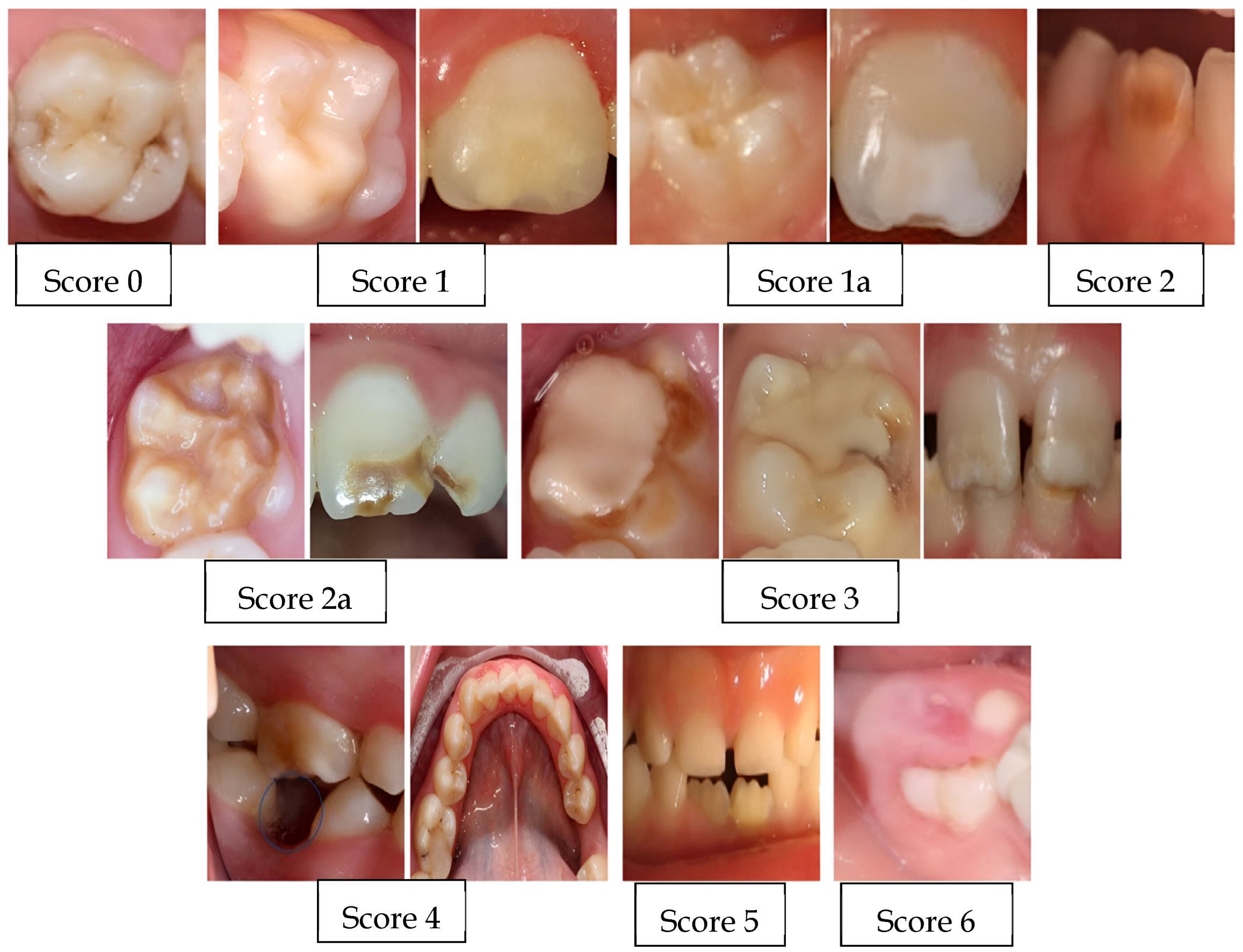
| 0 | Enamel defect free |
| 1 | White/creamy demarcated opacities, no PEB |
| 1a | White/creamy demarcated opacities, with PEB |
| 2 | Yellow/brown demarcated opacities, no PEB |
| 2a | Yellow/brown demarcated opacities, with PEB |
| 3 | Atypical restoration |
| 4 | Missing because of MIH |
| 5 | Partially erupted (i.e., less than one-third of the crown high) with evidence of MIH |
| 6 | Unerupted/partially erupted with no evidence of MIH |
| 7 | Diffuse opacities (not MIH) |
| 8 | Hypoplasia (not MIH) |
| 9 | Combined lesion (diffuse opacities/hypoplasia with MIH) |
| 10 | Demarcated opacities in incisors only |
| Prenatal Factors |
|
| Perinatal Factors |
|
| Postnatal Factors |
|
| Group n (%) | X2 | p | Odds Ratio (%95 L-U) | |||
|---|---|---|---|---|---|---|
| Control | Study | |||||
| Did you have any illnesses or birth complications in the last 3 months of your pregnancy? | Yes | 6 (5.8) | 7 (6.7) | 0.082 | 0.775 | 1.179 (0.382–3.634) |
| No | 98 (94.2) | 97 (93.3) | ||||
| Was your child born prematurely? | Yes | 6 (5.8) | 11 (10.6) | 1.601 | 0.206 | 1.932 (0.687–5.435) |
| No | 98 (94.2) | 93 (89.4) | ||||
| What was your child’s birth weight? | 1.5–2.5 kg | 10 (9.6) | 11 (10.6) | 0.053 | 0.818 | 1.112 (0.451–2.743) |
| >2.5 kg | 94 (90.4) | 93 (89.4) | ||||
| How long did you breastfeed your child? | >12 months | 20 (19.2) | 17 (16.3) | 1.992 | 0.574 | - |
| 8–12 months | 40 (38.5) | 50 (48.1) | ||||
| <8 months | 44 (42.3) | 37 (35.6) | ||||
| Did you give your child fluoride tablets, calcium tablets, or vitamin tablets before the age of 4? | Yes | 34 (32.7) | 30 (28.8) | 0.361 | 0.548 | 0.835 (0.463–1.505) |
| No | 70 (67.3) | 74 (71.2) | ||||
| Did your child have severe diarrhea by the age of 4? | Yes | 5 (4.8) | 14 (13.5) | 4.692 | 0.030 * | 3.080 * (1.067–8.892) |
| No | 99 (95.2) | 90 (86.5) | ||||
| Did your child have any digestive problems until the age of 4? | Yes | 3 (2.9) | 3 (2.9) | 0.000 | 0.999 | 1.000 (0.197–5.073) |
| No | 101(97.1) | 101 (97.1) | ||||
| Did your child have asthma since birth until the age of 4? | Yes | 3 (2.9) | 13 (12.5) | 6.771 | 0.009 * | 4.810 * (1.328–17.419) |
| No | 101 (97.1) | 91 (87.5) | ||||
| Did your child have pneumonia by the age of 4? | Yes | 6 (5.8) | 15 (14.4) | 4.290 | 0.038 * | 2.753 * (1.024–7.403) |
| No | 98 (94.2) | 89 (85.6) | ||||
| Did your child have lower respiratory tract infections such as bronchitis, bronchiolitis, and laryngitis until the age of 4? | Yes | 19 (18.3) | 32 (30.8) | 4.390 | 0.036 * | 1.988 * (1.039–3.804) |
| No | 85 (81.7) | 72 (69.2) | ||||
| Did your child have seizures by the age of 4? | Yes | 5 (4.8) | 13 (12.5) | 3.892 | 0.049 * | 2.829 * (1.007–8.246) |
| No | 99 (95.2) | 91 (87.5) | ||||
| Did your child have frequent fevers until the age of 4? | Yes | 49 (47.1) | 64 (61.5) | 4.360 | 0.037 * | 1.796 * (1.034–3.118) |
| No | 55 (52.9) | 40 (38.5) | ||||
| Did your child have a middle-ear infection by the age of 4? | Yes | 13 (12.5) | 11 (10.6) | 0.188 | 0.664 | 0.828 (0.353–1.944) |
| No | 91 (87.5) | 93 (89.4) | ||||
| Did your child have chronic kidney failure or another kidney disease until the age of 4? | Yes | 4 (3.8) | 4 (3.8) | 0.000 | 0.999 | 1.000 (0.243–4.110) |
| No | 100 (96.2) | 100 (96.2) | ||||
| Did your child have a urinary tract infection by the age of 4? | Yes | 18 (17.3) | 18 (17.3) | 0.000 | 0.999 | 1.000 (0.488–2.051) |
| No | 86 (82.7) | 86 (82.7) | ||||
| What childhood illnesses did your child have until the age of 4? | None | 76 (73.1) | 73 (70.2) | 0.307 | 0.858 | - |
| Chick pox | 20 (19.2) | 21 (20.2) | ||||
| Other | 8 (7.7) | 10 (9.6) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seloğlu, A.; Kahvecioğlu, F. Investigation of the Etiology of Molar Incisor Hypomineralization in Children Residing in Konya Province and Surrounding Areas, Türkiye. Children 2024, 11, 1399. https://doi.org/10.3390/children11111399
Seloğlu A, Kahvecioğlu F. Investigation of the Etiology of Molar Incisor Hypomineralization in Children Residing in Konya Province and Surrounding Areas, Türkiye. Children. 2024; 11(11):1399. https://doi.org/10.3390/children11111399
Chicago/Turabian StyleSeloğlu, Aslı, and Firdevs Kahvecioğlu. 2024. "Investigation of the Etiology of Molar Incisor Hypomineralization in Children Residing in Konya Province and Surrounding Areas, Türkiye" Children 11, no. 11: 1399. https://doi.org/10.3390/children11111399
APA StyleSeloğlu, A., & Kahvecioğlu, F. (2024). Investigation of the Etiology of Molar Incisor Hypomineralization in Children Residing in Konya Province and Surrounding Areas, Türkiye. Children, 11(11), 1399. https://doi.org/10.3390/children11111399





