A Systematic Review and Meta-Analysis of Conditioned Pain Modulation in Children and Young People with Chronic Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Study Quality Assessment
2.5. Meta Analytic Procedures
3. Results
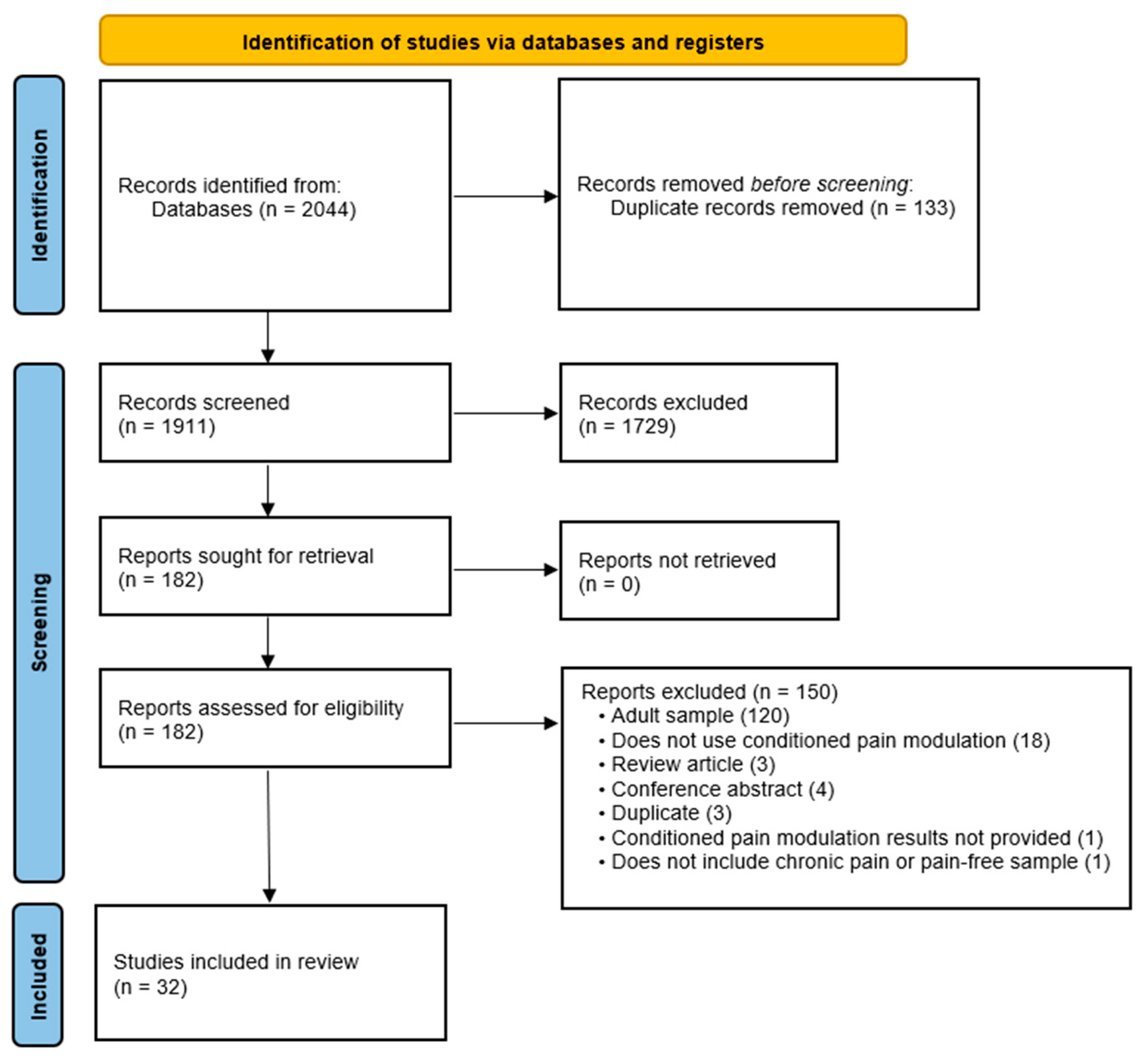
3.1. Search Results
3.2. Summary of Identified Studies
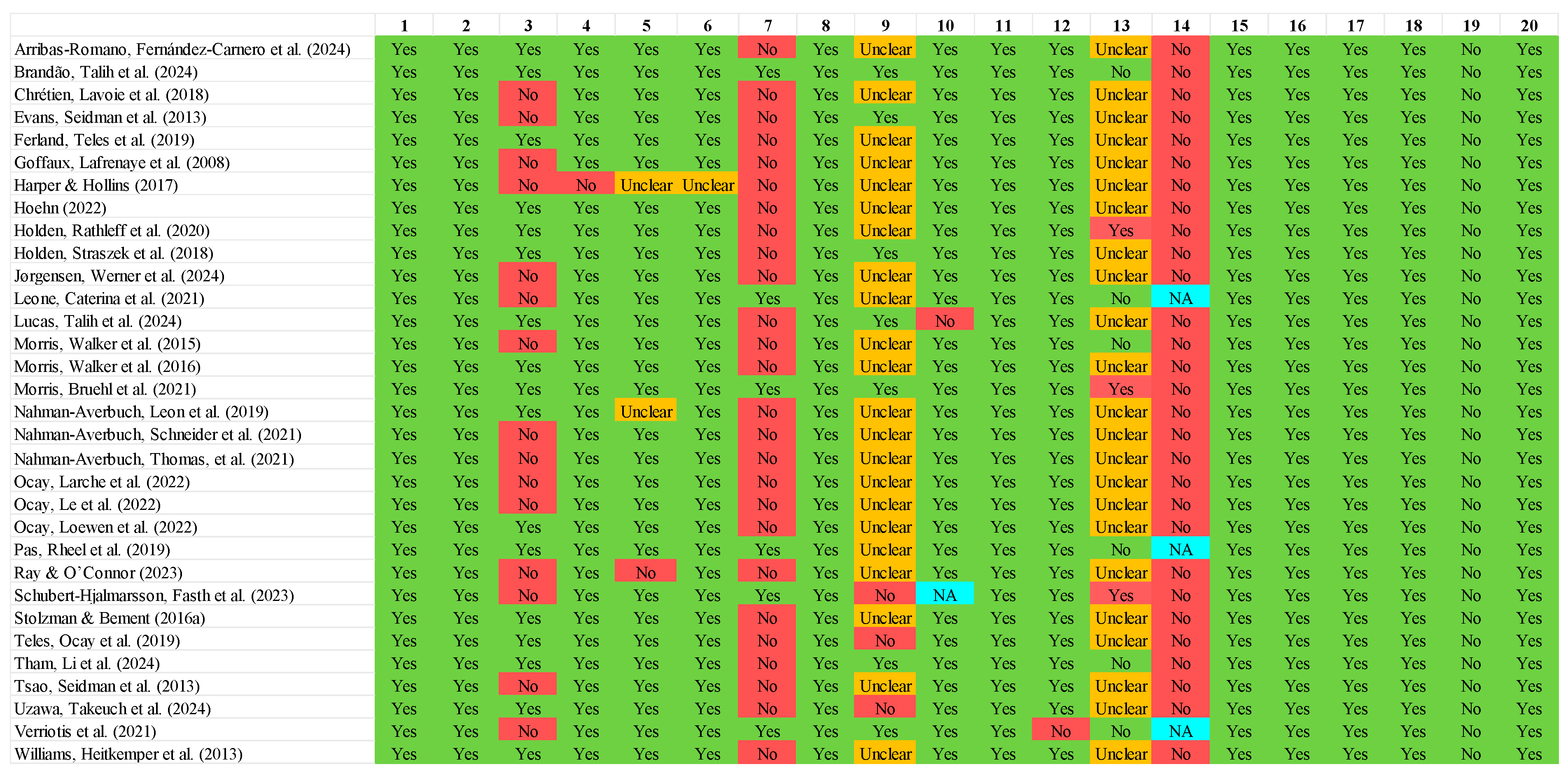
3.3. Methodological Quality
3.4. Narrative Summary
3.5. Psychometric Properties of Outcome Measures
3.6. Meta-Analysis of CPM Response in CYP with Chronic Pain Compared to Healthy Controls
3.7. The Influence of Experimental and Demographic Variables on CPM Outcomes
3.8. Psychometric Properties of CPM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Conditioned Pain Modulation Parameters and Overall Results for Each Study Included in the Systematic Review and Meta-Analysis of Conditioned Pain Modulation in Children and Adolescents
| Study | Sample Details | CPM Test Stimulus | Test and Conditioning Sites | CPM Testing Procedure | Main CPM Findings |
| Arribas-Romano, Fernández-Carnero et al. (2024) [68] | 30 pain-free adolescents | Test: Pressure pain threshold via handheld pressure algometerConditioning: Pressure pain via sphygmomanometer | Test site: Right thumb nail bedConditioning site: Left arm | Pressure pain test stimulus administered before, during, and one minute after conditioning pressure stimulus | Reported pain intensity to the test stimulus was lower during the presence of the conditioning stimulus than at baseline. |
| Brandão, Talih et al. (2024) [51] | Participants from the Generation XXI birth cohort.1496 adolescents. At age thirteen, 883 reported having any pain, of which 401 reported having musculoskeletal pain as their principal pain site.143 adolescents reported current musculoskeletalpain with more than 3 months’ duration. 50 reported current musculoskeletal painin addition to musculoskeletal painin one or more of the previous evaluation waves at ages 7and 10. | Test: Pressure pain threshold and tolerance via automated cuff algometerConditioning: Pressure pain via automated cuff algometer | Test site: Right legConditioning site: Left leg | Pressure pain test stimulus administered before and during conditioning pressure stimulus | For adolescents with musculoskeletal pain at 13 plus a history of pain, the CPM effect was slightly increased for pain detection threshold and pain tolerance compared to other participants, although this effect was not statistically significant. Impaired CPM was not detected among adolescents with musculoskeletal pain. The same pattern of results was found for adolescents with musculoskeletal pain at seven and ten with a history of pain. |
| Chrétien, Lavoie et al. (2018) [43] | 16 teenage girls with chronic pain and 25 healthy adolescent girls | Test: Heat pain intensity rating via thermodeConditioning: Cold pressor | Test site: Volar part of the left forearmConditioning site: Right forearm | Heat pain administered before and immediately after cold pressor | Pain intensity produced by heat pain stimulationssignificantly decreased following cold pressor pain in healthy girls, but not in girls with chronic pain. CPM magnitude was significantly greater in healthy girls compared to those with chronic pain. |
| Evans, Seidman et al. (2013) [58] | 133 healthy children and adolescents | Test: Pressure pain intensity rating via hydraulic piston connected to a computer activated pumpConditioning: Cold pressor | Test site: Thumbnail of left handConditioning site: Right hand | Pressure pain administered before, during, and after cold pressor | For boys, but not for girls, higher material anxiety was significantly associated with lower CPM (less pain inhibition). CPM magnitude was similar for boys and girls. |
| Ferland, Teles et al. (2019) [56] | 105 paediatric patients with chronic back pain | Test: Heat pain intensity via thermodeConditioning: Cold pressor | Test site: Right forearmConditioning site: Left forearm | Heat pain administered before and during cold pressor | High blood metanephrine was a significant predictor of poorer CPM efficiency, explaining 53% of CPM variation in males and 7% of CPM variation in females. |
| Goffaux, Lafrenaye et al. (2008) [61] | 26 children term-born or born pre-term exposed to numerous painful interventions or few painful interventions | Test: Heat pain intensity via thermodeConditioning: Cold pressor | Test site: Left calf and left forearmConditioning site: Right hand | Heat pain administered before and during cold pressor | Cold pressor pain was found to significantly reduce test heat pain intensity for full-term and low-pain pre-term groups with strong inhibitory responses. The high-pain pre-term group demonstrated a complete absence of CPM effect. |
| Harper & Hollins (2017) [64] | 37 healthy undergraduate students | Test: Heat pain intensity via thermal grillConditioning: Cold pressor | Test site: Right volar forearmConditioning site: Left hand | Heat pain administered during cold pressor run and control run (neutral temperature water), the order of which was counterbalanced | CPM produced significant and comparable reductions in pain in noxious heat and thermal grill conditions. |
| Hoehn (2022) [67] | 54 healthy children | Test: Pressure pain threshold via algometerConditioning: Cold pressor | Test site: Right thumbnailConditioning site: Left hand | Pressure pain administered before, during, and after cold pressor | A significant CPM effect was observed, with no significant main effect of age (6–12 years) |
| Holden, Rathleff et al. (2020) [48] | 151 adolescents with patellofemoral pain and 50 healthy controls | Test: Pressure pain threshold via computerised cuff pressure algometerConditioning: Pressure pain via computerised cuff pressure algometer | Test site: LegConditioning site: Leg(For those with patellofemoral pain, the test limb was the knee with pain or the most painful knee in the case of bilateralpain. The test limb was randomly selected for controls) | Test pressure pain stimulus administered before and during the conditioning stimulus | The healthy control group had a significant increase in pain detection threshold during the painful conditioning stimulus compared to without conditioning. No significant difference was found for the patellofemoral pain group, indicating no efficient CPM response. |
| Holden, Straszek et al. (2018) [49] | 36 young females with patellofemoral pain, 22 recovered from patellofemoral pain, and 29 healthy controls | Test: Pressure pain threshold and tolerance via computerised cuff pressure algometerConditioning: Pressure pain via computerised cuff pressure algometer | Test site: LegConditioning site: Leg(For those with or recovered from patellofemoral pain, the test limb was the knee with pain or the most painful knee in the case of bilateralpain. The test limb was randomly selected for controls) | Test pressure pain stimulus administered before and during the conditioning stimulus | Considering pressure tolerance threshold, females with patellofemoral pain had significantly impaired CPM compared to those recovered from patellofemoral pain, but no differences in CPM compared to healthy controls. No differences were found in CPM when pressure pain threshold was considered. |
| Jørgensen, Werner et al. (2024) [50] | 25 children and adolescents with cerebral palsy (9 with chronic pain and 16 without chronic pain) and 26 typically developed children (TDC) and adolescents (14 with chronic pain and 12 without chronic pain) | Test: Pressure pain threshold via handheld pressure algometerConditioning: Cold pressor | Test sites: Hand and footConditioning sites: Hand and foot(Test and conditioning sites were (i) both hands, and (ii) both feet)For all participants except TDC without chronic pain, the primary test site was the most affected limb.The non-dominant hand or foot site was randomlychosen in TDCwithout chronic pain. | Pressure pain stimulus administered before and during the cold pressor | Across all participants, pressure pain threshold consistently increased by a median of approximately 30 kPa from unconditioned to conditioned test stimuli. No other effects were found. |
| Leone, Caterina et al. (2021) [63] | 30 adolescents with non-suicidal self-injury (NSSI) and 20 healthy controls | Test: Heat pain intensity via thermodeConditioning: Heat pain via thermode | Test site: Non-dominant volar forearmConditioning site: Dominant forearm | Test heat pain administered before and during conditioning heat pain | Adolescents with NSSI showed significantly deficient CPM compared to healthy adolescents. |
| Lucas, Talih et al. (2024) [66] | 1727 children and adolescents classified based on a bullying scale as aggressor only, victim only, both victim and aggressor, or not involved | Test: Pressure pain threshold and tolerance threshold via computerised cuff pressure algometerConditioning; Pressure pain via computerised cuff pressure algometer | Test site: Lower right legConditioning site: Lower left leg | Test pressure pain stimulus administered before and during the conditioning stimulus | Children classified as aggressors only had higher CPM for pressure detection thresholds compared to those not involved in bullying. |
| Morris, Bruehl et al. (2021) [52] | 63 adolescents with functional abdominal pain (FAP) receivinginternet-delivered cognitive behaviour therapy (n = 90) or pain education (n = 93) | Test: Heat pain intensity via thermodeConditioning: Hot water immersion | Test site: Ventral forearm of non-dominant handConditioning site: Dominant hand | Test heat pain administered before and during hot water immersion | Beyond any effects of the interventions, pain-related interference declined significantly over time for children with stronger baseline CPM. |
| Morris, Walker et al. (2015) [59] | 78 healthy children | Test: Heat pain intensity via thermodeConditioning: Hot water immersion | Test site: Ventral forearm of non-dominant handConditioning site: Dominant hand | Test heat pain administered before and during hot water immersion | Significantly stronger CPM effects were found in African-American children than Non-Hispanic White children |
| Morris, Walker et al. (2016) [44] | 63 youth with functional abdominal pain (FAP) and 77 healthy controls | Test: Heat pain intensity via thermodeHot water immersion | Test site: Ventral forearm of non-dominant handConditioning site: Dominant hand | Test heat pain administered before and during hot water immersion | Children with FAP showed significantly weaker CPM responses than healthy controls |
| Nahman-Averbuch, Leon et al. (2019) [30] | 19 adolescentswith migraine, 20 healthy adolescents with a family history of migraine, and 29 healthy adolescents without a family history of migraine | Test: Heat pain intensity via thermode, pressure pain threshold via algometerConditioning: Cold pressor | Test site: Both stimuli at the lower dominant legConditioning site: Non-dominant foot | Heat/pressure pain administered before and during cold pressor | No significant difference in CPM magnitude was found between the three groups. |
| Nahman-Averbuch, Schneider et al. (2021) [57] | 20 adolescentswith migraine | Test: Pressure pain threshold via algometerConditioning: Cold pressor | Test site: Lower dominant leg and trapeziusConditioning site: Non-dominant foot | Pressure pain administered before and during cold pressor | A significant CPM response was seen in children before CBT, although the effect was variable. |
| Nahman-Averbuch, Thomas, et al. (2021) [40]—Secondary analysis of above 2021 [57] study, recruited additional healthy controls | 19 adolescentswith migraine and 20 healthy adolescents | Test: Pressure pain threshold via algometerConditioning: Cold pressor | Test site: Lower dominant leg and trapeziusConditioning site: Non-dominant foot | Pressure pain administered before and during cold pressor | No significant difference in CPM magnitude was found between adolescents with migraine and healthy controls. |
| Ocay, Larche et al. (2022) [41] | 302 adolescents with chronic musculoskeletal pain and 80 healthy controls | Test: Heat pain intensity via thermodeConditioning: Cold pressor | Test site: Right volar forearmConditioning site: Left forearm | Heat pain administered before and after cold pressor | CPM efficiency was significantly weaker in children with musculoskeletal pain than in healthy controls. Adolescents with chronic musculoskeletal pain were heterogenous, with distinct subgroups identified, including patients who did and did not display optimal CPM efficiency |
| Ocay, Ye et al. (2022) [47] | 639 children and adolescents with chronic pain and 60 healthy controls (regrouped data from multiple former studies) | Test: Heat pain intensity via thermodeConditioning: Cold pressor | Test site: Right volar forearmConditioning site: Left forearm | Heat pain administered before and after cold pressor | Mean CPM efficiency did not significantly differ between those with chronic pain and healthy controls. Cluster analysis was performed, which revealed heterogeneity amongst patients in their responses to CPM |
| Ocay, Loewen et al. (2022) [53] | 198 adolescents with chronic back pain | Test: Heat pain intensity via thermodeConditioning: Cold pressor | Test site: Right forearmConditioning site: Left arm | Heat pain administered before and after cold pressor | CPM efficiency was optimal in 51.5% of adolescents, suboptimal in 22.7% of adolescents, and inefficient in 25.8% of adolescents. |
| Pas, Rheel et al. (2019) [45] | 39 children and youth with functional abdominal pain disorder and 36 healthy controls | Test: Pressure pain threshold via algometerConditioning: Cold pressor | Test site: Dominant trapezial regionConditioning site: Non-dominant hand | Pressure pain administered before and during cold pressor | Children with functional abdominal pain showed significantly lower CPM responses than healthy children |
| Ray & O’Connor (2023) [62] | 54 healthy young women randomised to receive yoga/no yoga and slow-breathing/normal breathing (n = 11–15 per group) | Test: Heat pain intensity via thermodeConditioning: Cold pressor | Test site: Right forearmConditioning site: Left hand | Test heat pain administered before and during cold pressor | No significant effects of either yoga or slow breathing on CPM efficiency were found. |
| Schubert-Hjalmarsson, Fasth et al. (2023) [42] | 10 children with hypermobility spectrum disorderor hypermobile Ehlers–Danlos syndrome and 9 healthy controls | Test: Pressure pain threshold via handheld pressure algometerConditioning: Cold pressor | Test site: Trapezius (side not stated)Conditioning site: Non-dominant hand | Pressure pain stimulus administered before and during (30s and 50s) the cold pressor | Only descriptive statistics are reported. A similar CPM outcome was found for patient and control groups. |
| Stolzman & Bement (2016) [60] | 32 normal-weightadolescents and 24 overweight/obese adolescents | Test: Pressure pain threshold via algometerConditioning: Cold pressor | Test site: Left 4th digit nailbed and left middle deltoid muscleConditioning site: Right foot | Pressure pain administered during cold pressor and during cool water (control condition) | CPM responses were similar across weight status between boys and girls. |
| Teles, Ocay et al. (2019) [54] | Ninety-four adolescents diagnosed with idiopathic scoliosis and chronic back pain | Test: Heat pain intensity via thermodeConditioning: Cold pressor | Test site: Left volar forearm (control area), most painful location on the back (affected area)Conditioning site: Hand and arm | Heat pain administered before and after the cold pressor | Efficient pain inhibitory response was shown by 51.1% of children, while 21.3% had sub-optimal CPM and 27.7% had inefficient CPM |
| Tham, Li et al. (2024) [29] | 77 adolescents with functional abdominal pain | Test: Heat pain threshold via thermodeConditioning: Cold pressor | Test site: Volar surface of the dominant forearmConditioning site: Non-dominant hand and wrist | Heat pain administered during the cold pressor conditioning stimulus | Higher CPM was significantly correlated with lower abdominal pain intensity. When adjusted for age and sex, this was no longer significant, although it was marginally associated with increased pain interference. |
| Tsao, Seidman et al. (2013) [31] | 133 healthy children | Test: Pressure pain intensity via hydraulic piston connected to a computer-activated pumpConditioning: Cold pressor | Test site: Thumbnail of left handConditioning site: Right hand | Pressure pain administered before, during, and after the cold pressor | A significant CPM effect was revealed for the whole sample, although younger children (8–11 years) showed significantly less CPM than adolescents (12–17 years). No significant difference in CPM was found between boys and girls. |
| Uzawa, Takeuch et al. (2024) [65] | 32 healthy young people (14 female, 18 male) | Test: Pressure pain threshold via handheld pressure algometerConditioning: Cold pressor | Test site: Left trapeziusConditioning site: Right hand | Pressure pain stimulus administered before and after the cold pressor | No significant differences in CPM indices were found between males and females. Sympathetic and parasympathetic nervous system activitieswere significantly associated with CPM effects across all participants. Descending pain modulations in women might be more associated with autonomic activities than those in men. |
| Verriotis et al. (2021) [55] | 52 adolescents with neuropathic pain and 14 adolescents with complex region pain syndrome | Test: Pressure pain threshold via algometerConditioning: Cold pressor | Test site: Head of fibulaConditioning site: Contralateral handTypically, pressure test pain was applied on the right knee with the lefthand for conditioning, but if patients reported pain in the left hand orright knee, this was reversed. | Pressure pain administered before, during, and after the cold pressor | CPM was found to be inhibitory in 54% of children and facilitatory in 14% of children. |
| Williams, Heitkemper et al. (2013) [46] | 22 girls with irritable bowel syndrome (IBS) and 21 healthy girls | Test: Heat pain threshold via thermodeConditioning: Cold pressor | Test site: Volar surface of right forearmConditioning site: Left hand | Heat pain administered before and during cold pressor | Girls with IBS did not show significant endogenous pain inhibition. A significant difference between the two groups was observed for endogenous pain inhibition, which was deficient in girls with IBS compared to healthy controls. |
Appendix B. Between-Groups Forest Plots for Individual Analyses Conditioned Pain Modulation Response in Children and Young People with to Healthy Controls
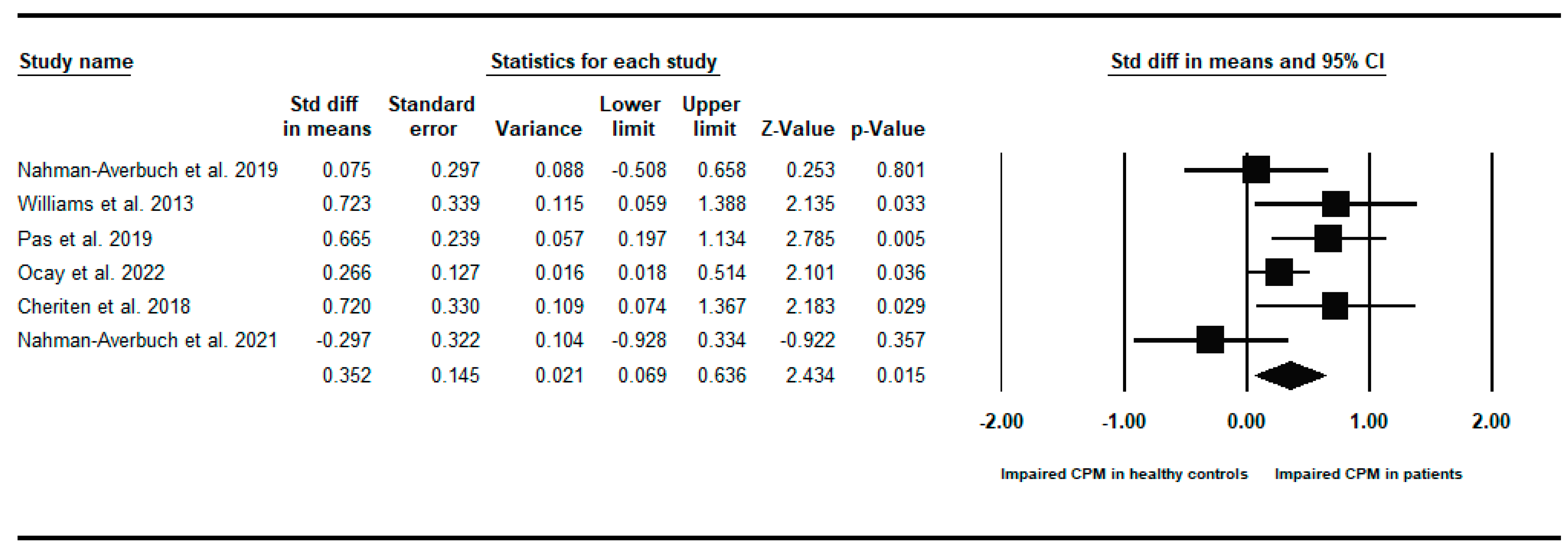
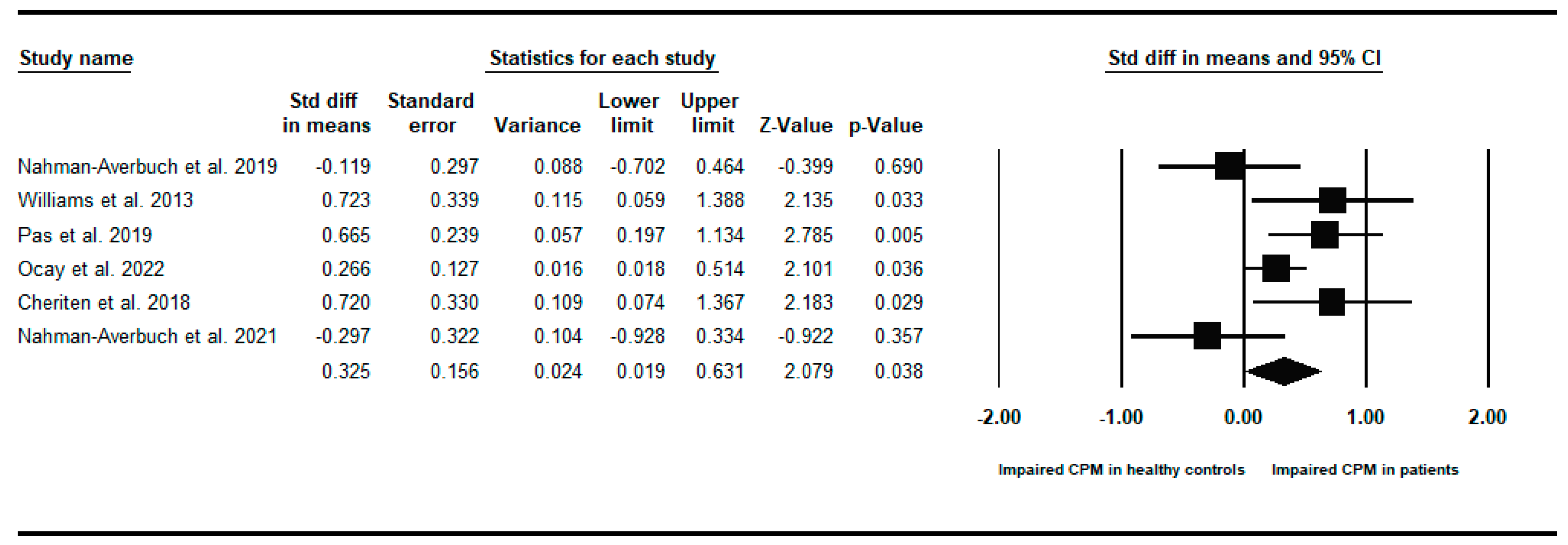
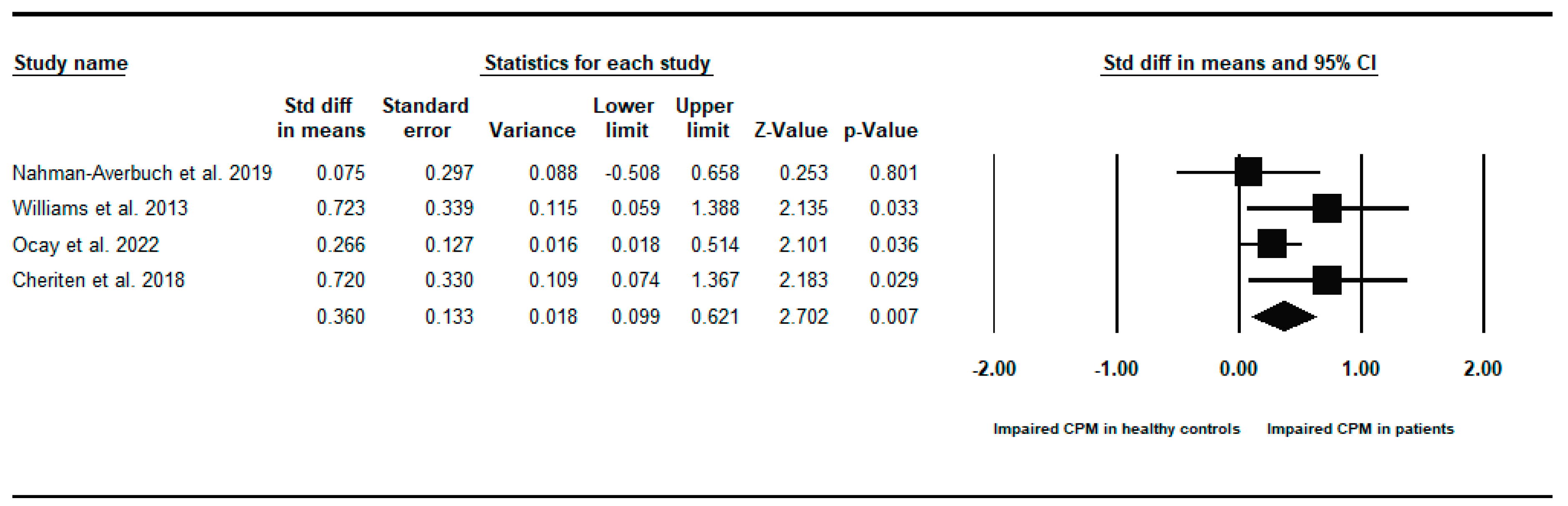

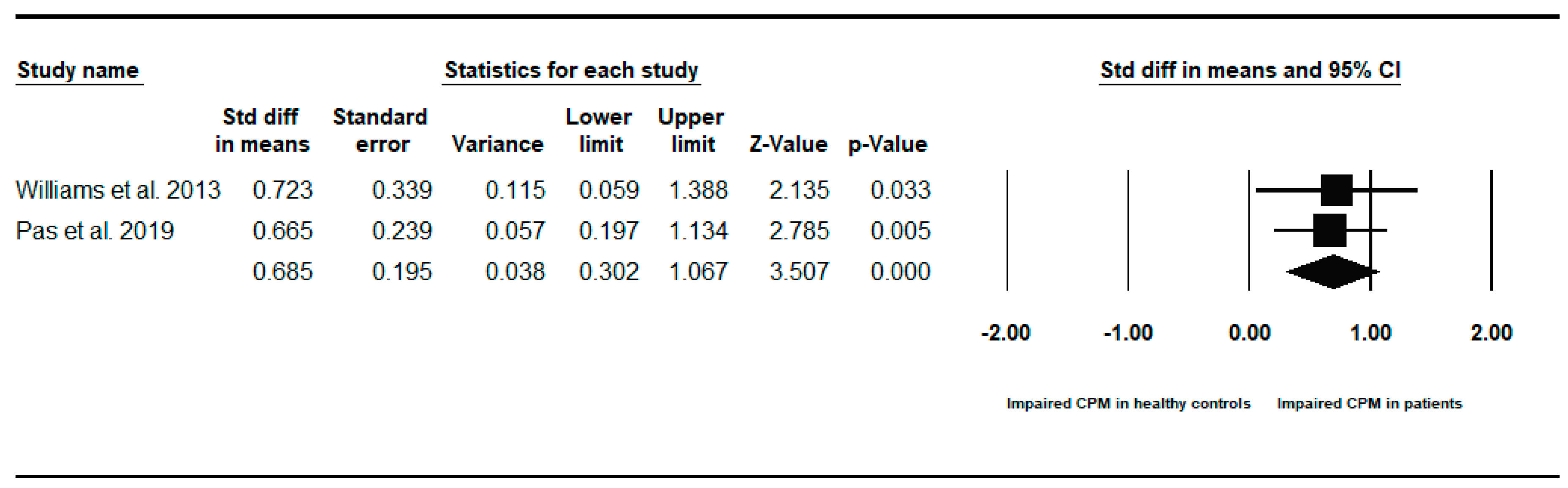

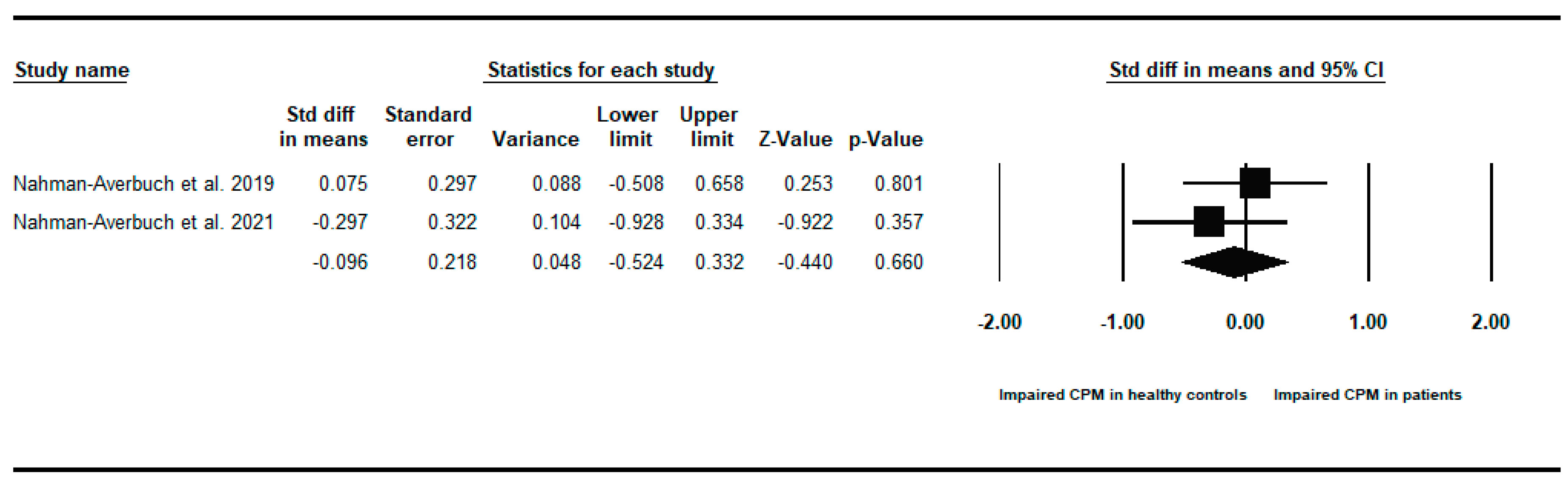
References
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. A classification of chronic pain for ICD-11. Pain 2015, 156, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.T.; Dol, J.; Tutelman, P.R.; Langley, C.L.; Parker, J.A.; Cormier, B.T.; Macfarlane, G.J.; Jones, G.T.; Chapman, D.; Proudfoot, N.; et al. The prevalence of chronic pain in children and adolescents: A systematic review update and meta-analysis. Pain 2024, 165, 2215–2234. [Google Scholar] [CrossRef]
- Murray, C.B.; de la Vega, R.; Murphy, L.K.; Kashikar-Zuck, S.; Palermo, T.M. The prevalence of chronic pain in young adults: A systematic review and meta-analysis. Pain 2022, 163, e972–e984. [Google Scholar] [CrossRef]
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. Pain 2022, 163, e328–e332. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Liossi, C.; Clinch, J. Preventing and Treating Chronic Headache Disorders in Children and Young People. In Managing Pain in Children and Young People: A Clinical Guide; Tywcross, A., Stinson, J., Zempsey, W.T., Jordan, A., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 170–181. [Google Scholar]
- Miró, J.; Roman-Juan, J.; Sánchez-Rodríguez, E.; Solé, E.; Castarlenas, E.; Jensen, M.P. Chronic Pain and High Impact Chronic Pain in Children and Adolescents: A Cross-Sectional Study. J. Pain 2023, 24, 812–823. [Google Scholar] [CrossRef]
- Logan, D.E.; Simons, L.E.; Carpino, E.A. Too sick for school? Parent influences on school functioning among children with chronic pain. Pain 2012, 153, 437–443. [Google Scholar] [CrossRef]
- Ragnarsson, S.; Myleus, A.; Hurtig, A.-K.; Sjöberg, G.; Rosvall, P.-Å.; Petersen, S. Recurrent Pain and Academic Achievement in School-Aged Children: A Systematic Review. J. Sch. Nurs. 2020, 36, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.S.; Palermo, T.M.; Stinson, J.; Handley, S.; Chambers, C.T. Systematic Review of Family Functioning in Families of Children and Adolescents with Chronic Pain. J. Pain 2010, 11, 1027–1038. [Google Scholar] [CrossRef]
- Klein, S.; Chiu, K.; Clinch, J.; Liossi, C. Musculoskeletal Pain in Children and Young People. In Managing Pain in Children and Young People: A Clinical Guide; Tywcross, A., Stinson, J., Zempsey, W.T., Jordan, A., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 147–169. [Google Scholar]
- Tran, S.T.; Mano, K.E.J.; Hainsworth, K.R.; Medrano, G.R.; Khan, K.A.; Weisman, S.J.; Davies, W.H. Distinct Influences of Anxiety and Pain Catastrophizing on Functional Outcomes in Children and Adolescents with Chronic Pain. J. Pediatr. Psychol. 2015, 40, 744–755. [Google Scholar] [CrossRef]
- Vervoort, T.; Goubert, L.; Eccleston, C.; Bijttebier, P.; Crombez, G. Catastrophic Thinking About Pain is Independently Associated with Pain Severity, Disability, and Somatic Complaints in School Children and Children with Chronic Pain. J. Pediatr. Psychol. 2006, 31, 674–683. [Google Scholar] [CrossRef]
- Liossi, C.; Howard, R.F. Pediatric Chronic Pain: Biopsychosocial Assessment and Formulation. Pediatrics 2016, 138, e20160331. [Google Scholar] [CrossRef]
- Liossi, C.; Johnstone, L.; Lilley, S.; Caes, L.; Williams, G.; Schoth, D.E. Interdisciplinary interventions for pediatric chronic pain: A systematic review and meta-analysis. Br. J. Anaesth. 2019, 123, e359–e371. [Google Scholar] [CrossRef]
- Schoth, D.E.; Blankenburg, M.; Wager, J.; Broadbent, P.; Zhang, J.; Zernikow, B.; Liossi, C. Association between quantitative sensory testing and pain or disability in paediatric chronic pain: Protocol for a systematic review and meta-analysis. BMJ Open 2019, 9, e031861. [Google Scholar] [CrossRef] [PubMed]
- Yarnitsky, D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr. Opin. Anaesthesiol. 2010, 23, 611–615. [Google Scholar] [CrossRef]
- Yarnitsky, D.; Arendt-Nielsen, L.; Bouhassira, D.; Edwards, R.R.; Fillingim, R.B.; Granot, M.; Hansson, P.; Lautenbacher, S.; Marchand, S.; Wilder-Smith, O. Recommendations on terminology and practice of psychophysical DNIC testing. Eur. J. Pain 2010, 14, 339. [Google Scholar] [CrossRef]
- Baron, R.; Maier, C.; Attal, N.; Binder, A.; Bouhassira, D.; Cruccu, G.; Finnerup, N.B.; Haanpää, M.; Hansson, P.; Hüllemann, P. Peripheral neuropathic pain: A mechanism-related organizing principle based on sensory profiles. Pain 2017, 158, 261–272. [Google Scholar] [CrossRef]
- Yarnitsky, D.; Granot, M.; Nahman-Averbuch, H.; Khamaisi, M.; Granovsky, Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 2012, 153, 1193–1198. [Google Scholar] [CrossRef]
- Lewis, G.N.; Rice, D.A.; McNair, P.J. Conditioned Pain Modulation in Populations with Chronic Pain: A Systematic Review and Meta-Analysis. J. Pain 2012, 13, 936–944. [Google Scholar] [CrossRef]
- Marcuzzi, A.; Chakiath, R.J.; Siddall, P.J.; Kellow, J.E.; Hush, J.M.; Jones, M.P.; Costa, D.S.; Wrigley, P.J. Conditioned pain modulation (CPM) is reduced in irritable bowel syndrome: A systematic review and meta-analysis of CPM and the role of psychological factors. J. Clin. Gastroenterol. 2019, 53, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Albusoda, A.; Ruffle, J.K.; Friis, K.A.; Gysan, M.R.; Drewes, A.M.; Aziz, Q.; Farmer, A.D. Systematic review with meta-analysis: Conditioned pain modulation in patients with the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Neelapala, Y.R.; Bhagat, M.; Frey-Law, L. Conditioned pain modulation in chronic low back pain: A systematic review of literature. Clin. J. Pain 2020, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Romano, A.; Fernández-Carnero, J.; Beltran-Alacreu, H.; Alguacil-Diego, I.M.; Cuenca-Zaldívar, J.N.; Rodríguez-Lagos, L.; Runge, N.; Mercado, F. Conditioned Pain Modulation and Temporal Summation of Pain in Patients with Traumatic and Non-Specific Neck Pain: A Systematic Review and Meta-Analysis. J. Pain 2023, 25, 312–330. [Google Scholar] [CrossRef] [PubMed]
- Nuwailati, R.; Bobos, P.; Drangsholt, M.; Curatolo, M. Reliability of conditioned pain modulation in healthy individuals and chronic pain patients: A systematic review and meta-analysis. Scand. J. Pain 2022, 22, 262–278. [Google Scholar] [CrossRef]
- Hackett, J.; Naugle, K.E.; Naugle, K.M. The decline of endogenous pain modulation with aging: A meta-analysis of temporal summation and conditioned pain modulation. J. Pain 2020, 21, 514–528. [Google Scholar] [CrossRef]
- Hermans, L.; Van Oosterwijck, J.; Goubert, D.; Goudman, L.; Crombez, G.; Calders, P.; Meeus, M. Inventory of Personal Factors Influencing Conditioned Pain Modulation in Healthy People: A Systematic Literature Review. Pain Pract. 2016, 16, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.; Roviello, G.; Thacker, M.; Willett, M.; Bannister, K.; Smith, T. The association between conditioned pain modulation and psychological factors in people with chronic spinal pain: A systematic review. Br. J. Pain 2024, 18, 314–324. [Google Scholar] [CrossRef]
- Tham, S.W.; Li, R.; Edwards, R.R.; Palermo, T.M. Pain Catastrophizing Moderates the Relationship Between Pain Sensitivity and Clinical Pain in Adolescents with Functional Abdominal Pain. J. Pain 2024, 25, 104549. [Google Scholar] [CrossRef]
- Nahman-Averbuch, H.; Leon, E.; Hunter, B.M.; Ding, L.; Hershey, A.D.; Powers, S.W.; King, C.D.; Coghill, R.C. Increased pain sensitivity but normal pain modulation in adolescents with migraine. Pain 2019, 160, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Tsao, J.C.; Seidman, L.C.; Evans, S.; Lung, K.C.; Zeltzer, L.K.; Naliboff, B.D. Conditioned Pain Modulation in Children and Adolescents: Effects of Sex and Age. J. Pain 2013, 14, 558–567. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions 6.4 (Updated August 2023); The Cochrane Collaboration: London, UK, 2023. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions 6.3; The Cochrane Collaboration: London, UK, 2019. [Google Scholar]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H.R. Comprehensive Meta-Analysis Version 3; Biostat: Englewood, NJ, USA, 2013. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.; Rothstein, H.R. Introduction to Meta-Analysis; Wiley Online Library: Cornwell, UK, 2009. [Google Scholar]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2008. [Google Scholar]
- Arribas-Romano, A.; Fernández-Carnero, J.; González-Zamorano, Y.; Rodríguez-Lagos, L.; Gurdiel-Álvarez, F.; Molina-Álvarez, M.; Tejera, D.M.; Mercado, F. Conditioned pain modulation and psychological factors in young adults with recurrent or chronic neck pain. Pain Pract. 2023, 24, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Brandão, M.; Talih, M.; Holden, S.; Fernandes, F.; Graven-Nielsen, T.; Lucas, R. Pain history and experimental pressure pain responses in adolescents: Results from a population-based birth cohort. Eur. J. Pain 2024, 28, 70–82. [Google Scholar] [CrossRef]
- Chrétien, R.; Lavoie, S.; Chalaye, P.; de Vette, E.; Counil, F.-P.; Dallaire, F.; Lafrenaye, S. Reduced endogenous pain inhibition in adolescent girls with chronic pain. Scand. J. Pain 2018, 18, 711–717. [Google Scholar] [CrossRef]
- Tsao, J.; Evans, S.; Seidman, L.; Lung, K.C.; Zeltzer, L. Sex differences in the relationship between maternal fear of pain and children's conditioned pain modulation. J. Pain Res. 2013, 6, 231–238. [Google Scholar] [CrossRef]
- Ferland, C.E.; Teles, A.R.; Ingelmo, P.; Saran, N.; Marchand, S.; Ouellet, J.A. Blood monoamines as potential biomarkers for conditioned pain modulation efficacy: An exploratory study in paediatrics. Eur. J. Pain 2019, 23, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Goffaux, P.; Lafrenaye, S.; Morin, M.; Patural, H.; Demers, G.; Marchand, S. Preterm births: Can neonatal pain alter the development of endogenous gating systems? Eur. J. Pain. 2008, 12, 945–951. [Google Scholar] [CrossRef]
- Harper, D.; Hollins, M. Conditioned pain modulation dampens the thermal grill illusion. Eur. J. Pain 2017, 21, 1591–1601. [Google Scholar] [CrossRef]
- Hoehn, J.L.; Dahlquist, L.M.; Zeroth, J.A. Conditioned Pain Modulation in Children: The Effects of Painful and Nonpainful Conditioning Stimuli. J. Pain 2022, 23, 1208–1219. [Google Scholar] [CrossRef]
- Holden, S.; Rathleff, M.S.; Thorborg, K.; Holmich, P.; Graven-Nielsen, T. Mechanistic pain profiling in young adolescents with patellofemoral pain before and after treatment: A prospective cohort study. Pain 2020, 161, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Holden, S.; Straszek, C.L.; Rathleff, M.S.; Petersen, K.K.; Roos, E.M.; Graven-Nielsen, T. Young females with long-standing patellofemoral pain display impaired conditioned pain modulation, increased temporal summation of pain, and widespread hyperalgesia. Pain 2018, 159, 2530–2537. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.V.; Werner, M.U.; Michelsen, J.S.; Tierp-Wong, C.N.E. Assessment of somatosensory profiles by quantitative sensory testing in children and adolescents with and without cerebral palsy and chronic pain. Eur. J. Paediatr. Neurol. 2024, 51, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Leone, C.; Galosi, S.; Mollica, C.; Fortunato, M.; Possidente, C.; Milone, V.; Misuraca, S.; Berillo, L.; Truini, A.; Cruccu, G.; et al. Dissecting pain processing in adolescents with Non-Suicidal Self Injury: Could suicide risk lurk among the electrodes? Eur. J. Pain 2021, 25, 1815–1828. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Talih, M.; Soares, S.; Fraga, S. Bullying Involvement and Physical Pain Between Ages 10 and 13 Years: Reported History and Quantitative Sensory Testing in a Population-Based Cohort. J. Pain 2023, 25, 1012–1023. [Google Scholar] [CrossRef]
- Morris, M.C.; Walker, L.; Bruehl, S.; Hellman, N.; Sherman, A.L.; Rao, U. Race Effects on Conditioned Pain Modulation in Youth. J. Pain 2015, 16, 873–880. [Google Scholar] [CrossRef]
- Morris, M.C.; Walker, L.S.; Bruehl, S.; Stone, A.L.; Mielock, A.S.; Rao, U. Impaired conditioned pain modulation in youth with functional abdominal pain. Pain 2016, 157, 2375–2381. [Google Scholar] [CrossRef]
- Morris, M.C.; Bruehl, S.; Stone, A.L.; Garber, J.; Smith, C.; Palermo, T.M.; Walker, L.S. Does Quantitative Sensory Testing Improve Prediction of Chronic Pain Trajectories? A Longitudinal Study of Youth with Functional Abdominal Pain Participating in a Randomized Controlled Trial of Cognitive Behavioral Treatment. Clin. J. Pain 2021, 37, 648–656. [Google Scholar] [CrossRef]
- Nahman-Averbuch, H.; Schneider, V.J.I.; Chamberlin, L.A.; Van Diest, A.M.K.; Peugh, J.L.; Lee, G.R.; Radhakrishnan, R.; Hershey, A.D.; Powers, S.W.; Coghill, R.C.; et al. Identification of neural and psychophysical predictors of headache reduction after cognitive behavioral therapy in adolescents with migraine. Pain 2021, 162, 372–381. [Google Scholar] [CrossRef]
- Nahman-Averbuch, H.; Thomas, P.L.; Schneider, V.J.; Chamberlin, L.A.; Peugh, J.L.; Hershey, A.D.; Powers, S.W.; Coghill, R.C.; King, C.D. Spatial aspects of pain modulation are not disrupted in adolescents with migraine. Headache 2020, 61, 485–492. [Google Scholar] [CrossRef]
- Ocay, D.D.; Larche, C.L.; Betinjane, N.; Jolicoeur, A.; Beaulieu, M.J.; Saran, N.; A Ouellet, J.; Ingelmo, P.M.; E Ferland, C. Phenotyping Chronic Musculoskeletal Pain in Male and Female Adolescents: Psychosocial Profiles, Somatosensory Profiles and Pain Modulatory Profiles. J. Pain Res. 2022, 15, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Ocay, D.D.; Ye, D.-L.; Larche, C.L.; Potvin, S.; Marchand, S.; Ferland, C.E. Clusters of facilitatory and inhibitory conditioned pain modulation responses in a large sample of children, adolescents, and young adults with chronic pain. PAIN Rep. 2022, 7, e1032. [Google Scholar] [CrossRef] [PubMed]
- Ocay, D.D.; Loewen, A.; Premachandran, S.; Ingelmo, P.M.; Saran, N.; Ouellet, J.A.; Ferland, C.E. Psychosocial and psychophysical assessment in paediatric patients and young adults with chronic back pain: A cluster analysis. Eur. J. Pain 2022, 26, 855–872. [Google Scholar] [CrossRef] [PubMed]
- Pas, R.; Rheel, E.; Van Oosterwijck, S.; Leysen, L.; Van De Vijver, E.; Nijs, J.; Ickmans, K.; Meeus, M. Endogenous pain modulation in children with functional abdominal pain disorders. Pain 2019, 160, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Ray, L.N.; O'Connor, P.J. Session of yoga, with and without slow (Ujjayi) breathing, reduces anxiety; no change on acute pain sensitivity and endogenous pain modulation. Explore 2023, 19, 362–370. [Google Scholar] [CrossRef]
- Schubert-Hjalmarsson, E.; Fasth, A.; Ickmans, K.; Mårdbrink, E.-L.; Söderpalm, A.-C.; Lundberg, M. Central sensitization in adolescents with hypermobility spectrum disorder or hypermobile Ehlers-Danlos syndrome—A feasibility study. Pilot Feasibility Stud. 2023, 9, 97. [Google Scholar] [CrossRef]
- Stolzman, S.; Bement, M.H. Lean mass predicts conditioned pain modulation in adolescents across weight status. Eur. J. Pain 2016, 20, 967–976. [Google Scholar] [CrossRef]
- Teles, A.R.; Ocay, D.D.; Bin Shebreen, A.; Tice, A.; Saran, N.; Ouellet, J.A.; Ferland, C.E. Evidence of impaired pain modulation in adolescents with idiopathic scoliosis and chronic back pain. Spine J. 2019, 19, 677–686. [Google Scholar] [CrossRef]
- Uzawa, H.; Takeuch, S.; Nishida, Y. Sex differences in conditioned pain modulation effects and its associations with autonomic nervous system activities in healthy, younger individuals: A pilot study. PAIN Rep. 2024, 9, e1123. [Google Scholar] [CrossRef]
- Verriotis, M.; Peters, J.; Sorger, C.; Walker, S.M. Phenotyping peripheral neuropathic pain in male and female adolescents: Pain descriptors, somatosensory profiles, conditioned pain modulation, and child–parent reported disability. Pain 2021, 162, 1732–1748. [Google Scholar] [CrossRef]
- Williams, A.E.; Heitkemper, M.; Self, M.M.; Czyzewski, D.I.; Shulman, R.J. Endogenous Inhibition of Somatic Pain Is Impaired in Girls with Irritable Bowel Syndrome Compared with Healthy Girls. J. Pain 2013, 14, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Teepker, M.; Kunz, M.; Peters, M.; Kundermann, B.; Schepelmann, K.; Lautenbacher, S. Endogenous pain inhibition during menstrual cycle in migraine. Eur. J. Pain 2014, 18, 989–998. [Google Scholar] [CrossRef]
- Perrotta, A.; Serrao, M.; Sandrini, G.; Burstein, R.; Sances, G.; Rossi, P.; Bartolo, M.; Pierelli, F.; Nappi, G. Sensitisation of spinal cord pain processing in medication overuse headache involves supraspinal pain control. Cephalalgia 2010, 30, 272–284. [Google Scholar] [CrossRef]
- Sandrini, G.; Rossi, P.; Milanov, I.; Serrao, M.; Cecchini, A.; Nappi, G. Abnormal Modulatory Influence of Diffuse Noxious Inhibitory Controls in Migraine and Chronic Tension-Type Headache Patients. Cephalalgia 2006, 26, 782–789. [Google Scholar] [CrossRef]
- Nahman-Averbuch, H.; Granovsky, Y.; Coghill, R.C.; Yarnitsky, D.; Sprecher, E.; Weissman-Fogel, I. Waning of “Conditioned Pain Modulation”: A Novel Expression of Subtle Pronociception in Migraine. Headache: J. Head Face Pain 2013, 53, 1104–1115. [Google Scholar] [CrossRef]
- de Tommaso, M.; Difruscolo, O.; Sardaro, M.; Libro, G.; Pecoraro, C.; Serpino, C.; Lamberti, P.; Livrea, P. Effects of remote cutaneous pain on trigeminal laser-evoked potentials in migraine patients. J. Headache Pain 2007, 8, 167–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Williams, A.E.; Miller, M.M.; Bartley, E.J.; McCabe, K.M.; Kerr, K.L.; Rhudy, J.L. Impairment of Inhibition of Trigeminal Nociception via Conditioned Pain Modulation in Persons with Migraine Headaches. Pain Med. 2019, 20, 1600–1610. [Google Scholar] [CrossRef] [PubMed]
- Lemley, K.J.; Hunter, S.K.; Bement, M.K.H. Conditioned Pain Modulation Predicts Exercise-Induced Hypoalgesia in Healthy Adults. Med. Sci. Sports Exerc. 2015, 47, 176–184. [Google Scholar] [CrossRef]
- Vaegter, H.B.; Handberg, G.; Graven-Nielsen, T. Hypoalgesia After Exercise and the Cold Pressor Test is Reduced in Chronic Musculoskeletal Pain Patients with High Pain Sensitivity. Clin. J. Pain 2016, 32, 58–69. [Google Scholar] [CrossRef]
- Clinch, J.; Eccleston, C. Chronic musculoskeletal pain in children: Assessment and management. Rheumatology 2009, 48, 466–474. [Google Scholar] [CrossRef]
- Rajapakse, D.; Liossi, C.; Howard, R.F. Presentation and management of chronic pain. Arch. Dis. Child. 2014, 99, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Blankenburg, M.; Boekens, H.; Hechler, T.; Maier, C.; Krumova, E.; Scherens, A.; Magerl, W.; Aksu, F.; Zernikow, B. Reference values for quantitative sensory testing in children and adolescents: Developmental and gender differences of somatosensory perception. Pain 2010, 149, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Birnie, K.A.; Caes, L.; Wilson, A.C.; E Williams, S.; Chambers, C.T. A Practical Guide and Perspectives on the Use of Experimental Pain Modalities with Children and Adolescents. Pain Manag. 2014, 4, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; McCartin, M.; Liu, W.; Zhang, Q.; Kenefati, G.; Chen, Z.S.; Wang, J. Temporal pain processing in the primary somatosensory cortex and anterior cingulate cortex. Mol. Brain 2023, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, R.; Fauchon, C.; Kim, J.A.; Firouzian, S.; Osborne, N.R.; Besik, A.; Mills, E.P.; Bhatia, A.; Davis, K.D. The Potential Clinical Utility of Pressure-Based vs. Heat-Based Paradigms to Measure Conditioned Pain Modulation in Healthy Individuals and Those with Chronic Pain. Front. Pain Res. 2021, 2, 784362. [Google Scholar] [CrossRef]
- Zangaro, G.A. Importance of Reporting Psychometric Properties of Instruments Used in Nursing Research. West. J. Nurs. Res. 2019, 41, 1548–1550. [Google Scholar] [CrossRef]
- American Psychological Association. Publication Manual of the American Psychological Association 2020: The Official Guide to APA Style, 7th ed.; American Psychological Association: Washington, DC, USA, 2020. [Google Scholar]
- Toepoel, V.; Schonlau, M. Dealing with nonresponse: Strategies to increase participation and methods for postsurvey adjustments. Math. Popul. Stud. 2017, 24, 79–83. [Google Scholar] [CrossRef]
- Schoth, D.E.; Blankenburg, M.; Wager, J.; Zhang, J.; Broadbent, P.; Radhakrishnan, K.; van Jole, O.; Lyle, G.L.; Laycock, H.; Zernikow, B.; et al. Quantitative sensory testing in paediatric patients with chronic pain: A systematic review and meta-analysis. Br. J. Anaesth. 2022, 129, e94–e97. [Google Scholar] [CrossRef]
- Thurston, K.L.; Zhang, S.J.; Wilbanks, B.A.; Billings, R.; Aroke, E.N. A Systematic Review of Race, Sex, and Socioeconomic Status Differences in Postoperative Pain and Pain Management. J. PeriAnesthesia Nurs. 2022, 38, 504–515. [Google Scholar] [CrossRef]
- Ruau, D.; Liu, L.Y.; Clark, J.D.; Angst, M.S.; Butte, A.J. Sex Differences in Reported Pain Across 11,000 Patients Captured in Electronic Medical Records. J. Pain 2012, 13, 228–234. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Peters, J.H.; Heesen, M.; Scheel, J.; Kunz, M. Age changes in pain perception: A systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci. Biobehav. Rev. 2017, 75, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.L., 3rd; King, C.D.; Wong, F.; Fillingim, R.B.; Mauderli, A.P. Lack of endogenous modulation and reduced decay of prolonged heat pain in older adults. Pain 2010, 150, 153–160. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Wodehouse, T. Conditioned pain modulation—A comprehensive review. Neurophysiol. Clin. 2021, 51, 197–208. [Google Scholar] [CrossRef]
- Fernandes, C.; Pidal-Miranda, M.; Samartin-Veiga, N.; Carrillo-De-La-Peña, M.T. Conditioned pain modulation as a biomarker of chronic pain: A systematic review of its concurrent validity. Pain 2019, 160, 2679–2690. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liossi, C.; Laycock, H.; Radhakrishnan, K.; Hussain, Z.; Schoth, D.E. A Systematic Review and Meta-Analysis of Conditioned Pain Modulation in Children and Young People with Chronic Pain. Children 2024, 11, 1367. https://doi.org/10.3390/children11111367
Liossi C, Laycock H, Radhakrishnan K, Hussain Z, Schoth DE. A Systematic Review and Meta-Analysis of Conditioned Pain Modulation in Children and Young People with Chronic Pain. Children. 2024; 11(11):1367. https://doi.org/10.3390/children11111367
Chicago/Turabian StyleLiossi, Christina, Helen Laycock, Kanmani Radhakrishnan, Zara Hussain, and Daniel Eric Schoth. 2024. "A Systematic Review and Meta-Analysis of Conditioned Pain Modulation in Children and Young People with Chronic Pain" Children 11, no. 11: 1367. https://doi.org/10.3390/children11111367
APA StyleLiossi, C., Laycock, H., Radhakrishnan, K., Hussain, Z., & Schoth, D. E. (2024). A Systematic Review and Meta-Analysis of Conditioned Pain Modulation in Children and Young People with Chronic Pain. Children, 11(11), 1367. https://doi.org/10.3390/children11111367






