The Reliability of Salivary Cortisol Compared to Serum Cortisol for Diagnosing Adrenal Insufficiency with the Gold Standard ACTH Stimulation Test in Children
Abstract
:1. Introduction
2. Materials and Methods
3. Results
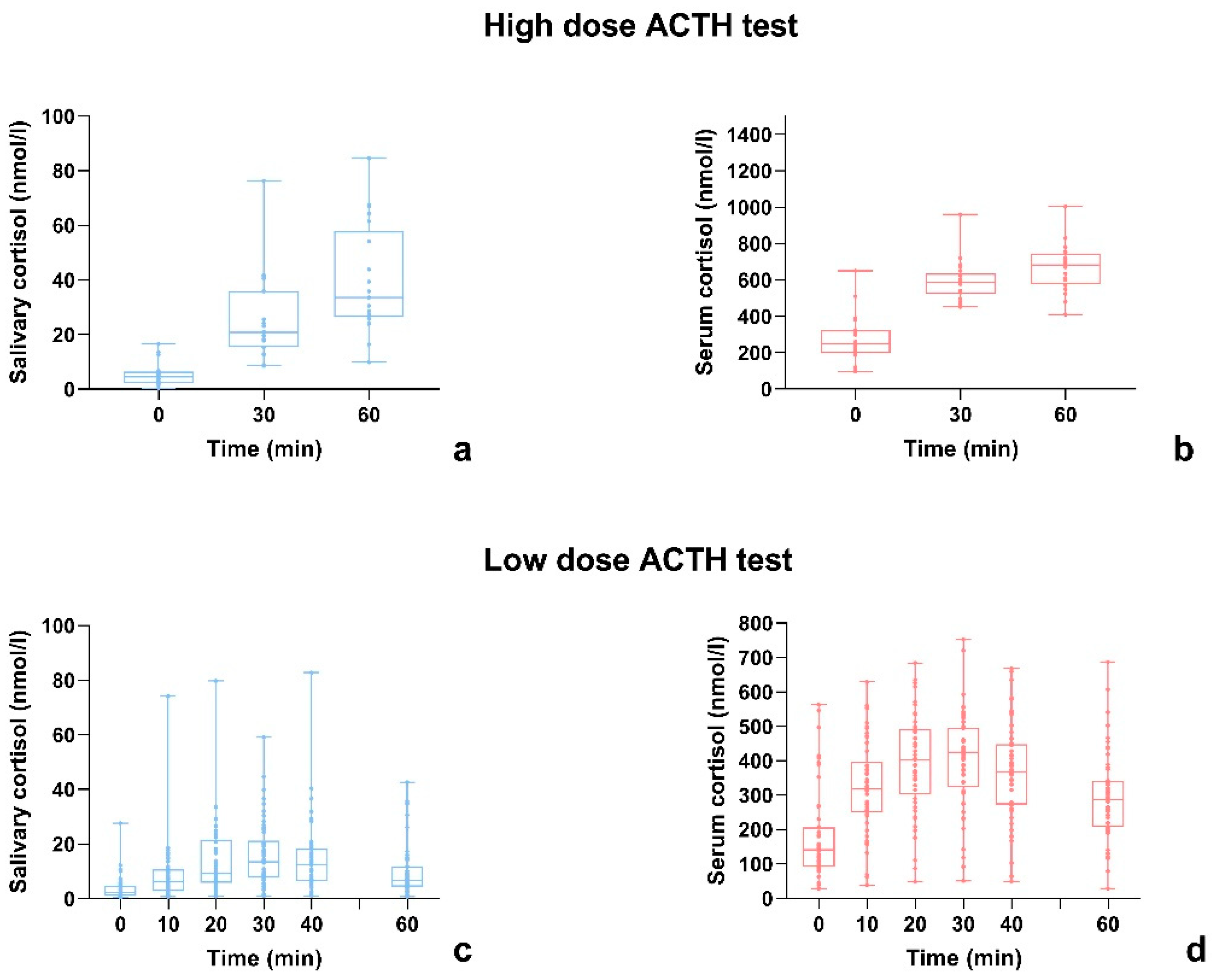
3.1. High-Dose ACTH Stimulation Test
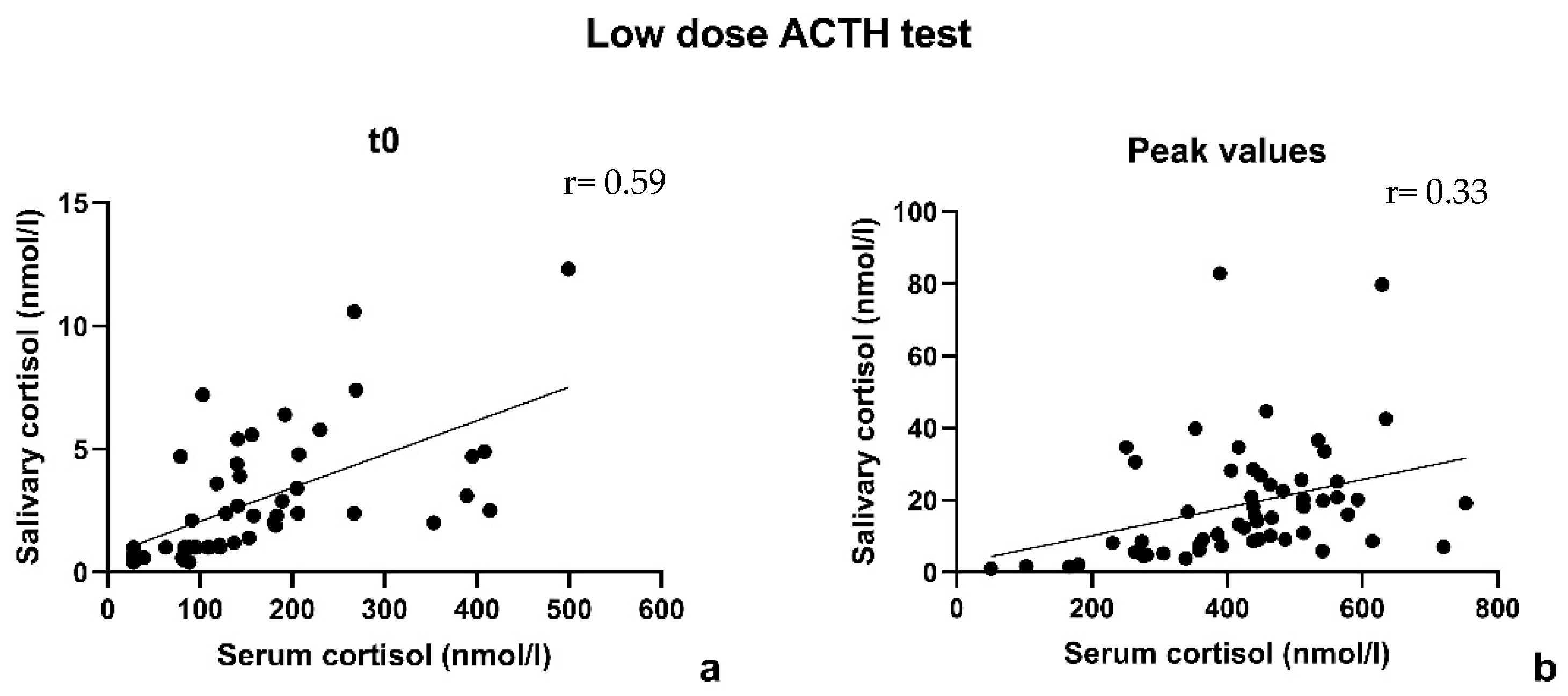
3.2. Low-Dose ACTH Stimulation Test
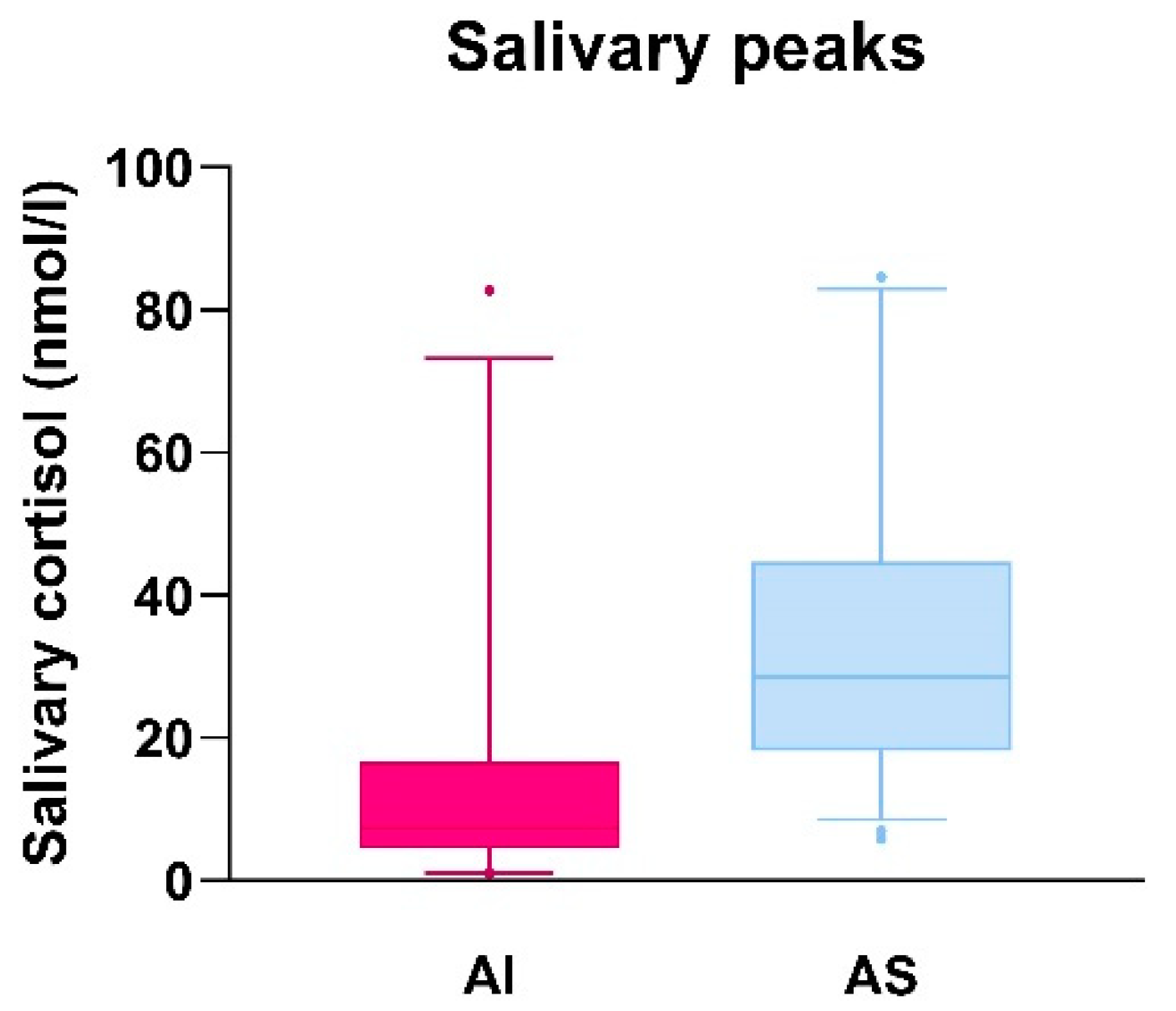
3.3. Comparison of Salivary Cortisol Peak Values in Adrenal Insufficient and Adrenal Sufficient Patients and Evaluation of Sensitivity and Specificity of the Derived Salivary Cortisol Cut-Off
3.4. Serum and Salivary Cortisol Time Response to ACTH Administration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Husebye, E.S.; Pearce, S.H.; Krone, N.P.; Kämpe, O. Adrenal insufficiency. Lancet 2021, 397, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Chabre, O.; Goichot, B.; Zenaty, D.; Bertherat, J. Group 1. Epidemiology of primary and secondary adrenal insufficiency: Prevalence and incidence, acute adrenal insufficiency, long-term morbidity and mortality. Ann. Endocrinol. 2017, 78, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Betterle, C.; Presotto, F.; Furmaniak, J. Epidemiology, pathogenesis, and diagnosis of Addison’s disease in adults. J. Endocrinol. Investig. 2019, 42, 1407–1433. [Google Scholar] [CrossRef]
- Wijaya, M.; Huamei, M.; Jun, Z.; Du, M.; Li, Y.; Chen, Q.; Chen, H.; Song, G. Etiology of primary adrenal insufficiency in children: A 29-year single-center experience. J. Pediatr. Endocrinol. Metab. 2019, 32, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Eyal, O.; Levin, Y.; Oren, A.; Zung, A.; Rachmiel, M.; Landau, Z.; Schachter-Davidov, A.; Segev-Becker, A.; Weintrob, N. Adrenal crises in children with adrenal insufficiency: Epidemiology and risk factors. Eur. J. Pediatr. 2019, 178, 731–738. [Google Scholar] [CrossRef]
- Chung, S.; Son, G.H.; Kim, K. Circadian rhythm of adrenal glucocorticoid: Its regulation and clinical implications. Biochim. Biophys. Acta—Mol. Basis Dis. 2011, 1812, 581–591. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Allolio, B.; Arlt, W.; Barthel, A.; Don-Wauchope, A.; Hammer, G.D.; Husebye, E.S.; Merke, D.P.; Murad, M.H.; Stratakis, C.A.; et al. Diagnosis and treatment of primary adrenal insufficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2016, 101, 364–389. [Google Scholar] [CrossRef]
- El-Farhan, N.; Pickett, A.; Ducroq, D.; Bailey, C.; Mitchem, K.; Morgan, N.; Armston, A.; Jones, L.; Evans, C.; Rees, D.A. Method-specific serum cortisol responses to the adrenocorticotrophin test: Comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clin. Endocrinol. 2013, 78, 673–680. [Google Scholar] [CrossRef]
- Ospina, N.S.; Nofal AAl Bancos, I.; Javed, A.; Benkhadra, K.; Kapoor, E.; Lteif, A.N.; Natt, N.; Murad, M.H. ACTH stimulation tests for the diagnosis of adrenal insufficiency: Systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2016, 101, 427–434. [Google Scholar] [CrossRef]
- El-Farhan, N.; Rees, D.A.; Evans, C. Measuring cortisol in serum, urine and saliva—Are our assays good enough? Ann. Clin. Biochem. 2017, 54, 308–322. [Google Scholar] [CrossRef]
- Bastin, P.; Maiter, D.; Gruson, D. Salivary cortisol testing: Preanalytic and analytic aspects. Ann. Biol. Clin. 2018, 76, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Kosák, M.; Hána, V.; Hill, M.; Šimůnková, K.; Lacinová, Z.; Kršek, M.; Marek, J. Serum cortisol seems to be a more appropriate marker for adrenocortical reserve evaluation in ACTH test in comparison to salivary cortisol. Physiol. Res. 2014, 63, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, J.H.; Hong, A.R.; Park, K.S.; Kim, S.W.; Shin, C.S.; Kim, S.Y. Stimulated Salivary Cortisol as a Noninvasive Diagnostic Tool for Adrenal Insufficiency. Endocrinol. Metab. 2020, 35, 628–635. [Google Scholar] [CrossRef]
- Langelaan, M.L.P.; Kisters, J.M.H.; Oosterwerff, M.M.; Boer, A.K. Salivary cortisol in the diagnosis of adrenal insufficiency: Cost efficient and patient friendly. Endocr. Connect. 2018, 7, 560–566. [Google Scholar] [CrossRef]
- Chao, C.S.; Shi, R.Z.; Kumar, R.B.; Aye, T. Salivary cortisol levels by tandem mass spectrometry during high dose ACTH stimulation test for adrenal insufficiency in children. Endocrine 2020, 67, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Van der Kamp, H.J.; Kamphuis, S.S.M.; Merkus, P.J.F.M.; Schroor, E.J. Richtlijn Afbouwen Glucocorticoïden bij Kinderen; Nederlandse Vereniging voor Kindergeneeskunde: Utrecht, The Netherlands, 2019. [Google Scholar]
- Cornes, M.P.; Ashby, H.L.; Khalid, Y.; Buch, H.N.; Ford, C.; Gama, R. Salivary cortisol and cortisone responses to tetracosactrin (synacthen). Ann. Clin. Biochem. 2015, 52, 606–610. [Google Scholar] [CrossRef]
- Deutschbein, T.; Unger, N.; Mann, K.; Petersenn, S. Diagnosis of secondary adrenal insufficiency in patients with hypothalamic-pituitary disease: Comparison between serum and salivary cortisol during the high-dose short synacthen test. Eur. J. Endocrinol. 2009, 160, 9–16. [Google Scholar] [CrossRef]
- Šimůnková, K.; Hampl, R.; Hill, M.; Doucha, J.; Stárka, L.; Vondra, K. Salivary cortisol in low dose (1 μg) ACTH test in healthy women: Comparison with serum cortisol. Physiol. Res. 2007, 56, 449–453. [Google Scholar] [CrossRef]
- Marcus-Perlman, Y.; Tordjman, K.; Greenman, Y.; Limor, R.; Shenkerman, G.; Osher, E.; Stern, N. Low-dose ACTH (1 μg) salivary test: A potential alternative to the classical blood test. Clin. Endocrinol. 2006, 64, 215–218. [Google Scholar] [CrossRef]
- Schindhelm, R.K.; Van De Leur, J.J.C.M.; Rondeel, J.M.M. Salivary cortisol as an alternative for serum cortisol in the low-dose adrenocorticotropic hormone stimulation test? J. Endocrinol. Investig. 2010, 33, 92–95. [Google Scholar] [CrossRef]
- Vaiani, E.; Lazzati, J.M.; Ramirez, P.; Costanzo, M.; Gil, S.; Dratler, G.; Zaidman, V.; Chaler, E.; Belgorosky, A. The Low-Dose ACTH Test: Usefulness of Combined Analysis of Serum and Salivary Maximum Cortisol Response in Pediatrics. J. Clin. Endocrinol. Metab. 2019, 104, 4323–4330. [Google Scholar] [CrossRef] [PubMed]
- Elder, C.J.; Harrison, R.F.; Cross, A.S.; Vilela, R.; Keevil, B.G.; Wright, N.P.; Ross, R.J. Use of salivary cortisol and cortisone in the high- and low-dose synacthen test. Clin. Endocrinol. 2018, 88, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.; Midgley, P.; Lone, N.; Michael Wallace, A.; Nicol, M.; Smith, J.; McIntosh, N. A re-investigation of saliva collection procedures that highlights the risk of potential positive interference in cortisol immunoassay. Steroids 2009, 74, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Poll, E.M.; Kreitschmann-Andermahr, I.; Langejuergen, Y.; Stanzel, S.; Gilsbach, J.M.; Gressner, A.; Yagmur, E. Saliva collection method affects predictability of serum cortisol. Clin. Chim. Acta 2007, 382, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.; Leitch, M.M.; Massey, A.E.; McAllister-Williams, R.H.; Young, A.H. Assessing cortisol and dehydroepiandrosterone (DHEA) in saliva: Effects of collection method. J. Psychopharmacol. 2006, 20, 643–649. [Google Scholar] [CrossRef]
- Harmon, A.G.; Hibel, L.C.; Rumyantseva, O.; Granger, D.A. Measuring salivary cortisol in studies of child development: Watch out—What goes in may not come out of saliva collection devices. Dev. Psychobiol. 2007, 49, 495–500. [Google Scholar] [CrossRef]
- Gill, H.; Barrowman, N.; Webster, R.; Ahmet, A. Evaluating the Low-Dose ACTH Stimulation Test in Children: Ideal Times for Cortisol Measurement. J. Clin. Endocrinol. Metab. 2019, 104, 4587–4593. [Google Scholar] [CrossRef]

| HDT (n = 24) | LDT (n = 56) | |
|---|---|---|
| Adrenal insufficient tests | 0 | 23 |
| Peak time | 60 min | 20–40 min |
| R coefficient at t0 | 0.80 | 0.59 |
| R coefficient at peak | 0.75 | 0.33 |
| Sensitivity * | NA | 73.9% |
| Specificity * | NA | 69.6% |
| Positive predictive value * | NA | 62.9% |
| Negative predictive value * | NA | 79.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciancia, S.; van den Berg, S.A.A.; van den Akker, E.L.T. The Reliability of Salivary Cortisol Compared to Serum Cortisol for Diagnosing Adrenal Insufficiency with the Gold Standard ACTH Stimulation Test in Children. Children 2023, 10, 1569. https://doi.org/10.3390/children10091569
Ciancia S, van den Berg SAA, van den Akker ELT. The Reliability of Salivary Cortisol Compared to Serum Cortisol for Diagnosing Adrenal Insufficiency with the Gold Standard ACTH Stimulation Test in Children. Children. 2023; 10(9):1569. https://doi.org/10.3390/children10091569
Chicago/Turabian StyleCiancia, Silvia, Sjoerd A. A. van den Berg, and Erica L. T. van den Akker. 2023. "The Reliability of Salivary Cortisol Compared to Serum Cortisol for Diagnosing Adrenal Insufficiency with the Gold Standard ACTH Stimulation Test in Children" Children 10, no. 9: 1569. https://doi.org/10.3390/children10091569
APA StyleCiancia, S., van den Berg, S. A. A., & van den Akker, E. L. T. (2023). The Reliability of Salivary Cortisol Compared to Serum Cortisol for Diagnosing Adrenal Insufficiency with the Gold Standard ACTH Stimulation Test in Children. Children, 10(9), 1569. https://doi.org/10.3390/children10091569







