Feasibility, Safety and Reliability of Surgeon-Directed Transcranial Motor Evoked Potentials Monitoring in Scoliosis Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Population Criteria
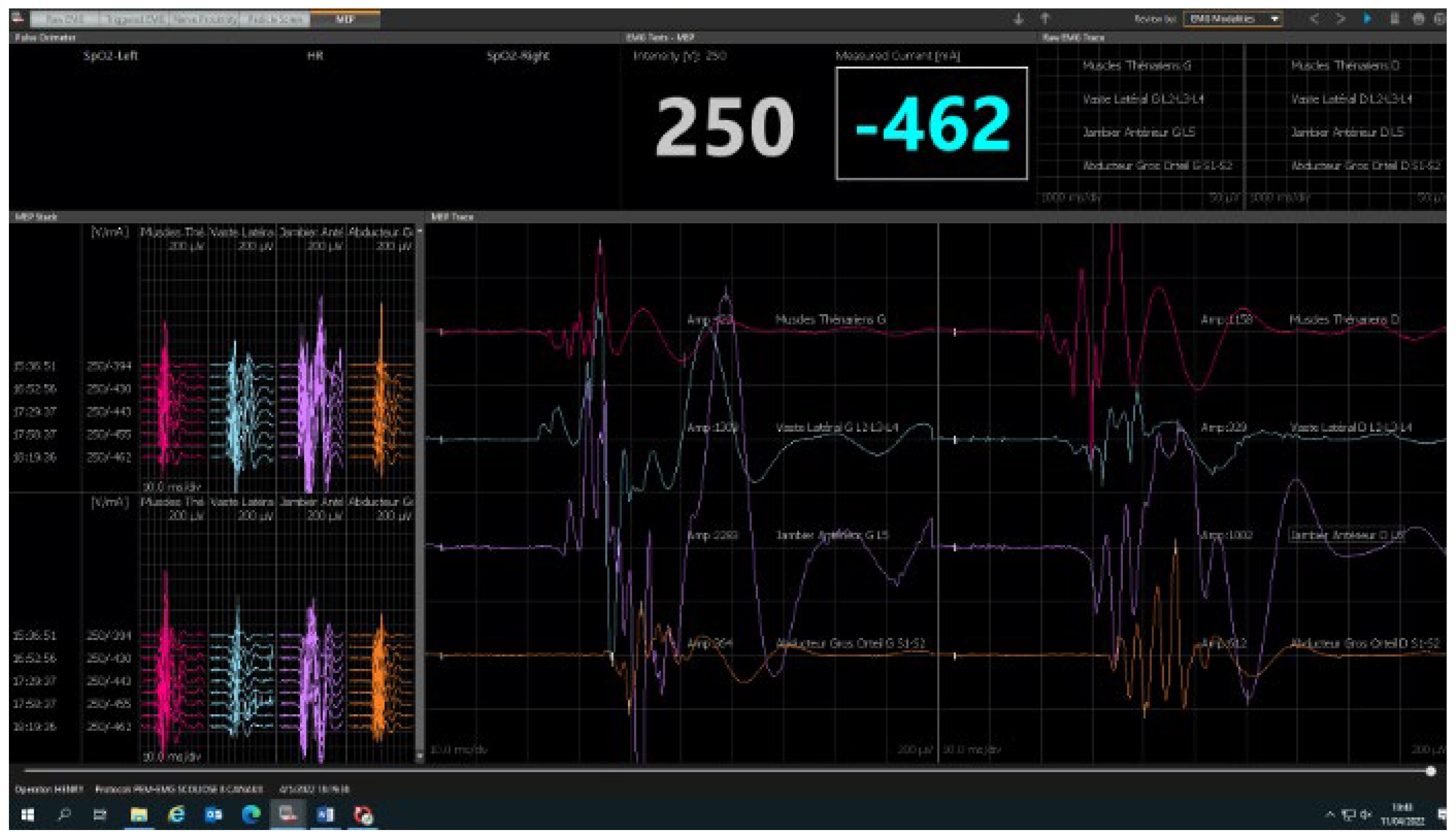
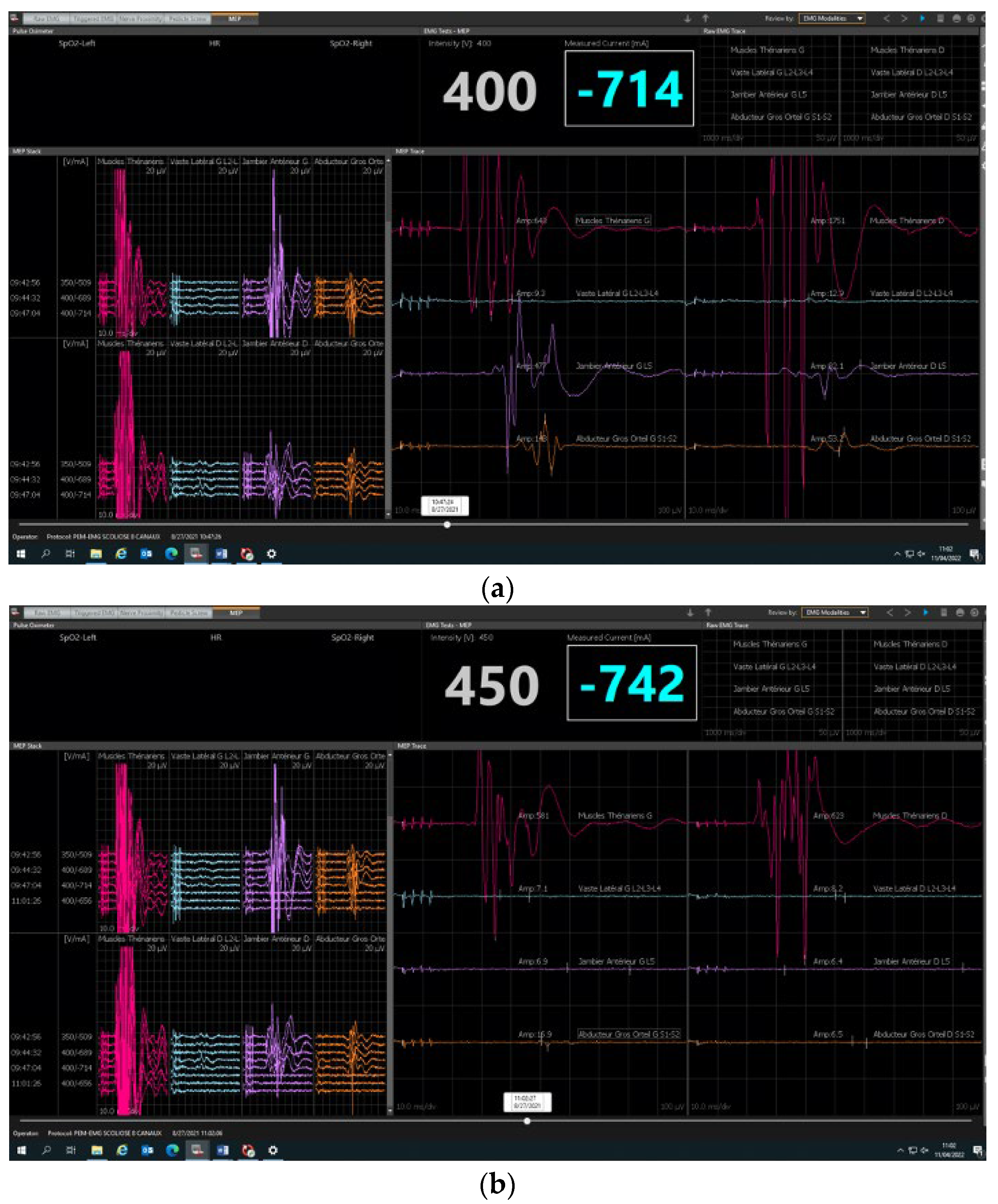
2.2. Neuromonitoring Procedure
2.3. Statistical Analysis
3. Results
3.1. Feasibility
3.2. Safety and Reliability Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamilton, D.K.; Smith, J.S.; Sansur, C.A.; Glassman, S.D.; Ames, C.P.; Berven, S.H.; Polly, D.W., Jr.; Perra, J.H.; Knapp, D.R.; Boachie-Adjei, O.; et al. Rates of new neurological deficit associated with spine surgery based on 108,419 procedures: A report of the scoliosis research society morbidity and mortality committee. Spine 2011, 36, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.Y.H.; Koh, H.Y.; Blanke, K.M.; Cheung, K.M.C. Complications following surgery for adolescent idiopathic scoliosis over a 13-year period. Bone Jt. J. 2020, 102, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.K.; Loh, K.W.; Chung, W.H.; Chiu, C.K.; Hasan, M.S.; Chan, C.Y.W. Perioperative outcome and complications following single-staged Posterior Spinal Fusion (PSF) using pedicle screw instrumentation in Adolescent Idiopathic Scoliosis (AIS): A review of 1057 cases from a single centre. BMC Musculoskelet. Disord. 2021, 22, 413. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.-R.; Goodall, D. Rate of complications in scoliosis surgery—A systematic review of the Pub Med literature. Scoliosis 2008, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Accadbled, F.; Henry, P.; de Gauzy, J.S.; Cahuzac, J.P. Spinal cord monitoring in scoliosis surgery using an epidural electrode. results of a prospective, consecutive series of 191 cases. Spine 2006, 31, 2614–2623. [Google Scholar] [CrossRef]
- Scoliosis Research Society. Scoliosis Research Society Position Statement. Somatosensory Evoked Potential Monitoring of Neurologic Spinal Cord Function during Spinal Surgery; Scoliosis Research Society: Kansas City, MO, USA, 1992. [Google Scholar]
- Gavaret, M.; Jouve, J.; Péréon, Y.; Accadbled, F.; André-Obadia, N.; Azabou, E.; Blondel, B.; Bollini, G.; Delécrin, J.; Farcy, J.-P.; et al. Intraoperative neurophysiologic monitoring in spine surgery. Developments and state of the art in France in 2011. Orthop. Traumatol. Surg. Res. 2013, 99, S319–S327. [Google Scholar] [CrossRef]
- Merton, P.A.; Morton, H.B. Stimulation of the cerebral cortex in the intact human subject. Nature 1980, 285, 227. [Google Scholar] [CrossRef]
- Ito, Z.; Matsuyama, Y.; Ando, M.; Kawabata, S.; Kanchiku, T.; Kida, K.; Fujiwara, Y.; Yamada, K.; Yamamoto, N.; Kobayashi, S.; et al. What is the best multimodality combination for intraoperative spinal cord monitoring of motor function? A multicenter study by the monitoring committee of the Japanese society for spine surgery and related research. Glob. Spine J. 2016, 6, 234–241. [Google Scholar] [CrossRef]
- Pelosi, L.; Lamb, J.; Grevitt, M.; Mehdian, S.M.; Webb, J.K.; Blumhardt, L.D. Combined monitoring of motor and somatosensory evoked potentials in orthopaedic spinal surgery. Clin. Neurophysiol. 2002, 113, 1082–1091. [Google Scholar] [CrossRef]
- Magampa, R.S.; Dunn, R. Surgeon-directed transcranial motor evoked potential spinal cord monitoring in spinal deformity surgery. Bone Jt. J. 2021, 103, 547–552. [Google Scholar] [CrossRef]
- Chan, A.; Banerjee, P.; Lupu, C.; Bishop, T.; Bernard, J.; Lui, D. Surgeon-Directed Neuromonitoring in Adolescent Spinal Deformity Surgery Safely Assesses Neurological Function. Cureus 2021, 13, e19843. [Google Scholar] [CrossRef] [PubMed]
- Langlais, T.; Rougereau, G.; Bruncottan, B.; Bolzinger, M.; Accadbled, F.; Compagnon, R.; Sales de Gauzy, J. Proximal Fixation in Adolescent Scoliosis Lenke 1 and 3 Treated by Posteromedial Translation Using Sublaminar Bands: Transverse-pedicular Hook Claw Versus Transverse Hook-pedicular Screw Claw. Clin. Spine Surg. 2021, 34, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Laumonerie, P.; Tibbo, M.E.; Kerezoudis, P.; Langlais, T.; de Gauzy, J.S.; Accadbled, F. Influence of the sublaminar band density in the treatment of Lenke 1 adolescent idiopathic scoliosis. Orthop. Traumatol. Surg. Res. 2020, 106, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Huss, G.K.; Betz, R.R.; Clancy, M. A draping technique for spinal surgery using the Stagnara wake-up test. AORN J. 1988, 48, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Noonan, K.J.; Walker, T.; Feinberg, J.R.; Nagel, M.; Didelot, W.; Lindseth, R. Factors related to false- versus true-positive neuromonitoring changes in adolescent idiopathic scoliosis surgery. Spine 2002, 27, 825–830. [Google Scholar] [CrossRef]
- Shrader, M.W.; DiCindio, S.; Kenny, K.G.; Franco, A.J.; Zhang, R.; Theroux, M.C.; Rogers, K.J.; Shah, S.A. Transcranial electric motor evoked potential monitoring during scoliosis surgery in children with cerebral palsy and active seizure disorder: Is it feasible and safe? Spine Deform. 2023; epub ahead of print. [Google Scholar]
- Ushirozako, H.; Yoshida, G.; Hasegawa, T.; Yamato, Y.; Yasuda, T.; Banno, T.; Arima, H.; Oe, S.; Yamada, T.; Ide, K.; et al. Characteristics of false-positive alerts on transcranial motor evoked potential monitoring during pediatric scoliosis and adult spinal deformity surgery: An “anesthetic fade” phenomenon. J. Neurosurg. Spine 2019, 32, 423–431. [Google Scholar] [CrossRef]
- Bhagat, S.; Durst, A.; Grover, H.; Blake, J.; Lutchman, L.; Rai, A.S.; Crawford, R. An evaluation of multimodal spinal cord monitoring in scoliosis surgery: A single centre experience of 354 operations. Eur. Spine J. 2015, 24, 1399–1407. [Google Scholar] [CrossRef]
- Feng, B.; Qiu, G.; Shen, J.; Zhang, J.; Tian, Y.; Li, S.; Zhao, H.; Zhao, Y. Impact of multimodal intraoperative monitoring during surgery for spine deformity and potential risk factors for neurological monitoring changes. J. Spinal Disord. Tech. 2012, 25, E108–E114. [Google Scholar] [CrossRef]
- Tsirikos, A.I.; Duckworth, A.D.; Henderson, L.E.; Michaelson, C. Multimodal Intraoperative Spinal Cord Monitoring during Spinal Deformity Surgery: Efficacy, Diagnostic Characteristics, and Algorithm Development. Med. Princ. Pract. 2019, 29, 6–17. [Google Scholar] [CrossRef]
- Nassef, M.; Splinter, W.; Lidster, N.; Al-Kalbani, A.; Nashed, A.; Ilton, S.; Vanniyasingam, T.; Paul, J. Intraoperative neurophysiologic monitoring in idiopathic scoliosis surgery: A retrospective observational study of new neurologic deficits. Can. J. Anaesth. 2021, 68, 477–484. [Google Scholar] [CrossRef]
- Hero, N.; Vengust, R.; Topolovec, M. Comparative Analysis of Combined (First Anterior, Then Posterior) Versus Only Posterior Approach for Treating Severe Scoliosis: A Mean Follow Up of 8.5 Years. Spine 2017, 42, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Skaggs, D.L.; Lee, C.; Myung, K.S. Neuromonitoring Changes Are Common and Reversible With Temporary Internal Distraction for Severe Scoliosis. Spine Deform. 2014, 2, 61–69. [Google Scholar] [CrossRef] [PubMed]
- McClung, A.; Mundis, G.; Pawelek, J.; Garg, S.; Yaszay, B.; Boachie-Adjei, O.; James, O.S.; Sponseller, P.; Sánchez Pérez-Grueso, F.J.; Lavelle, W.; et al. Paper #19: Utilization and Reliability of Intraoperative Neuromonitoring in Vertebral Column Resections for Severe Early-Onset Scoliosis. Spine Deform. 2017, 5, 448–449. [Google Scholar]
- MacDonald, D.B. Intraoperative motor evoked potential monitoring: Overview and update. J. Clin. Monit. Comput. 2006, 20, 347–377. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Chen, L.; Yang, J.-F.; Deng, Y.-L.; Sui, W.-Y.; Yang, J.-L. Multimodality Intraoperative Neuromonitoring in Severe Thoracic Deformity Posterior Vertebral Column Resection Correction. World Neurosurg. 2019, 127, e416–e426. [Google Scholar] [CrossRef] [PubMed]


| Etiology | ADC * Coronal Pre-Operative | Cincinnati Correction Index | ADC * Coronal Post Op | |
|---|---|---|---|---|
| Idiopathic | Average | 49 | 2.17 | 16 |
| SD ** | 15 | 1.69 | 10 | |
| Min-max | 12–95 | 0.4–11 | 0–72 | |
| Secondary | Average | 60 | 3.3 | 29 |
| SD ** | 22 | 3.3 | 21 | |
| Min-max | 23–115 | 1–18 | 6–80 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerdoncuff, A.; Henry, P.; Compagnon, R.; Accadbled, F.; Sales de Gauzy, J.; Langlais, T. Feasibility, Safety and Reliability of Surgeon-Directed Transcranial Motor Evoked Potentials Monitoring in Scoliosis Surgery. Children 2023, 10, 1560. https://doi.org/10.3390/children10091560
Kerdoncuff A, Henry P, Compagnon R, Accadbled F, Sales de Gauzy J, Langlais T. Feasibility, Safety and Reliability of Surgeon-Directed Transcranial Motor Evoked Potentials Monitoring in Scoliosis Surgery. Children. 2023; 10(9):1560. https://doi.org/10.3390/children10091560
Chicago/Turabian StyleKerdoncuff, Aude, Patrice Henry, Roxane Compagnon, Franck Accadbled, Jérôme Sales de Gauzy, and Tristan Langlais. 2023. "Feasibility, Safety and Reliability of Surgeon-Directed Transcranial Motor Evoked Potentials Monitoring in Scoliosis Surgery" Children 10, no. 9: 1560. https://doi.org/10.3390/children10091560
APA StyleKerdoncuff, A., Henry, P., Compagnon, R., Accadbled, F., Sales de Gauzy, J., & Langlais, T. (2023). Feasibility, Safety and Reliability of Surgeon-Directed Transcranial Motor Evoked Potentials Monitoring in Scoliosis Surgery. Children, 10(9), 1560. https://doi.org/10.3390/children10091560






