Effect of Ankle-Foot Orthoses in Pediatric Patients with Hereditary Motor-Sensory Neuropathy: A Case Series Study
Abstract
1. Introduction
2. Case Series Presentation
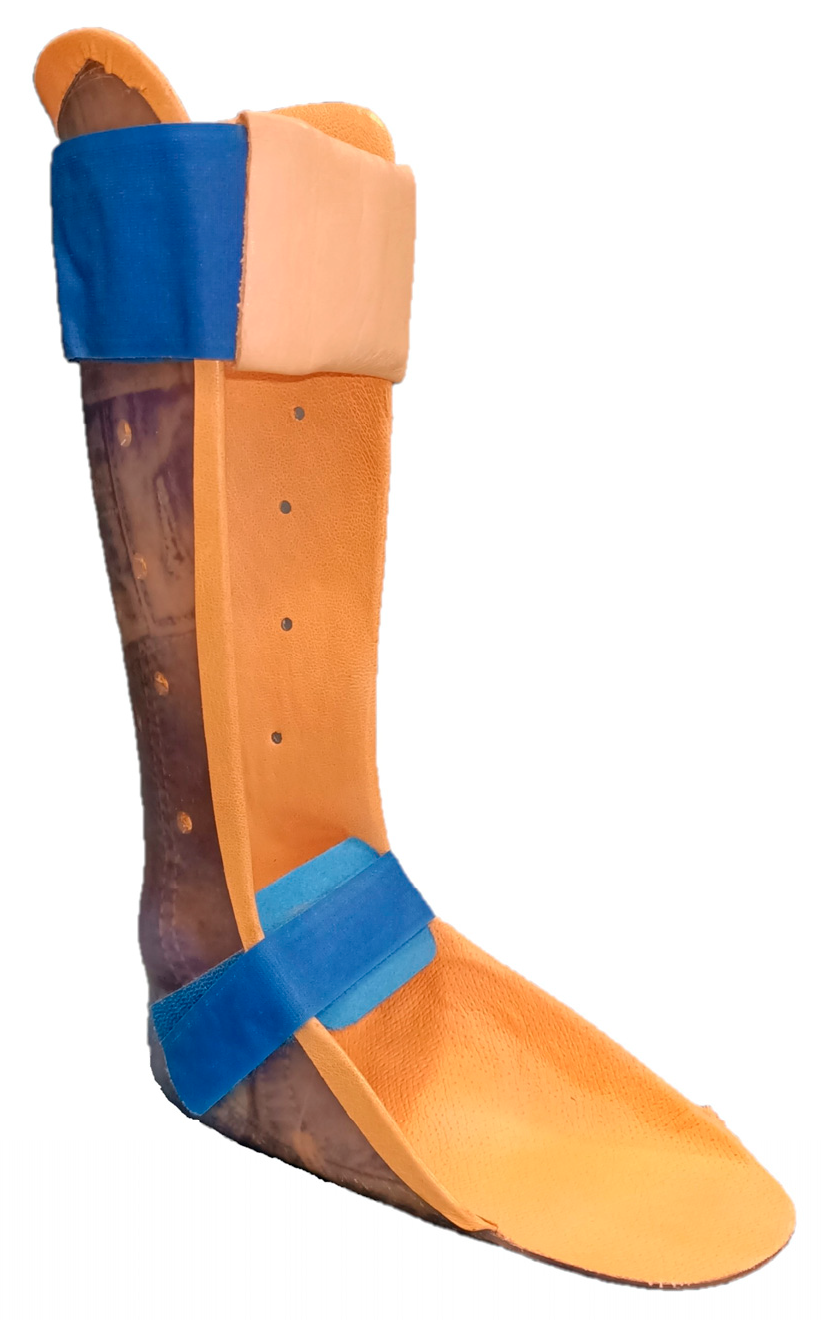
3. Interventions
4. Assessments
- Maximum ankle dorsiflexion in the stance: inability to support the weight, mainly due to plantar-flexor weakness.
- Maximum plantarflexion in the swing: measurement of foot drop, determined by dorsal-flexor weakness.
- Maximum knee flexion in the swing: compensation strategy to increase the clearance in the presence of foot drop.
- Maximum hip flexion in the swing: compensation strategy to increase the clearance in the presence of foot drop.
- Range of motion (ROM) of hip flexion in the terminal swing (measured as the difference between the maximum flexion in the swing and flexion at initial contact): represents the pass retract, that is, a quick extension of the hip before initial contact used to compensate for a toe-first landing in the presence of foot drop; this quick movement utilizes the inertia of the foot to obtain a passive dorsiflexion and, therefore, a better placement of the foot.
- Minimal knee flexion during the single support phase: measure of possible hyperextension of the knee to passively stabilize the knee itself and compensate for quadriceps weakness.
- Maximum power at the hip at the push-off: hip “pull-up”, that is, a compensation for a weak propulsion at the ankle level to flex the hip.
- Total ROM of pelvic rotation in the gait cycle (0–100%): rotation of the upper body to advance, used to compensate for a weak distal push-off power generation.
5. Outcomes
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pipis, M.; Rossor, A.M.; Laura, M.; Reilly, M.M. Next-generation sequencing in Charcot–Marie–Tooth disease: Opportunities and challenges. Nat. Rev. Neurol. 2019, 15, 644–656. [Google Scholar] [CrossRef]
- Howcroft, D.W.J.; Kumar, S.; Makwana, N. Charcot Marie Tooth Disease. Orthop. Trauma 2009, 23, 274–277. [Google Scholar] [CrossRef]
- de Jong, L.A.; Kerkum, Y.L.; Altmann, V.C.; Geurts, A.C.; Keijsers, N.L. Orthopedic footwear has a positive influence on gait adaptability in individuals with hereditary motor and sensory neuropathy. Gait Posture 2023, 106, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Ramdharry, G. Peripheral nerve disease. In Handbook of Clinical Neurology, 1st ed.; Elsevier BV: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Õunpuu, S.; Garibay, E.; Acsadi, G.; Brimacombe, M.; Pierz, K. The impact of orthoses on gait in children with Charcot-Marie-Tooth disease. Gait Posture 2021, 85, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Pareyson, D.; Marchesi, C. Diagnosis, natural history, and management of Charcot–Marie–Tooth disease. Lancet Neurol. 2009, 8, 654–667. [Google Scholar] [CrossRef]
- Vinci, P.; Paoloni, M.; Ioppolo, F.; Gargiulo, P.; Santilli, V. Gait analysis in a patient with severe Charcot-Marie-Tooth disease: A case study with a new orthotic device for footdrop. Eur. J. Phys. Rehabil. Med. 2010, 46, 355–361. [Google Scholar]
- Newman, C.J.; Walsh, M.; O’Sullivan, R.; Jenkinson, A.; Bennett, D.; Lynch, B.; O’brien, T. The characteristics of gait in Charcot-Marie-Tooth disease types I and II. Gait Posture 2007, 26, 120–127. [Google Scholar] [CrossRef]
- Nonnekes, J.; Hofstad, C.; de Rotteveel, A.; van Wielen, H.; Gelder, J.; Plaats, C.; Altmann, V.; Krause, F.; Keijsers, N.; Geurts, A.; et al. Management of gait impairments in people with Charcot-Marie-Tooth disease: A treatment algorithm. J. Rehabilit. Med. 2021, 53, jrm00194. [Google Scholar] [CrossRef]
- Õunpuu, S.; Garibay, E.; Solomito, M.; Bell, K.; Pierz, K.; Thomson, J.; Acsadi, G.; DeLuca, P. A comprehensive evaluation of the variation in ankle function during gait in children and youth with Charcot–Marie–Tooth disease. Gait Posture 2013, 38, 900–906. [Google Scholar] [CrossRef]
- Ferrarin, M.; Bovi, G.; Rabuffetti, M.; Mazzoleni, P.; Montesano, A.; Pagliano, E.; Marchi, A.; Magro, A.; Marchesi, C.; Pareyson, D.; et al. Gait pattern classification in children with Charcot–Marie–Tooth disease type 1A. Gait Posture 2012, 35, 131–137. [Google Scholar] [CrossRef][Green Version]
- Bigelow, K.E.; Jackson, K. Immediate influence of carbon composite ankle-foot orthoses on balance and gait in individuals with peripheral neuropathy: A pilot study. J. Prosthetics Orthot. 2014, 26, 220–227. [Google Scholar] [CrossRef]
- Johnson, N.; McCorquodale, D.; Pucillo, E. Management of Charcot–Marie–Tooth disease: Improving long-term care with a multidisciplinary approach. J. Multidiscip. Health 2016, 9, 7–19. [Google Scholar] [CrossRef]
- Fink, J.K. The hereditary spastic paraplegias. Handb. Clin. Neurol. 2023, 196, 59–88. [Google Scholar] [CrossRef]
- Scheffers, G.; Hiller, C.; Refshauge, K.; Burns, J. Prescription of foot and ankle orthoses for children with Charcot–Marie–Tooth disease: A review of the evidence. Phys. Ther. Rev. 2012, 17, 79–90. [Google Scholar] [CrossRef]
- Kenis-Coskun, O.; Matthews, D.J. Rehabilitation issues in Charcot-Marie-Tooth disease. J. Pediatr. Rehabilit. Med. 2016, 9, 31–34. [Google Scholar] [CrossRef]
- Zuccarino, R.; Anderson, K.M.; Shy, M.E.; Wilken, J.M. Satisfaction with ankle foot orthoses in individuals with Charcot-Marie-Tooth disease. Muscle Nerve 2021, 63, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Dufek, J.S.; Neumann, E.S.; Hawkins, M.C.; O’toole, B. Functional and dynamic response characteristics of a custom composite ankle foot orthosis for Charcot–Marie–Tooth patients. Gait Posture 2014, 39, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Waterval, N.F.J.; Nollet, F.; Harlaar, J.; Brehm, M.-A. Modifying ankle foot orthosis stiffness in patients with calf muscle weakness: Gait responses on group and individual level. J. Neuroeng. Rehabilit. 2019, 16, 120. [Google Scholar] [CrossRef]
- Leardini, A.; Sawacha, Z.; Paolini, G.; Ingrosso, S.; Nativo, R.; Benedetti, M.G. A new anatomically based protocol for gait analysis in children. Gait Posture 2007, 26, 560–571. [Google Scholar] [CrossRef]
- Wojciechowski, E.; Sman, A.; Cornett, K.; Raymond, J.; Refshauge, K.; Menezes, M.P.; Burns, J. Gait patterns of children and adolescents with Charcot-Marie-Tooth disease. Gait Posture 2017, 56, 89–94. [Google Scholar] [CrossRef]
- Aboutorabi, A.; Arazpour, M.; Ahmadi Bani, M.; Saeedi, H.; Head, J.S. Efficacy of ankle foot orthoses types on walking in children with cerebral palsy: A systematic review. Ann. Phys. Rehabilit. Med. 2017, 60, 393–402. [Google Scholar] [CrossRef]
- Jonkers, I.; Delp, S.; Patten, C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture 2009, 29, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.H.; Rozumalski, A.; Trost, J.P. The effect of walking speed on the gait of typically developing children. J. Biomech. 2008, 41, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Scoppa, F.; Capra, R.; Gallamini, M.; Shiffer, R. Clinical stabilometry standardization: Basic definitions—Acquisition interval—Sampling frequency. Gait Posture 2013, 37, 290–292. [Google Scholar] [CrossRef]
- Terra, M.B.; Da Silva, R.A.; Bueno, M.E.B.; Ferraz, H.B.; Smaili, S.M. Center of pressure-based balance evaluation in individuals with Parkinson’s disease: A reliability study. Physiother. Theory Pract. 2020, 36, 826–833. [Google Scholar] [CrossRef]
- Lin, D.; Seol, H.; Nussbaum, M.A.; Madigan, M.L. Reliability of COP-based postural sway measures and age-related differences. Gait Posture 2008, 28, 337–342. [Google Scholar] [CrossRef]
- Palmieri, R.M.; Ingersoll, C.D.; Stone, M.B.; Krause, B.A. Center-of-Pressure Parameters Used in the Assessment of Postural Control. J. Sport Rehabil. 2002, 11, 51–66. [Google Scholar] [CrossRef]
- Nardone, A.; Tarantola, J.; Miscio, G.; Pisano, F.; Schenone, A.; Schieppati, M. Loss of large-diameter spindle afferent fibres is not detrimental to the control of body sway during upright stance: Evidence from neuropathy. Exp. Brain Res. 2000, 135, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Lafond, D.; Corriveau, H.; Hébert, R.; Prince, F. Intrasession reliability of center of pressure measures of postural steadiness in healthy elderly people. Arch. Phys. Med. Rehabilit. 2004, 85, 896–901. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, M.H.; van der Linden, S.C.; Hendricks, H.T.; van Engelen, B.G.; Geurts, A.C. Postural instability in Charcot-Marie-Tooth type 1A patients is strongly associated with reduced somatosensation. Gait Posture 2010, 31, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Tozza, S.; Aceto, M.G.; Pisciotta, C.; Bruzzese, D.; Iodice, R.; Santoro, L.; Manganelli, F. Postural instability in Charcot-Marie-Tooth 1A disease. Gait Posture 2016, 49, 353–357. [Google Scholar] [CrossRef]
- Bravini, E.; Franchignoni, F.; Ferriero, G.; Giordano, A.; Bakhsh, H.; Sartorio, F.; Vercelli, S. Validation of the Italian version of the Client Satisfaction with Device module of the Orthotics and Prosthetics Users’ Survey. Disabil. Health J. 2014, 7, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Don, R.; Serrao, M.; Vinci, P.; Ranavolo, A.; Cacchio, A.; Ioppolo, F.; Paoloni, M.; Procaccianti, R.; Frascarelli, F.; De Santis, F.; et al. Foot drop and plantar flexion failure determine different gait strategies in Charcot-Marie-Tooth patients. Clin. Biomech. 2007, 22, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Ramdharry, G.M.; Day, B.L.; Reilly, M.M.; Marsden, J.F. Foot drop splints improve proximal as well as distal leg control during gait in Charcot-Marie-Tooth Disease. Muscle Nerve 2012, 46, 512–519. [Google Scholar] [CrossRef]
- Burdett, R.G.P.; Hassell, G.B. Effects of Three Types of Ankle-Foot Orthoses on the Gait and Bicycling of a Patient with Charcot-Marie-Tooth Disease. J. Prosthetics Orthot. 2004, 16, 25–30. [Google Scholar] [CrossRef]
- Esquenazi, A.; Ofluoglu, D.; Hirai, B.; Kim, S. The Effect of an Ankle-Foot Orthosis on Temporal Spatial Parameters and Asymmetry of Gait in Hemiparetic Patients. PM&R 2009, 1, 1014–1018. [Google Scholar] [CrossRef]
- Bregman, D.J.; Harlaar, J.; Meskers, C.G.; de Groot, V. Spring-like Ankle Foot Orthoses reduce the energy cost of walking by taking over ankle work. Gait Posture 2012, 35, 148–153. [Google Scholar] [CrossRef]


| S1 | S2 | S3 | |||||
|---|---|---|---|---|---|---|---|
| Gender | M | F | M | ||||
| Age [years] | 14 | 14 | 14 | ||||
| Weight [kg] | 39.5 | 47 | 42 | ||||
| Height [cm] | 161 | 159 | 150 | ||||
| Passive range of motion | Hip | preserved | preserved | preserved | |||
| Knee | with hyperextension (left > right) | preserved | with hyperextension (left > right) | ||||
| Ankle | preserved | preserved | preserved | ||||
| Muscle force [MRC scale] | Left | Right | Left | Right | Left | Right | |
| Gluteus maximus | 4 | 4 | 3 | 3 | 4 | 4 | |
| Gluteus medius | 3 | 3 | 3 | 3 | 4 | 4 | |
| Adduttors | 3 | 2 | 3 | 3 | 4 | 4 | |
| Quadriceps | 3 | 2 | 4 | 4 | 4 | 4 | |
| Hamstrings | 2 | 2 | 4 | 4 | 4 | 4 | |
| Psoas | 4 | 4 | 4 | 4 | 4 | 4 | |
| Plantar-flexors | 0 | 0 | 1 | 1 | 4 | 3 | |
| Dorsal-flexors | 0 | 0 | 1 | 1 | 2 | 1 | |
| S1 | S2 | S3 | Ref | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bar | SAFO | Botter | Bar | SAFO | Botter | Bar | Botter | |||||||||||
| L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | |||
| Push-off | Max Ankle push-off power [W] | 1 (1) | 5 (5) | 12 (3) | 38 (11) | 19 (2) | 45 (5) | 18 (1) | 31 (1) | 19 (1) | 20 (0) | 32 (1) | 34 (2) | 104 (9) | 44 (5) | 52 (3) | 52 (5) | 84 |
| Max pre-swing Ankle moment [Nm] | 1 (1) | 1 (0) | 5 (0) | 6 (1) | 4 (0) | 6 (1) | 2 (0) | 3 (1) | 5 (0) | 5 (1) | 3 (0) | 3 (0) | 9 (1) | 5 (0) | 9 (0) | 6 (0) | 8 | |
| Vertical GRF push-off peak [%BW] | 106 (0) | 117 (4) | 113 (4) | 125 (6) | 108 (2) | 113 (4) | 115 (1) | 101 (3) | 110 (1) | 110 (2) | 109 (3) | 109 (0) | 114 (1) | 90 (4) | 110 (3) | 98 (3) | 109 | |
| STP | Stride [%h] | 58 (1) | 78 (3) | 79 (2) | 56 (3) | 76 (2) | 69 (1) | 68 (2) | 75 (2) | 85 | ||||||||
| Speed [%h/s] | 37 (1) | 53 (5) | 51 (2) | 49 (4) | 67 (2) | 62 (1) | 64 (3) | 73 (3) | 78 | |||||||||
| Deficits | Max stance Ankle dorsiflexion [deg] | 18 (2) | 17 (1) | 14 (1) | 25 (0) | 13 (1) | 17 (1) | 20 (1) | 14 (1) | 18 (0) | 17 (1) | 23 (1) | 24 (1) | 16 (1) | 22 (2) | 18 (8) | 25 (1) | 8.6 |
| Max swing Ankle plantiflexion [deg] | 44 (1) | 49 (3) | −2 (0) | −10 (1) | 2 (1) | 2 (1) | 54 (2) | 37 (1) | −8 (1) | −9 (1) | 5 (2) | 3 (1) | 52 (2) | 50 (3) | −4 (2) | −2 (1) | −18 | |
| Compensations | Max Knee swing flexion [deg] | 71 (2) | 71 (12) | 73 (3) | 65 (4) | 72 (1) | 59 (1) | 67 (3) | 81 (3) | 66 (3) | 68 (1) | 65 (2) | 72 (2) | 73 (2) | 81 (1) | 63 (2) | 74 (1) | 74 |
| Max Hip swing flexion [deg] | 51 (2) | 50 (5) | 47 (3) | 37 (3) | 36 (1) | 26 (3) | 34 (1) | 35 (2) | 28 (2) | 30 (2) | 28 (3) | 32 (2) | 38 (1) | 40 (1) | 25 (2) | 31 (1) | 31 | |
| Hip terminal swing ROM [deg] | 20 (2) | 18 (6) | 19 (3) | 8 (2) | 17 (2) | 8 (1) | 18 (2) | 9 (2) | 5 (3) | 4 (1) | 4 (2) | 4 (1) | 15 (1) | 13 (2) | 7 (2) | 9 (2) | 9.0 | |
| Min single support Knee flexion [deg] | −13 (1) | 8 (0) | −14 (0) | 9 (1) | −20 (1) | −1 (1) | −7 (0) | 10 (9) | −13 (0) | −5 (1) | −10 (2) | −5 (2) | 11 (4) | 14 (3) | 3 (3) | −3 (2) | −3 | |
| Max Hip power at push-off [W] | 5 (0) | 22 (9) | 26 (13) | 19 (5) | 26 (2) | 18 (9) | 34 (8) | 32 (23) | 46 (2) | 45 (10) | 27 (5) | 27 (2) | 19 (6) | 33 (1) | 29 (7) | 35 (4) | 35 | |
| Pelvis rotation ROM [deg] | 44 (2) | 46 (2) | 39 (1) | 36 (4) | 37 (4) | 34 (1) | 21 (7) | 21 (2) | 13 (3) | 13 (4) | 10 (1) | 12 (2) | 37 (1) | 34 (1) | 12 (2) | 10 (2) | 10 | |
| Barefoot | SAFO | Botter | ||
|---|---|---|---|---|
| S1 | 2MWT [m] | NA | 72 | 110 |
| 10MWT [s] | 10.5 | 7.8 | 7.3 | |
| S2 | 2MWT [m] | 130 | 155 | 170 |
| 10MWT [s] | 7.6 | 6.0 | 6.2 | |
| S3 | 2MWT [m] | 160 | NA | 210 |
| 10MWT [s] | 6.9 | NA | 6.4 | |
| Barefoot | SAFO | Botter | ||
|---|---|---|---|---|
| S1 | 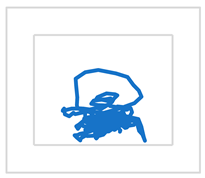 | 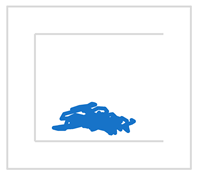 | ||
| MCV [mm/s] | 20 | 18 | ||
| RMS [mm] | 8 | 6 | ||
| S2 | 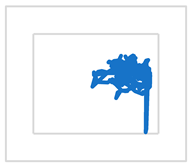 |  |  | |
| MCV [mm/s] | 50 | 19 | 21 | |
| RMS [mm] | 14 | 6 | 12 | |
| S3 |  | 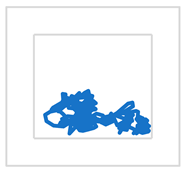 | ||
| MCV [mm/s] | 20 | 23 | ||
| RMS [mm] | 9 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borghi, C.; Sassi, S.; Pandarese, D.; Messori, S.; Faccioli, S. Effect of Ankle-Foot Orthoses in Pediatric Patients with Hereditary Motor-Sensory Neuropathy: A Case Series Study. Children 2023, 10, 1529. https://doi.org/10.3390/children10091529
Borghi C, Sassi S, Pandarese D, Messori S, Faccioli S. Effect of Ankle-Foot Orthoses in Pediatric Patients with Hereditary Motor-Sensory Neuropathy: A Case Series Study. Children. 2023; 10(9):1529. https://doi.org/10.3390/children10091529
Chicago/Turabian StyleBorghi, Corrado, Silvia Sassi, Daniela Pandarese, Samuele Messori, and Silvia Faccioli. 2023. "Effect of Ankle-Foot Orthoses in Pediatric Patients with Hereditary Motor-Sensory Neuropathy: A Case Series Study" Children 10, no. 9: 1529. https://doi.org/10.3390/children10091529
APA StyleBorghi, C., Sassi, S., Pandarese, D., Messori, S., & Faccioli, S. (2023). Effect of Ankle-Foot Orthoses in Pediatric Patients with Hereditary Motor-Sensory Neuropathy: A Case Series Study. Children, 10(9), 1529. https://doi.org/10.3390/children10091529





