Testicular Adrenal Rest Tumors in Congenital Adrenal Hyperplasia: Study of a Cohort of Patients from a Single Italian Center
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Population
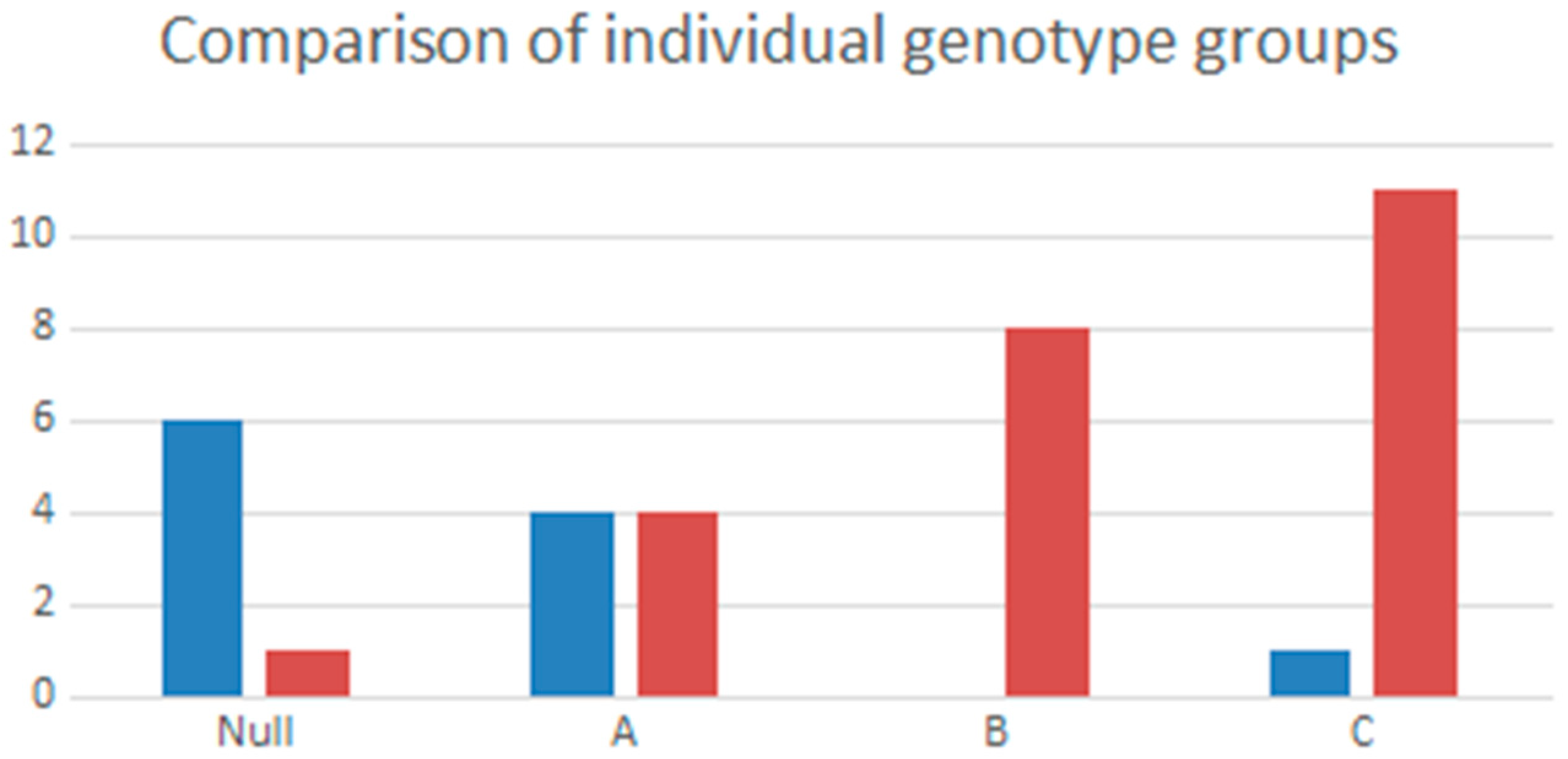
3.2. Genotype Association in Patients with and without TARTs
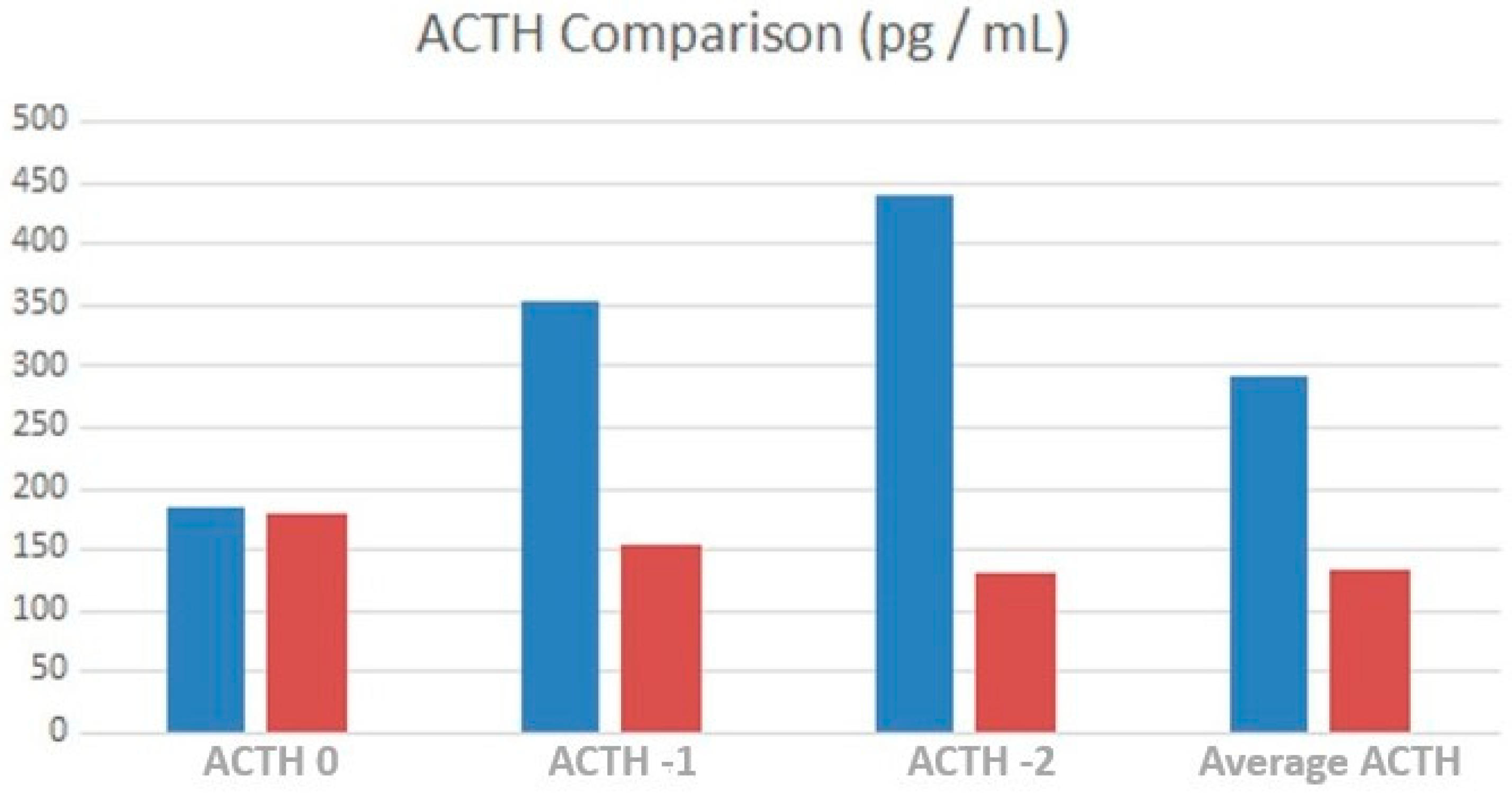
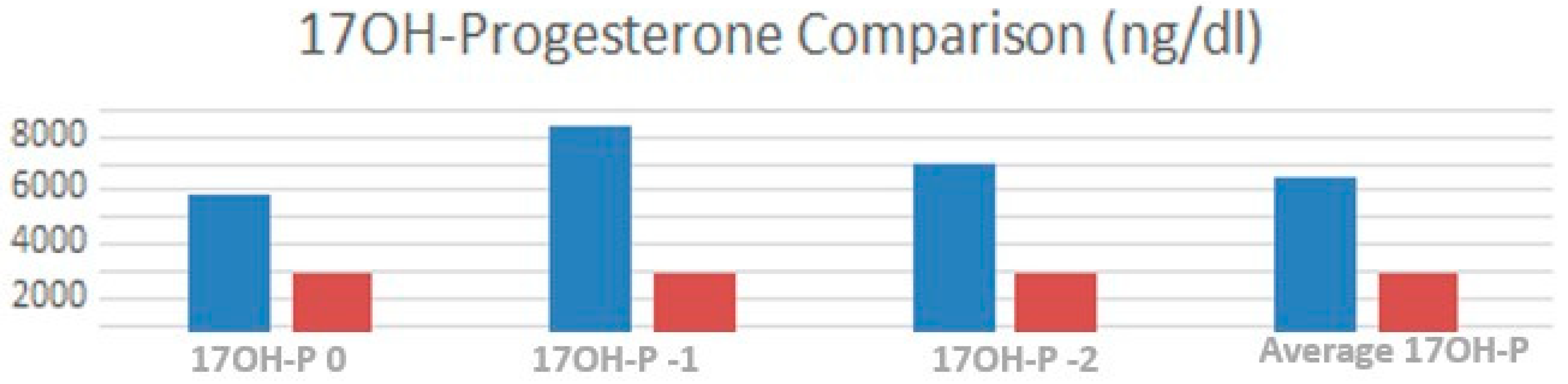
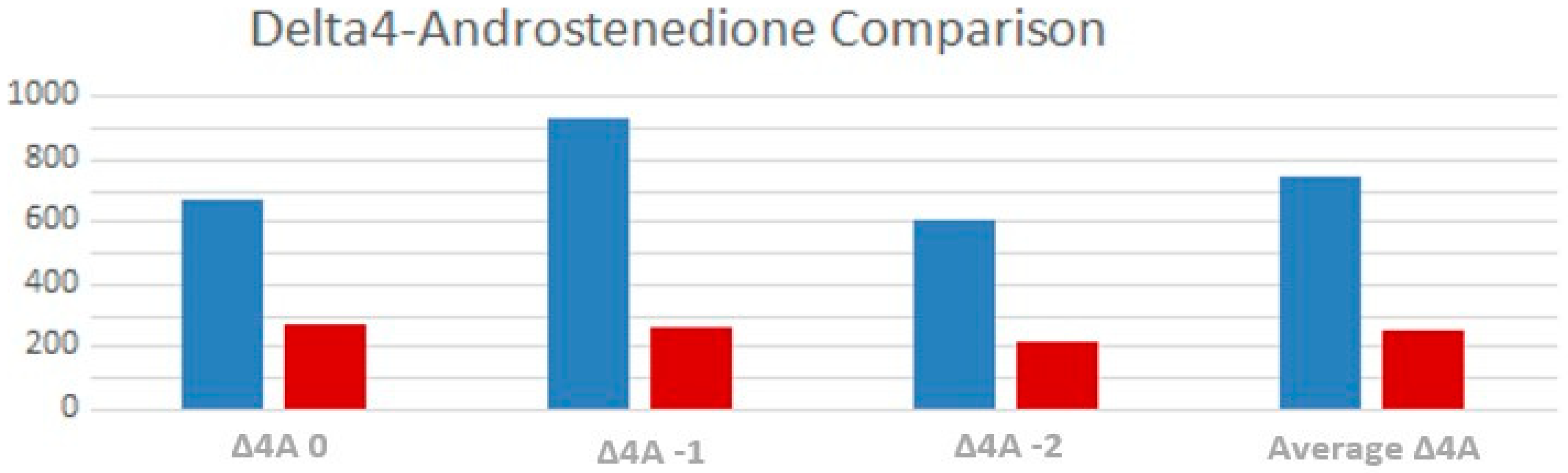
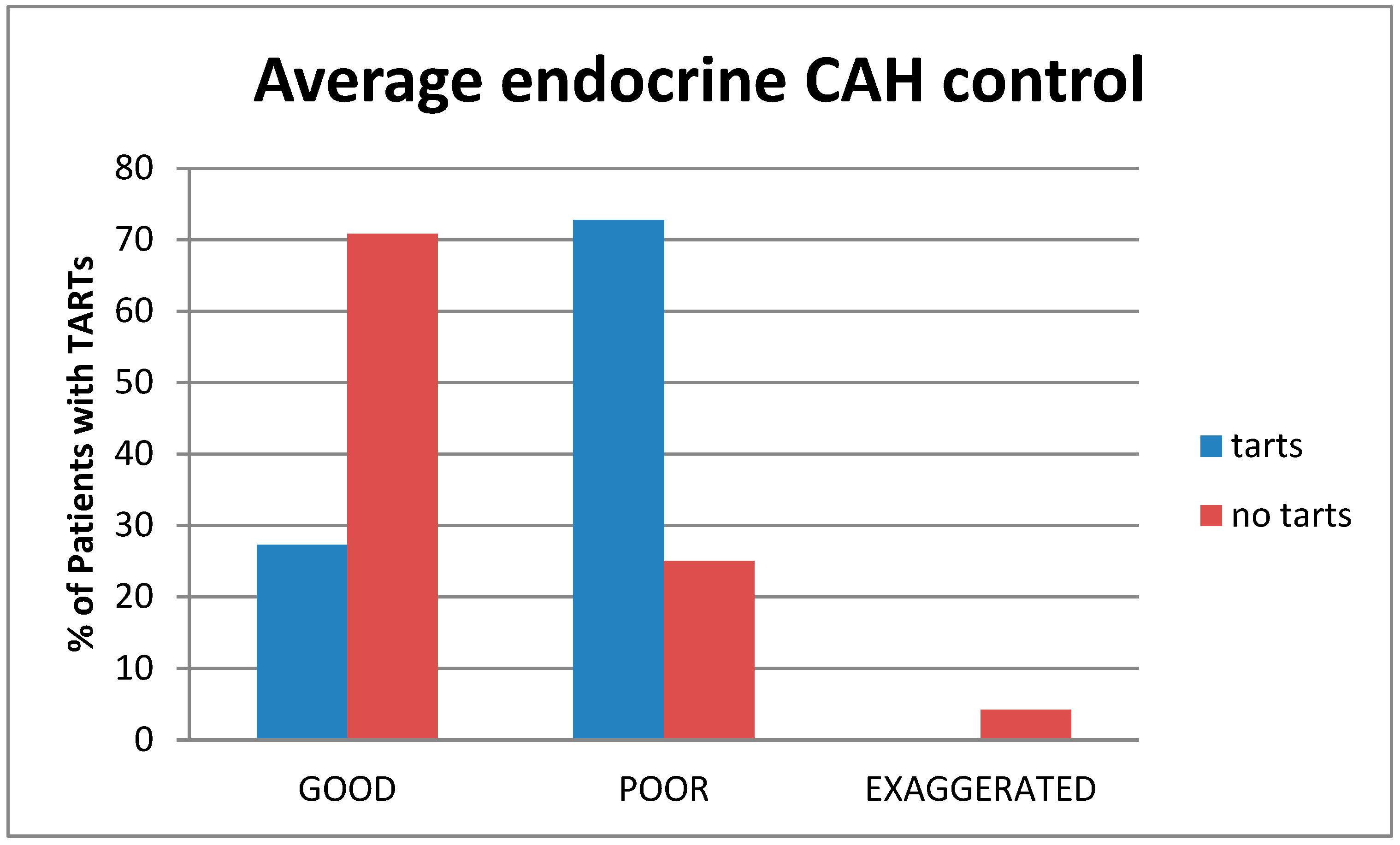
3.3. Endocrine Control
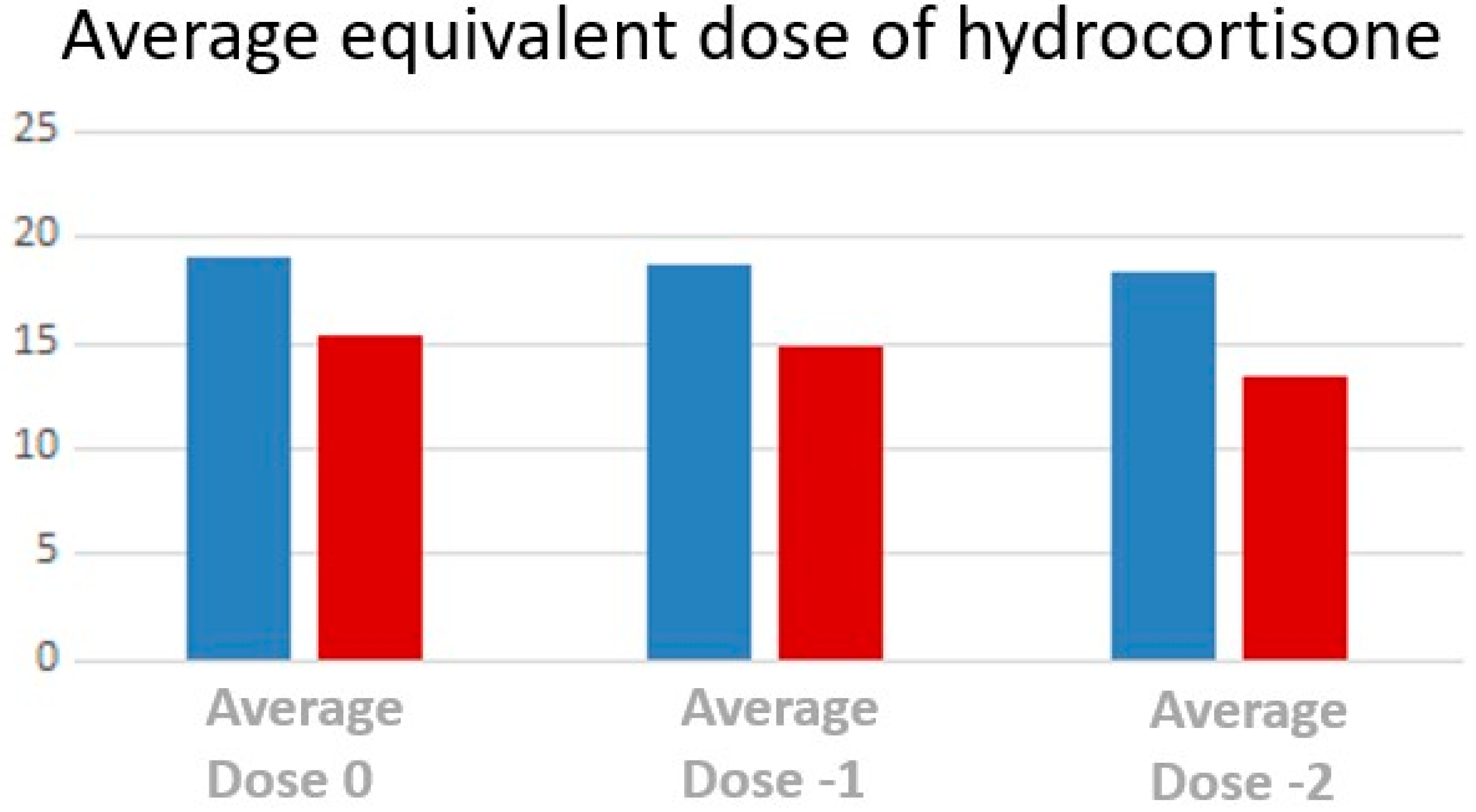
3.4. Treatment
3.5. Logistic Regression and Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Auer, M.K.; Nordenström, A.; Lajic, S.; Reisch, N. Congenital adrenal hyperplasia. Lancet 2023, 401, 227–244. [Google Scholar] [CrossRef]
- Claahsen-van der Grinten, H.L.; Speiser, P.W.; Ahmed, S.F.; Arlt, W.; Auchus, R.J.; Falhammar, H.; Flück, C.E.; Guasti, L.; Huebner, A.; Kortmann, B.B.M.; et al. Congenital Adrenal Hyperplasia-Current Insights in Pathophysiology, Diagnostics, and Management. Endocr. Rev. 2022, 43, 91–159. [Google Scholar] [CrossRef]
- Speiser, P.W.; Arlt, W.; Auchus, R.J.; Baskin, L.S.; Conway, G.S.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Murad, M.H.; Oberfield, S.E.; et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 4043–4088, Erratum in J. Clin. Endocrinol. Metab. 2019, 104, 39–40. [Google Scholar] [CrossRef]
- Engels, M.; Span, P.N.; van Herwaarden, A.E.; Sweep, F.C.G.J.; Stikkelbroeck, N.M.M.L.; Claahsen-van der Grinten, H.L. Testicular Adrenal Rest Tumors: Current Insights on Prevalence, Characteristics, Origin, and Treatment. Endocr. Rev. 2019, 40, 973–987. [Google Scholar] [CrossRef]
- Delfino, M.; Elia, J.; Imbrogno, N.; Argese, N.; Mazzilli, R.; Toscano, V.; Mazzilli, F. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia: Prevalence and sonographic, hormonal, and seminal characteristics. J. Ultrasound Med. 2012, 31, 383–388. [Google Scholar] [CrossRef]
- Stikkelbroeck, N.M.M.L.; Otten, B.J.; Pasic, A.; Jager, G.J.; Sweep, C.G.J.F.; Noordam, K.; Hermus, A.R.M.M. High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2001, 86, 5721–5728. [Google Scholar] [CrossRef]
- Falhammar, H.; Nyström, H.F.; Ekström, U.; Granberg, S.; Wedell, A.; Thorén, M. Fertility, sexuality and testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia. Eur. J. Endocrinol. 2012, 166, 441–449. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J.; et al. Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef]
- Bouvattier, C.; Esterle, L.; Renoult-Pierre, P.; de la Perrière, A.B.; Illouz, F.; Kerlan, V.; Pascal-Vigneron, V.; Drui, D.; Christin-Maitre, S.; Galland, F.; et al. Clinical Outcome, Hormonal Status, Gonadotrope Axis, and Testicular Function in 219 Adult Men Born With Classic 21-Hydroxylase Deficiency. A French National Survey. J. Clin. Endocrinol. Metab. 2015, 100, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Cacciari, E.; Milani, S.; Balsamo, A. Directive council of SIEDP 1995–1997. Eur. J. Clin. Nutr. 2002, 56, 171–180. [Google Scholar] [CrossRef]
- Candela, E.; Zagariello, M.; Di Natale, V.; Ortolano, R.; Righetti, F.; Assirelli, V.; Biasucci, G.; Cassio, A.; Pession, A.; Baronio, F. Cystathionine Beta-Synthase Deficiency: Three Consecutive Cases Detected in 40 Days by Newborn Screening in Emilia Romagna (Italy) and a Comprehensive Review of the Literature. Children 2023, 10, 396. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M.; Candela, E.; Di Palmo, E.; Miniaci, A.; Pession, A. Non-Cystic Fibrosis Bronchiectasis in Pediatric Age: A Case Series in a Metropolitan Area of Northern Italy. Children 2022, 9, 1420. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, A.; Cacciari, E.; Piazzi, S.; Cassio, A.; Bozza, D.; Pirazzoli, P.; Zappulla, F. Congenital adrenal hyperplasia: Neonatal mass screening compared with clinical diagnosis only in the Emilia-Romagna region of Italy, 1980–1995. Pediatrics 1996, 98 Pt 1, 362–367. [Google Scholar] [PubMed]
- Aycan, Z.; Keskin, M.; Lafcı, N.G.; Savaş-Erdeve, Ş.; Baş, F.; Poyrazoğlu, Ş.; Öztürk, P.; Parlak, M.; Ercan, O.; Güran, T.; et al. Genotype of congenital adrenal hyperplasia patients with testicular adrenal rest tumor. Eur. J. Med. Genet. 2022, 65, 104654. [Google Scholar] [CrossRef]
- Mendes-Dos-Santos, C.T.; Martins, D.L.; Guerra-Júnior, G.; Baptista, M.T.M.; De-Mello, M.P.; de Oliveira, L.C.; Morcillo, A.M.; Lemos-Marini, S.H.V. Prevalence of Testicular Adrenal Rest Tumor and Factors Associated with Its Development in Congenital Adrenal. Horm. Res. Paediatr. 2018, 90, 161–168. [Google Scholar] [CrossRef]
- Dumic, M.; Duspara, V.; Grubic, Z.; Oguic, S.K.; Skrabic, V.; Kusec, V. Testicular adrenal rest tumors in congenital adrenal hyperplasia—Cross-sectional study of 51 Croatian male patients. Eur. J. Pediatr. 2017, 176, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Engels, M.; Gehrmann, K.; Falhammar, H.; Webb, E.A.; Nordenström, A.; Sweep, F.C.; Span, P.N.; E van Herwaarden, A.; Rohayem, J.; Richter-Unruh, A.; et al. Gonadal function in adult male patients with congenital adrenal hyperplasia. Eur. J. Endocrinol. 2018, 178, 285–294. [Google Scholar] [CrossRef]
- Werneck, G.; Rodrigues, E.M.R.; Mantovani, R.M.; Lane, J.S.S.; Silva, I.N. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia: 6 years of follow-up. J. Pediatr. Endocrinol. Metab. 2019, 32, 519–526. [Google Scholar] [CrossRef]
- Saho, R.; Dolzan, V.; Zerjav Tansek, M.; Pastorakova, A.; Petrovic, R.; Knapkova, M.; Trebusak Podkrajsek, K.; Suput Omladic, J.; Bertok, S.; Avbelj Stefanija, M.; et al. Genetic and clinical characteristics including occurrence of testicular adrenal rest tumors in Slovak and Slovenian patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Front. Endocrinol. 2023, 14, 1134133. [Google Scholar] [CrossRef] [PubMed]
- Falhammar, H.; Nordenström, A. Nonclassic congenital adrenal hyperplasia due to 21-hydroxylase deficiency: Clinical presentation, diagnosis, treatment, and outcome. Endocrine 2015, 50, 32–50. [Google Scholar] [CrossRef]
- Kocova, M.; Janevska, V.; Anastasovska, V. Testicular adrenal rest tumors in boys with 21-hydroxylase deficiency, timely diagnosis and follow-up. Endocr. Connect. 2018, 7, 544–552. [Google Scholar] [CrossRef]
- Tresoldi, A.S.; Betella, N.; Hasenmajer, V.; Pozza, C.; Vena, W.; Fiamengo, B.; Negri, L.; Cappa, M.; Lania, A.G.A.; Lenzi, A.; et al. Bilateral testicular masses and adrenal insufficiency: Is congenital adrenal hyperplasia the only possible diagnosis? First two cases of TARTS described in Addison-only X-linked adrenoleukodystrophy and a brief review of literature. J. Endocrinol. Investig. 2021, 44, 391–402. [Google Scholar] [CrossRef]
- Corcioni, B.; Renzulli, M.; Marasco, G.; Baronio, F.; Gambineri, A.; Ricciardi, D.; Ortolano, R.; Farina, D.; Gaudiano, C.; Cassio, A.; et al. Prevalence and ultrasound patterns of testicular adrenal rest tumors in adults with congenital adrenal hyperplasia. Transl. Androl. Urol. 2021, 10, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Reisch, N.; Rottenkolber, M.; Greifenstein, A.; Krone, N.; Schmidt, H.; Reincke, M.; Schwarz, H.P.; Beuschlein, F. 2013 Testicular adrenal rest tumors develop independently of long-term disease control: A longitudinal analysis of 50 adult men with congenital adrenal hyperplasia due to classic 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2013, 98, E1820–E1826. [Google Scholar] [CrossRef] [PubMed]
- Claahsen-van der Grinten, H.L.; Stikkelbroeck, N.M.; Sweep, C.G.; Hermus, A.R.; Otten, B.J. Fertility in patients with congenital adrenal hyperplasia. J. Pediatr. Endocrinol. Metab. 2006, 19, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Falhammar, H.; Frisén, L.; Norrby, C.; Almqvist, C.; Hirschberg, A.L.; Nordenskjöld, A.; Nordenström, A. Reduced Frequency of Biological and Increased Frequency of Adopted Children in Males With 21-Hydroxylase Deficiency: A Swedish Population-Based National Cohort Study. J. Clin. Endocrinol. Metab. 2017, 102, 4191–4199. [Google Scholar] [CrossRef]
- Walker, B.R.; Skoog, S.J.; Winslow, B.H.; Canning, D.A.; Tank, E.S. Testis sparing surgery for steroid unresponsive testicular tumours of the adrenogenital syndrome. J. Urol. 1997, 157, 1460–1463. [Google Scholar] [CrossRef]
- Tiryaki, T.; Aycan, Z.; Huecumenglu, S. Testis sparing surgery for steroid unresponsive testicular tumors of the congenital adrenal hyperplasia. Pediatr. Surg. Int. 2005, 21, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.W.; Lacroix, A.; Rabin, D.; McKenna, T.J. Gonadal dysfunction in adult men with congenital adrenal hyper-plasia. Acta Endocrinol. 1980, 95, 185–193. [Google Scholar]

| Patient | Phenotype | Genotype | Genotype Group |
|---|---|---|---|
| 1 | CL (SW) | delB/delB | Null |
| 2 | CL (SW) | delB/delB | Null |
| 3 | CL (SW) | conv/delB | Null |
| 4 | CL (SW) | conv/delB | Null |
| 5 | CL (SW) | delB/delB | Null |
| 6 | CL (SW) | delB/delB | Null |
| 7 | CL (SW) | delB/delB | Null |
| 8 | CL (SV) | I172N/IN2 + Q318X | B |
| 9 | CL (SW) | IN2/delB | A |
| 10 | CL (SW) | IN2/Q318X | A |
| 11 | CL (SW) | IN2/R356W | A |
| 12 | CL (SW) | IN2/IN2 | A |
| 13 | CL (SW) | IN2/delB | A |
| 14 | CL (SW) | IN2/R356W | A |
| 15 | CL (SV) | IN2/R356Q | A |
| 16 | CL (SV) | IN2/I172N | B |
| 17 | CL (SV) | I172N/delB | B |
| 18 | CL (SV) | IN2/I172N | B |
| 19 | CL (SV) | I172N/D8bp | B |
| 20 | CL (SV) | I172N/R356W | B |
| 21 | CL (SV) | IN2/I172N | B |
| 22 | CL (SV) | IN2/I172N | B |
| 23 | CL (SV) | I172N/ClE6 | B |
| 24 | CL (SV) | conv(prom + P30L)/Q318X | B |
| 25 | NC | V281L/V281L + R366H | C |
| 26 | NC | IN2/NT2721 | C |
| 27 | NC | V281L/V281L | C |
| 28 | NC | V281L/V281L | C |
| 29 | NC | V281L/V281L | C |
| 30 | NC | V281L/P30L | C |
| 31 | NC | NT2721/V281L | C |
| 32 | NC | V281L/IL72N | C |
| 33 | NC | IN2/V281L | C |
| 34 | CL (SV) | L446P/R356W | C |
| 35 | NC | IN2 + Q318X/V281L | C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortolano, R.; Cassio, A.; Alqaisi, R.S.; Candela, E.; Di Natale, V.; Assirelli, V.; Bernardini, L.; Bortolamedi, E.; Cantarelli, E.; Corcioni, B.; et al. Testicular Adrenal Rest Tumors in Congenital Adrenal Hyperplasia: Study of a Cohort of Patients from a Single Italian Center. Children 2023, 10, 1457. https://doi.org/10.3390/children10091457
Ortolano R, Cassio A, Alqaisi RS, Candela E, Di Natale V, Assirelli V, Bernardini L, Bortolamedi E, Cantarelli E, Corcioni B, et al. Testicular Adrenal Rest Tumors in Congenital Adrenal Hyperplasia: Study of a Cohort of Patients from a Single Italian Center. Children. 2023; 10(9):1457. https://doi.org/10.3390/children10091457
Chicago/Turabian StyleOrtolano, Rita, Alessandra Cassio, Randa S. Alqaisi, Egidio Candela, Valeria Di Natale, Valentina Assirelli, Luca Bernardini, Elisa Bortolamedi, Erika Cantarelli, Beniamino Corcioni, and et al. 2023. "Testicular Adrenal Rest Tumors in Congenital Adrenal Hyperplasia: Study of a Cohort of Patients from a Single Italian Center" Children 10, no. 9: 1457. https://doi.org/10.3390/children10091457
APA StyleOrtolano, R., Cassio, A., Alqaisi, R. S., Candela, E., Di Natale, V., Assirelli, V., Bernardini, L., Bortolamedi, E., Cantarelli, E., Corcioni, B., Renzulli, M., Balsamo, A., & Baronio, F. (2023). Testicular Adrenal Rest Tumors in Congenital Adrenal Hyperplasia: Study of a Cohort of Patients from a Single Italian Center. Children, 10(9), 1457. https://doi.org/10.3390/children10091457








