The Most Valuable Predictive Factors for Bronchopulmonary Dysplasia in Very Preterm Infants
Abstract
1. Introduction
2. Methods
2.1. Subjects and Data Collection
2.2. Definitions
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Subjects
3.2. Factors Associated with Moderate to Severe BPD or Death
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, E.K.; Shin, S.H.; Kim, E.K.; Kim, H.S. Developmental outcomes of preterm infants with bronchopulmonary dysplasia-associated pulmonary hypertension at 18–24 months of corrected age. BMC Pediatr. 2019, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Sarici, S.Ü. Bronchopulmonary dysplasia: New insights about the definition, pathogenesis, epidemiology and pathology. Cocuk Sagligi Ve Hastalik. Dergisi 2006, 49, 60–70. [Google Scholar]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatr. Off. Publ. Am. Acad. Pediatr. 2010, 3, 126. [Google Scholar] [CrossRef]
- Fanaroff, A.A.; Stoll, B.J.; Wright, L.L.; Carlo, W.A.; Poole, W.K. Neonatal Research Network NICHD: Trends in neonatal morbidity and mortality for very low birthweight infants. Am. J. Obstet. Gynecol. 2007, 196, 147.e1–147.e8. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Lvarez-Fuente, M.; Ghazi, A.; Degraeuwe, P.; Villamor, E. Association of Chorioamnionitis with Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review, Meta-analysis, and Metaregression. JAMA Netw. Open 2019, 2, e1914611. [Google Scholar] [CrossRef]
- YaYang, T.; Shen, Q.; Wang, S.; Dong, T.; Liang, L.; Xu, F.; He, Y.; Li, C.; Luo, F.; Liang, J.; et al. Risk factors that affect the degree of bronchopulmonary dysplasia in very preterm infants: A 5-year retrospective study. BMC Pediatr. 2022, 22, 200. [Google Scholar] [CrossRef]
- Northway, W.H.; Jr Rosan, R.C.; Porter, D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, J.Y.; Yun, B.; Lee, B.; Choi, C.W.; Kim, B.I. Interstitial pneumonia pattern on day 7 chest radiograph predicts bronchopulmonary dysplasia in preterm infants. BMC Pediatr. 2017, 17, 125. [Google Scholar] [CrossRef]
- Jonduo, M.E.; Vallely, L.M.; Wand, H.; Sweeney, E.L.; Egli-Gany, D.; Kaldor, J.; Vallely, A.J.; Low, N. Adverse pregnancy and birth outcomes associated with Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum: A systematic review and meta-analysis. BMJ Open 2022, 12, e062990. [Google Scholar] [CrossRef]
- Sun, T.; Fu, J. Analysis of the Clinical Features of Intrauterine Ureaplasma urealyticum Infection in Preterm Infants: A Case-Control Study. Front. Pediatr. 2021, 9, 774150. [Google Scholar] [CrossRef]
- Beeton, M.L.; Maxwell, N.C.; Davies, P.L.; Nuttall, D.; McGreal, E.; Chakraborty, M.; Spiller, O.B.; Kotecha, S. Role of pulmonary infection in the development of chronic lung disease of prematurity. Eur. Respir. J. 2011, 37, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Glaser, K.; Gradzka-Luczewska, A.; Szymankiewicz-Breborowicz, M.; Kawczynska-Leda, N.; Henrich, B.; Waaga-Gasser, A.M.; Speer, C.P. Perinatal Ureaplasma Exposure Is Associated With Increased Risk of Late Onset Sepsis and Imbalanced Inflammation in Preterm Infants and May Add to Lung Injury. Front. Cell. Infect. Microbiol. 2019, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Panero, A.; Pacifico, L.; Rossi, N.; Roggini, M.; Chiesa, C. Ureaplasma urealyticum as a cause of pneumonia in preterm infants: Analysis of the white cell response. Arch. Dis. Child.-Fetal Neonatal Ed. 1995, 73, F37–F40. [Google Scholar] [CrossRef]
- Lorthe, E.; Torchin, H.; Delorme, P.; Ancel, P.-Y.; Marchand-Martin, L.; Foix-L’Hélias, L.; Benhammou, V.; Gire, C.; D’ercole, C.; Winer, N.; et al. Preterm premature rupture of membranes at 22-25 weeks’ gestation: Perinatal and 2-year outcomes within a national population-based study (EPIPAGE-2). Am. J. Obstet. Gynecol. 2018, 219, 298.e1–298.e14. [Google Scholar] [CrossRef]
- Fanczal, E.; Berecz, B.; Szijártó, A.; Gasparics, Á.; Varga, P. The Prognosis of Preterm Infants Born at the Threshold of Viability: Fog Over the Gray Zone-Population-Based Studies of Extremely Preterm Infants. Med. Sci. Monit. 2020, 26, e926947. [Google Scholar] [CrossRef]
- O’Reilly, M.; Sozo, F.; Harding, R. Impact of preterm birth and bronchopulmonary dysplasia on the developing lung: Long-term consequences for respiratory health. Clin. Exp. Pharmacol. Physiol. 2013, 40, 765–773. [Google Scholar] [CrossRef]
- Henderson-Smart, D.J.; Hutchinson, J.L.; Donoghue, D.A.; Evans, N.J.; Simpson, J.M.; Wright, I. Prenatal predictors of chronic lung disease in very preterm infants. Arch. Dis. Child.-Fetal Neonatal Ed. 2006, 91, F40–F45. [Google Scholar] [CrossRef]
- van Westering-Kroon, E.; Huizing, M.J.; Villamor-Martínez, E.; Villamor, E. Male Disadvantage in Oxidative Stress-Associated Complications of Prematurity: A Systematic Review, Meta-Analysis and Meta-Regression. Antioxidants 2021, 10, 1490. [Google Scholar] [CrossRef]
- Zozaya, C.; Avila-Alvarez, A.; Arruza, L.; Rodrigo, F.G.-M.; Fernandez-Perez, C.; Castro, A.; Cuesta, M.T.; Vacas, B.; Couce, M.L.; Torres, M.V.; et al. The Effect of Morbidity and Sex on Postnatal Growth of Very Preterm Infants: A Multicenter Cohort Study. Neonatology 2019, 115, 348–354. [Google Scholar] [CrossRef]
- Torday, J.S.N.H.; Fencl Mde, M.; Avery, M.E. Sex differences in fetal lung maturation. Am. Rev. Respir. Dis. 1981, 123, 205–208. [Google Scholar] [PubMed]
- Lien, Y.-C.; Zhang, Z.; Cheng, Y.; Polyak, E.; Sillers, L.; Falk, M.J.; Ischiropoulos, H.; Parry, S.; Simmons, R.A. Human Placental Transcriptome Reveals Critical Alterations in Inflammation and Energy Metabolism with Fetal Sex Differences in Spontaneous Preterm Birth. Int. J. Mol. Sci. 2021, 22, 7899. [Google Scholar] [CrossRef]
- Kotecha, S.J.; Lowe, J.; Kotecha, S. Does the sex of the preterm baby affect respiratory outcomes? Breathe 2018, 14, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Liggins, G.C.; Howie, R.N. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972, 50, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef]
- Luo, J.; Chen, J.; Li, Q.; Feng, Z. Differences in Clinical Characteristics and Therapy of Neonatal Acute Respiratory Distress Syndrome (ARDS) and Respiratory Distress Syndrome (RDS): A Retrospective Analysis of 925 Cases. Med. Sci. Monit. 2019, 25, 4992–4998. [Google Scholar] [CrossRef]
- Yuksel, B.; Greenough, A.; Karani, J. Prediction of chronic lung disease from the chest radiograph appearance at seven days of age. Acta Paediatr. 1993, 82, 944–947. [Google Scholar] [CrossRef]
- Almuntashiri, S.; Han, Y.; Zhu, Y.; Dutta, S.; Niazi, S.; Wang, X.; Siddiqui, B.; Zhang, D. CC16 Regulates Inflammation, ROS Generation and Apoptosis in Bronchial Epithelial Cells during Klebsiella pneumoniae Infection. Int. J. Mol. Sci. 2021, 22, 11459. [Google Scholar] [CrossRef]
- Lee, J.A.; Kim, M.J.; Oh, S.; Choi, B.M. Current Status of Therapeutic Strategies for Patent Ductus Arteriosus in Very-Low-Birth-Weight Infants in Korea. J. Korean Med. Sci. 2015, 30 (Suppl. S1), S59–S66. [Google Scholar] [CrossRef]
- Ognean, M.L.; Boantă, O.; Kovacs, S.; Zgârcea, C.; Dumitra, R.; Olariu, E.; Andreicuţ, D. Persistent Ductus Arteriosus in Critically Ill Preterm Infants. J. Crit. Care Med. 2016, 2, 175–184. [Google Scholar] [CrossRef]
| Variables | No BPD or Death n = 113 | BPD or Death n = 21 | t or χ2 | p |
|---|---|---|---|---|
| Gestational age (weeks) mean ± SD | 29.5 ± 1.4 | 27.2 ± 2.0 | 5.009 | <0.001 |
| Weight (g) mean ± SD | 1402 ± 275 | 976 ± 260 | 6.581 | <0.001 |
| Male (%) | 66 (58.4) | 17 (81.0) | 3.818 | 0.051 |
| UU colonization (%) | 22 (19.5) | 8 (38.1) | 2.545 | 0.111 |
| UU infection (%) | 6 (5.3) | 5 (23.8) | 5.776 | 0.016 |
| PDA (%) | 51 (45.1) | 16 (76.2) | 6.833 | 0.009 |
| PDA ≥ 1.5 mm (%) | 43 (38.1) | 14 (66.7) | 10.829 | 0.004 |
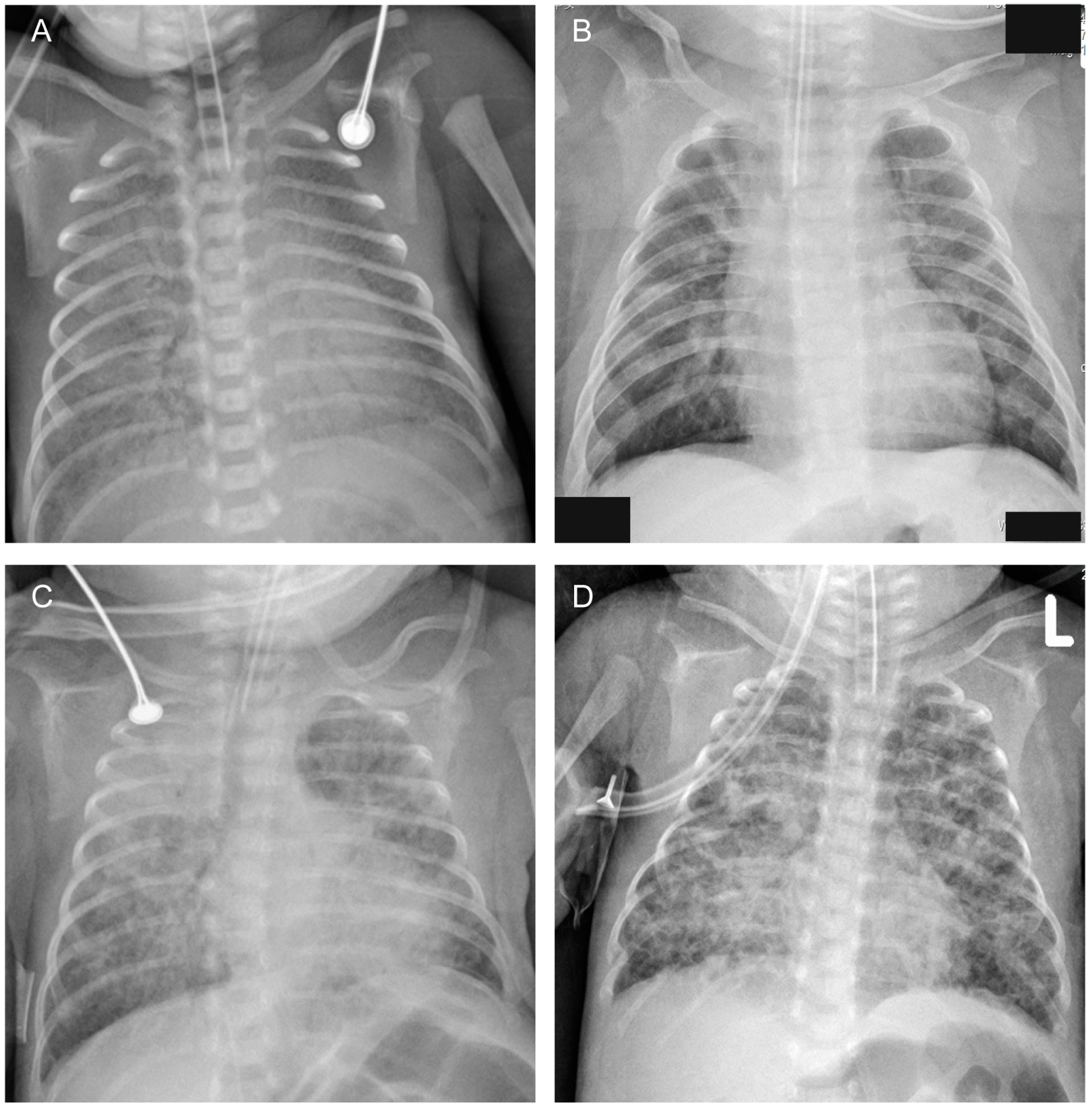
| Chest radiographs: diffuse opacities or grid shadows/interstitial opacities or mass opacities or cystic lucencies (%) | 13 (11.5) | 12 (57.1) | 21.391 | <0.001 |
| Serum CRP ≥ 10 mg/dl (%) | 12 (10.6) | 4 (19.0) | 0.529 | 0.467 |
| Factors | OR | 95%CI | p | |
|---|---|---|---|---|
| Upper Limit | Lower Limit | |||
| Gestational age (divided into 3 groups: <28 weeks, 28~29+6 weeks, 30~31+6 weeks) | 4.6 | 2.1 | 10.1 | <0.001 |
| Chest radiographs: diffuse opacities or grid shadows/interstitial opacities or mass opacities or cystic lucencies | 6.8 | 2 | 23.1 | 0.002 |
| Male sex | 3.9 | 1 | 15 | 0.049 |
| UU infection | - | - | - | 0.288 |
| PDA ≥ 1.5 mm | - | - | - | 0.118 |
| Variables | Cut-Off Value | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Gestational age | 28 weeks | 67% | 90% | 56% | 94% |
| 30 weeks | 81% | 60% | 27% | 95% | |
| Male | - | 81% | 42% | 20% | 92% |
| Chest radiographs: diffuse opacities or grid shadows/interstitial opacities or mass opacities or cystic lucencies | - | 57% | 88% | 48% | 92% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhang, Z.; Xu, L.; Chen, C. The Most Valuable Predictive Factors for Bronchopulmonary Dysplasia in Very Preterm Infants. Children 2023, 10, 1373. https://doi.org/10.3390/children10081373
Chen W, Zhang Z, Xu L, Chen C. The Most Valuable Predictive Factors for Bronchopulmonary Dysplasia in Very Preterm Infants. Children. 2023; 10(8):1373. https://doi.org/10.3390/children10081373
Chicago/Turabian StyleChen, Wenwen, Zhenhai Zhang, Liping Xu, and Chao Chen. 2023. "The Most Valuable Predictive Factors for Bronchopulmonary Dysplasia in Very Preterm Infants" Children 10, no. 8: 1373. https://doi.org/10.3390/children10081373
APA StyleChen, W., Zhang, Z., Xu, L., & Chen, C. (2023). The Most Valuable Predictive Factors for Bronchopulmonary Dysplasia in Very Preterm Infants. Children, 10(8), 1373. https://doi.org/10.3390/children10081373





