Group Changes in Cortisol and Heart Rate Variability of Children with Down Syndrome and Children with Autism Spectrum Disorder during Dog-Assisted Therapy
Abstract
1. Introduction
Physiological Measurements of Stress
2. Materials and Methods
2.1. Ethical Considerations
2.2. Design
2.3. Participants
2.4. Procedure
2.5. HRV Measurements
2.6. Cortisol Measurements
3. Results
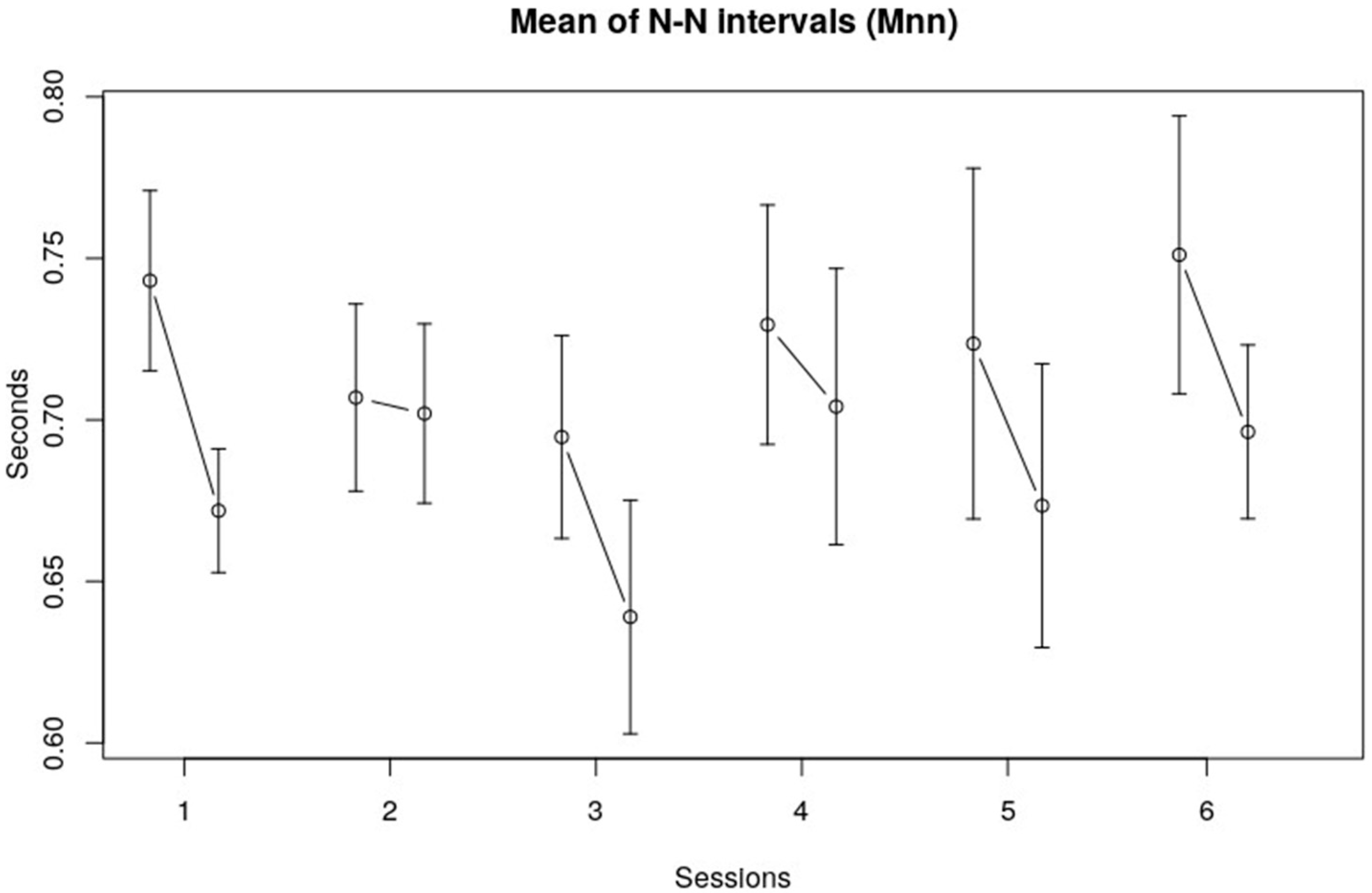
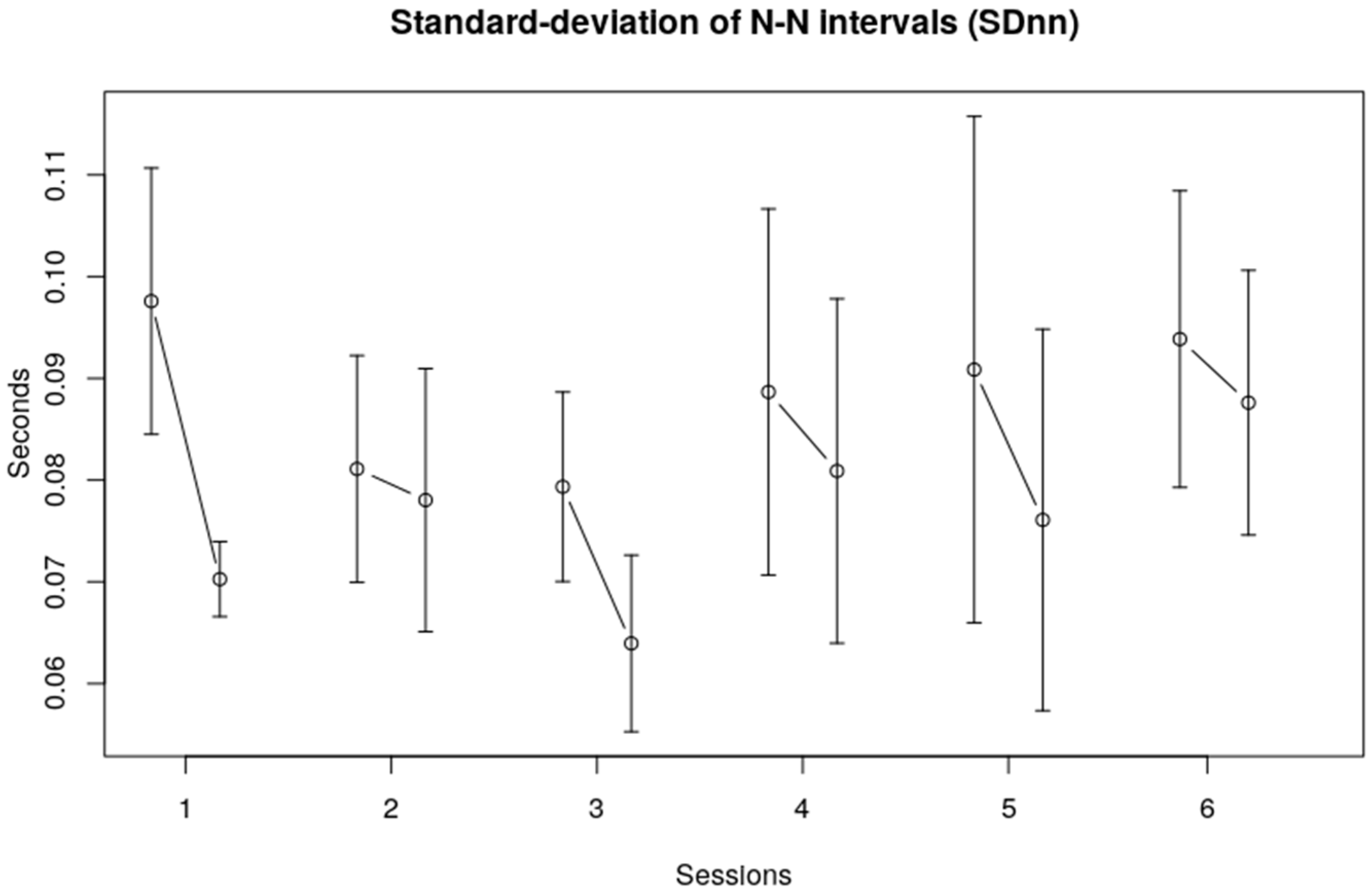
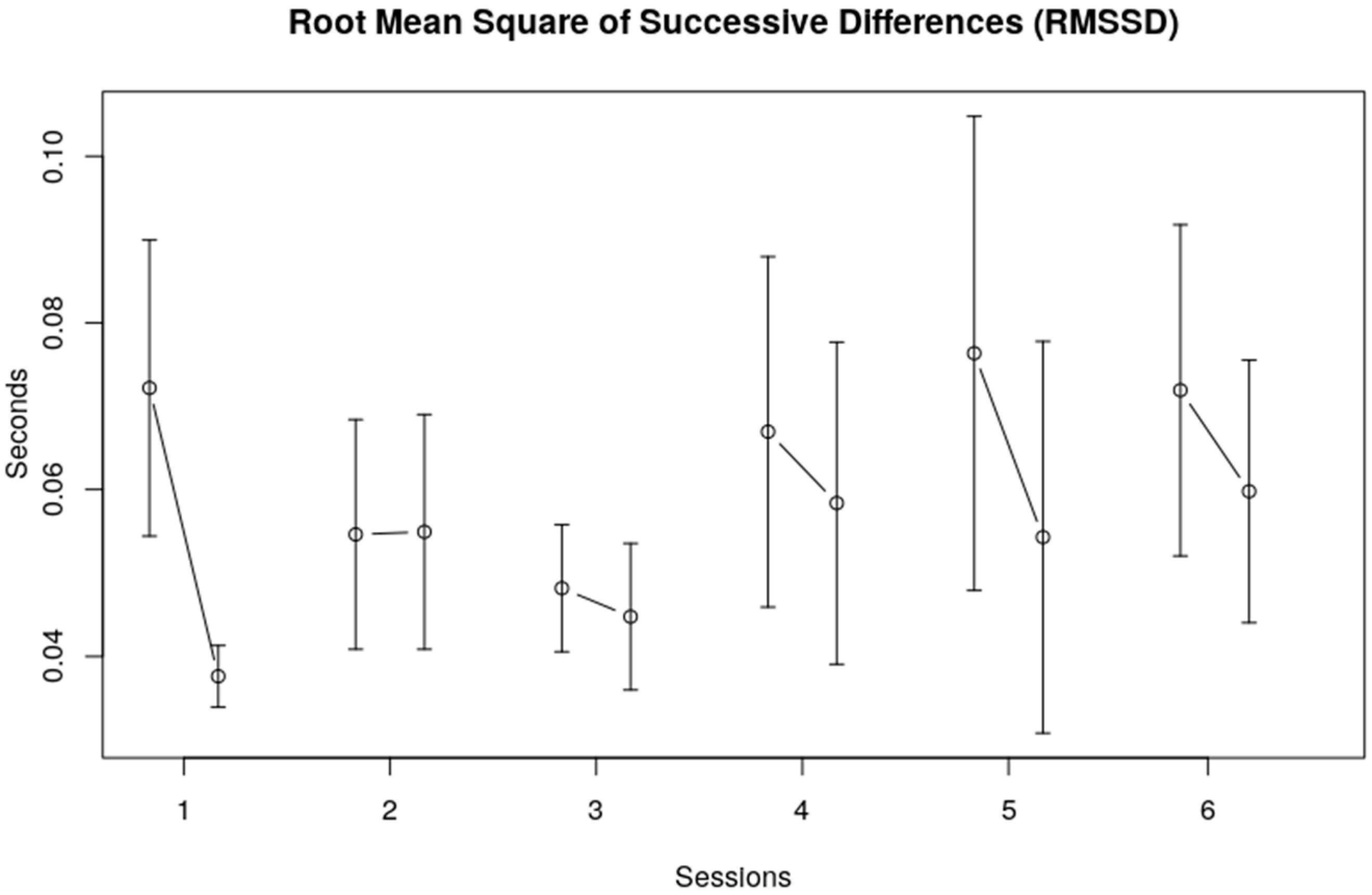
3.1. Heart Rate Variability, Time Domain
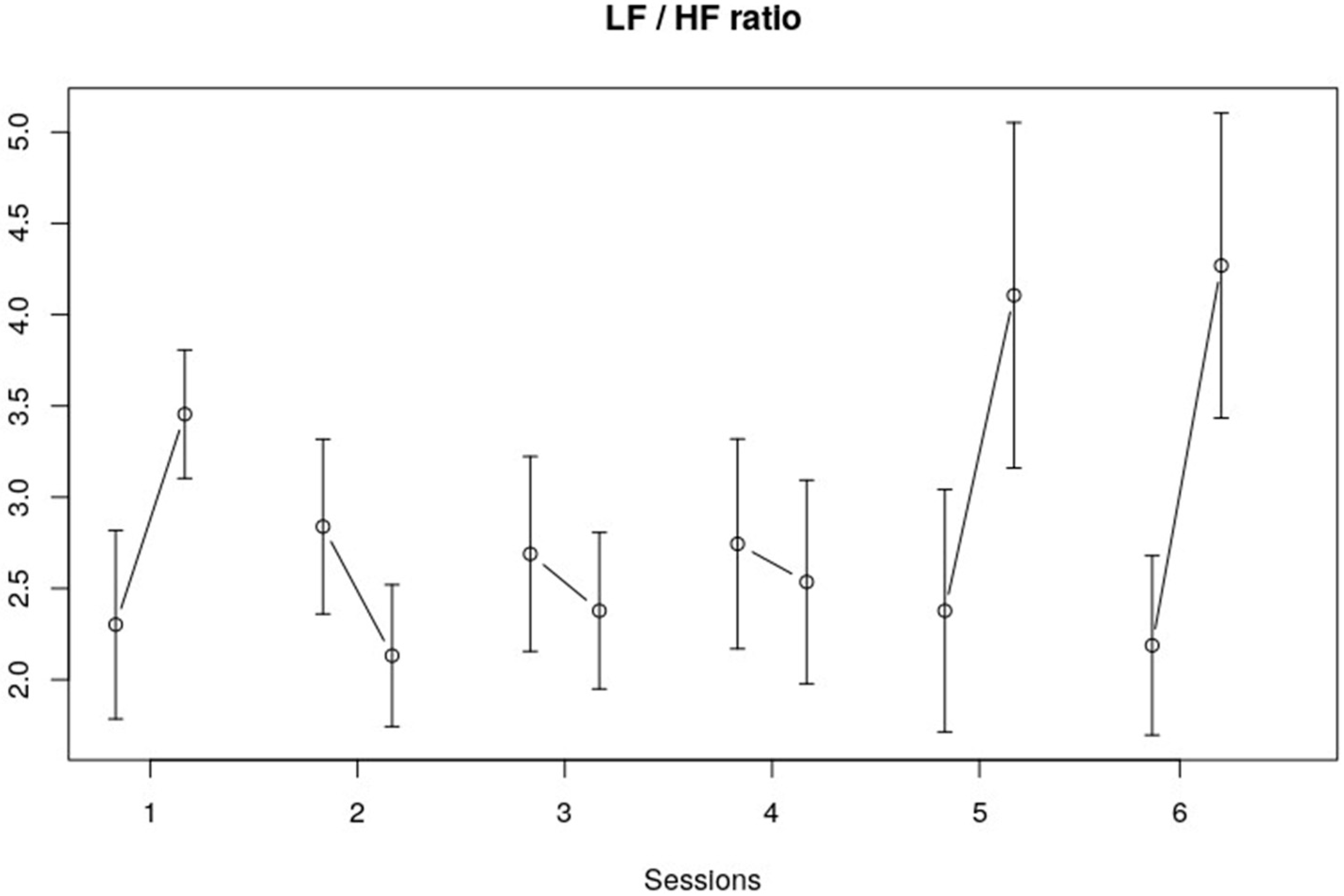
3.2. Frequency Domain
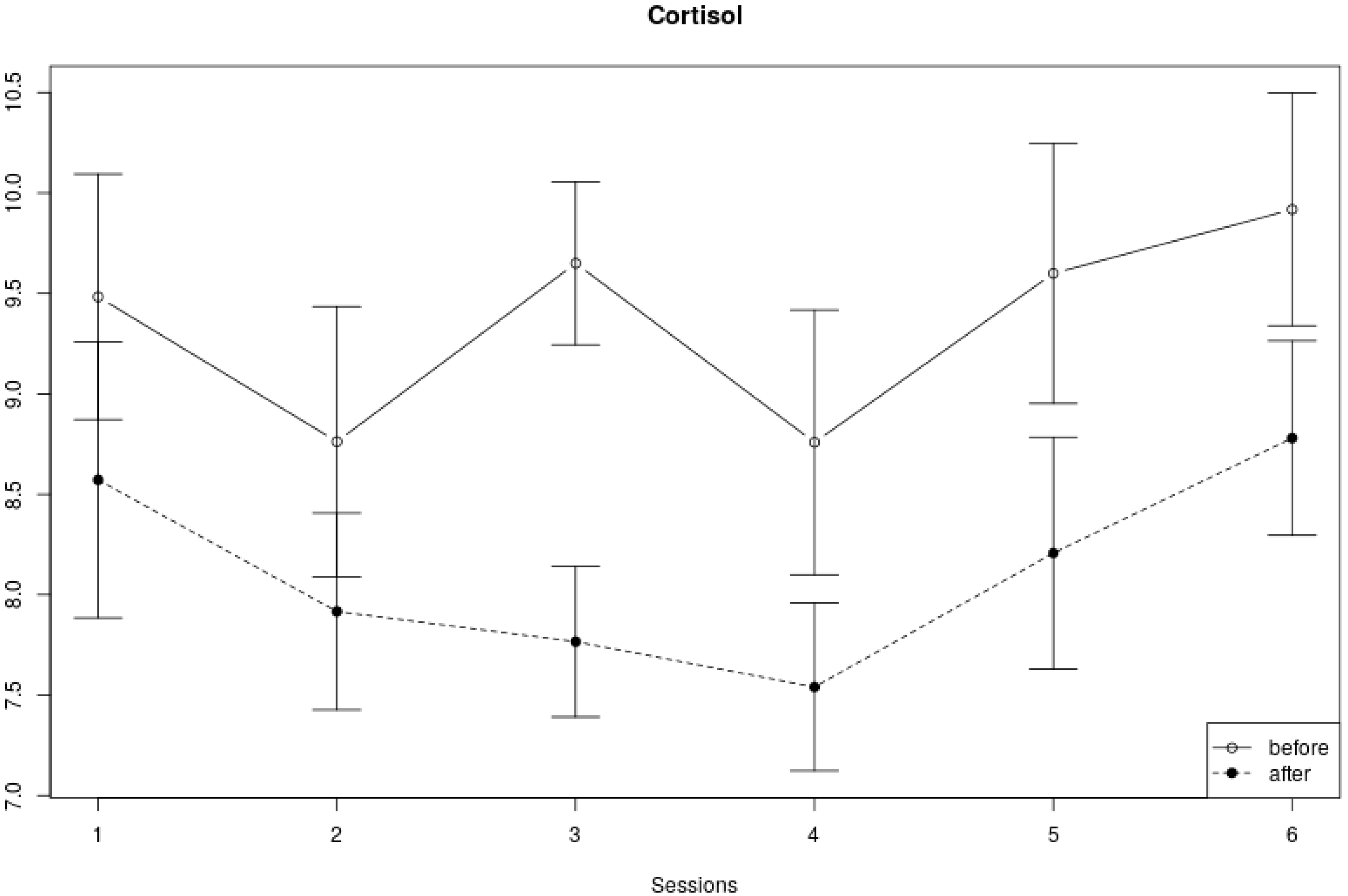
3.3. Cortisol Measures
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jansen, L.M.C.; Wied, C.C.G.-D.; van der Gaag, R.-J.; van Engeland, H. Differentiation between Autism and Multiple Complex Developmental Disorder in Response to Psychosocial Stress. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2003, 28, 582–590. [Google Scholar] [CrossRef]
- Jansen, L.M.C.; Wied, C.C.G.-D.; Wiegant, V.M.; Westenberg, H.G.M.; Lahuis, B.E.; van Engeland, H. Autonomic and Neuroendocrine Responses to a Psychosocial Stressor in Adults with Autistic Spectrum Disorder. J. Autism Dev. Disord. 2006, 36, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Wijker, C.; Kupper, N.; Leontjevas, R.; Spek, A.; Enders-Slegers, M.J. The effects of Animal Assisted Therapy on autonomic and endocrine activity in adults with autism spectrum disorder: A randomized controlled trial. Gen. Hosp. Psychiatry 2021, 72, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, S.Z.; Kratochwill, T.R. Self-Reported Fears in Children with and without Mental Retardation. Ment. Retard. 1997, 35, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.G.C.; Schuengel, C.; Stolk, J. Understanding Challenging Behaviour in People with Severe and Profound Intellectual Disability: A Stress-Attachment Model. J. Intellect. Disabil. Res. 2002, 46, 445–453. [Google Scholar] [CrossRef]
- Haruvi-Lamdan, N.; Horesh, D.; Zohar, S.; Kraus, M.; Golan, O. Autism spectrum disorder and post-traumatic stress disorder: An unexplored co-occurrence of conditions. Autism 2020, 24, 884–898. [Google Scholar] [CrossRef]
- Sánchez-Teruel, D.; Robles-Bello, M.A. Preliminary study on psychometric properties of an anxiety scale in Down syndrome with anxiety symptoms. Int. J. Psychol. Res. 2020, 13, 50–61. [Google Scholar] [CrossRef]
- Berry, A.; Borgi, M.; Francia, N.; Alleva, E.; Cirulli, F. Use of Assistance and Therapy Dogs for Children with Autism Spectrum Disorders: A Critical Review of the Current Evidence. J. Altern. Complement. Med. 2013, 19, 73–80. [Google Scholar] [CrossRef]
- Silva, K.; Correia, R.; Lima, M.; Magalhães, A.; de Sousa, L. Can Dogs Prime Autistic Children for Therapy? Evidence from a Single Case Study. J. Altern. Complement. Med. 2011, 17, 655–659. [Google Scholar] [CrossRef]
- Hall, S.S.; Wright, H.F.; Hames, A.; Mills, D.S. The Long-Term Benefits of Dog Ownership in Families with Children with Autism. J. Vet. Behav. 2016, 13, 46–54. [Google Scholar] [CrossRef]
- Wright, H.; Hall, S.; Hames, A.; Hardiman, J.; Mills, R.; PAWS Project Team; Mills, D. Pet Dogs Improve Family Functioning and Reduce Anxiety in Children with Autism Spectrum Disorder. Anthrozoos 2015, 28, 611–624. [Google Scholar] [CrossRef]
- Viau, R.; Arsenault-Lapierre, G.; Fecteau, S.; Champagne, N.; Walker, C.-D.; Lupien, S. Effect of Service Dogs on Salivary Cortisol Secretion in Autistic Children. Psychoneuroendocrinology 2010, 35, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Kertes, D.A.; Liu, J.; Hall, N.J.; Hadad, N.A.; Wynne, C.D.L.; Bhatt, S.S. Effect of Pet Dogs on Children’s Perceived Stress and Cortisol Stress Response. Soc. Dev. 2016, 26, 382–401. [Google Scholar] [CrossRef] [PubMed]
- Motomura, N.; Yagi, T.; Ohyama, H. Animal Assisted Therapy for People with Dementia. Psychogeriatrics 2004, 4, 40–42. [Google Scholar] [CrossRef]
- Allen, K.; Shykoff, B.E.; Izzo, J.L. Pet Ownership, but Not ACE Inhibitor Therapy, Blunts Home Blood Pressure Responses to Mental Stress. Hypertension 2001, 38, 815–820. [Google Scholar] [CrossRef]
- Beetz, A.; Julius, H.; Turner, D.; Kotrschal, K. Effects of Social Support by a Dog on Stress Modulation in Male Children with Insecure Attachment. Front. Psychol. 2012, 3, 352. [Google Scholar] [CrossRef]
- Beetz, A. Theories and Possible Processes of Action in Animal Assisted Interventions. Appl. Dev. Sci. 2017, 21, 139–149. [Google Scholar] [CrossRef]
- Hart, L.A. Psychosocial Benefits of Animal Companionship. In Handbook on Animal-Assisted Therapy; Elsevier: Amsterdam, The Netherlands, 2006; pp. 59–78. [Google Scholar] [CrossRef]
- Barker, S.B.; Knisely, J.S.; Schubert, C.M.; Green, J.D.; Ameringer, S. The Effect of an Animal-Assisted Intervention on Anxiety and Pain in Hospitalized Children. Anthrozoös 2015, 28, 101–112. [Google Scholar] [CrossRef]
- Germone, M.M.; Gabriels, R.L.; Guérin, N.A.; Pan, Z.; Banks, T.; O’haire, M.E. Animal-Assisted Activity Improves Social Behaviors in Psychiatrically Hospitalized Youth with Autism. Autism 2019, 23, 1740–1751. [Google Scholar] [CrossRef]
- Chur-Hansen, A.; McArthur, M.; Winefield, H.; Hanieh, E.; Hazel, S. Animal-Assisted Interventions in Children’s Hospitals: A Critical Review of the Literature. Anthrozoös 2014, 27, 5–18. [Google Scholar] [CrossRef]
- Martin, F.; Farnum, J. Animal-Assisted Therapy for Children with Pervasive Developmental Disorders. West. J. Nurs. Res. 2002, 24, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Redefer, L.A.; Goodman, J.F. Brief Report: Pet-Facilitated Therapy with Autistic Children. J. Autism Dev. Disord. 1989, 19, 461–467. [Google Scholar] [CrossRef] [PubMed]
- O’haire, M.E. Research on Animal-Assisted Intervention and Autism Spectrum Disorder, 2012–2015. Appl. Dev. Sci. 2017, 21, 200–216. [Google Scholar] [CrossRef]
- O’Haire, M.E.; McKenzie, S.J.; Beck, A.M.; Slaughter, V. Animals May Act as Social Buffers: Skin Conductance Arousal in Children with Autism Spectrum Disorder in a Social Context. Dev. Psychobiol. 2015, 57, 584–595. [Google Scholar] [CrossRef]
- Griffioen, R.E.; van der Steen, S.; Verheggen, T.; Enders-Slegers, M.J.; Cox, R. Changes in behavioural synchrony during dog-assisted therapy for children with autism spectrum disorder and children with down syndrome. J. Appl. Res. Intellect. Disabil. 2020, 33, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Tabares, C.; Vicente, F.; Sánchez, S.; Aparicio, A.; Alejo, S.; Cubero, J. Quantification of Hormonal Changes by Effects of Hippotherapy in the Autistic Population. Neurochem. J. 2012, 6, 311–316. [Google Scholar] [CrossRef]
- Meints, K.; Brelsford, V.L.; Dimolareva, M.; Maréchal, L.; Pennington, K.; Rowan, E.; Gee, N.R. Can dogs reduce stress levels in school children? effects of dog-assisted interventions on salivary cortisol in children with and without special educational needs using randomized controlled trials. PLoS ONE 2022, 17, e0269333. [Google Scholar] [CrossRef] [PubMed]
- Almela, M.; Hidalgo, V.; Villada, C.; van der Meij, L.; Espín, L.; Gómez-Amor, J.; Salvador, A. Salivary Alpha-Amylase Response to Acute Psychosocial Stress: The Impact of Age. Biol. Psychol. 2011, 87, 421–429. [Google Scholar] [CrossRef]
- Oberle, E.; Schonert-Reichl, K.A. Stress contagion in the classroom? The link between classroom teacher burnout and morning cortisol in elementary school students. Soc. Sci. Med. 2016, 159, 30–37. [Google Scholar] [CrossRef]
- Rai, B.; Kaur, J.; Foing, B.H. Salivary Amylase and Stress during Stressful Environment: Three Mars Analog Mission Crews Study. Neurosci. Lett. 2012, 518, 23–26. [Google Scholar] [CrossRef]
- Spratt, E.G.; Nicholas, J.S.; Brady, K.T.; Carpenter, L.A.; Hatcher, C.R.; Meekins, K.A.; Furlanetto, R.W.; Charles, J.M. Enhanced cortisol response to stress in children in autism. J. Autism Dev. Disord. 2012, 42, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Takai, N.; Yamaguchi, M.; Aragaki, T.; Eto, K.; Uchihashi, K.; Nishikawa, Y. Effect of Psychological Stress on the Salivary Cortisol and Amylase Levels in Healthy Young Adults. Arch. Oral Biol. 2004, 49, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, K.; McCarthy, A.M.; Kleiber, C.; Lutgendorf, S.; Tsalikian, E. Strategies for Salivary Cortisol Collection and Analysis in Research with Children. Appl. Nurs. Res. 2006, 19, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Rieger, A.; Stoll, R.; Kreuzfeld, S.; Behrens, K.; Weippert, M. Heart Rate and Heart Rate Variability as Indirect Markers of Surgeons’ Intraoperative Stress. Int. Arch. Occup. Environ. Heal. 2013, 87, 165–174. [Google Scholar] [CrossRef]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A Meta-Analysis of Heart Rate Variability and Neuroimaging Studies: Implications for Heart Rate Variability as a Marker of Stress and Health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef]
- van Boxtel, G.J.M.; Cluitmans, P.J.M.; Raymann, R.J.E.M.; Ouwerkerk, M.; Denissen, A.J.M.; Dekker, M.K.J.; Sitskoorn, M.M. Heart Rate Variability, Sleep, and the Early Detection of Post-Traumatic Stress Disorder. In Sleep and Combat-Related Post Traumatic Stress Disorder; Springer: New York, NY, USA, 2018; pp. 253–263. [Google Scholar] [CrossRef]
- Tsao, J.C.; Evans, S.; Seidman, L.C.; Lung, K.C.; Zeltzer, L.K.; Naliboff, B.D. Heart Rate Variability as a Biomarker for Autonomic Nervous System Response Differences between Children with Chronic Pain and Healthy Control Children. J. Pain Res. 2013, 6, 449–457. [Google Scholar] [CrossRef]
- Domènec, E.; Ristol, F. Animal Assisted Therapy: Techniques and Exercises for Dog Assisted Interventions; CTAC Group: Falls Church, VA, USA, 2012. [Google Scholar]
- Chamchad, D.; Horrow, J.; Nakhamchik, L.; Arkoosh, V. Heart Rate Variability Changes during Pregnancy: An Observational Study. Int. J. Obstet. Anesth. 2007, 16, 106–109. [Google Scholar] [CrossRef]
- May, D.K.; Seivert, N.P.; Cano, A.; Casey, R.J.; Johnson, A.; May, D. Animal-Assisted Therapy for Youth: A Systematic Methodological Critiqu. Hum.-Anim. Interact. Bull. 2016, 4, 1–18. [Google Scholar] [CrossRef]
- Handlin, L.; Hydbring-Sandberg, E.; Nilsson, A.; Ejdebäck, M.; Jansson, A.; Uvnäs-Moberg, K. Short-Term Interaction between Dogs and Their Owners: Effects on Oxytocin, Cortisol, Insulin and Heart Rate—An Exploratory Study. Anthrozoos A Multidiscip. J. Interact. People Anim. 2011, 24, 301–315. [Google Scholar] [CrossRef]
- Barker, S.B.; Barker, R.T.; McCain, N.L.; Schubert, C.M. A Randomized Cross-over Exploratory Study of the Effect of Visiting Therapy Dogs on College Student Stress before Final Exams. Anthrozoos 2016, 29, 35–46. [Google Scholar] [CrossRef]

| F | df | p | ||
|---|---|---|---|---|
| Mnn | Session | 2.0064 | 5, 113 | 0.0830 |
| Interval within session | 12.8576 | 1, 113 | 0.0005 * | |
| Session x within session | 0.6853 | 5, 113 | 0.6355 | |
| SDnn | Session | 1.5261 | 5, 113 | 0.1873 |
| Interval within session | 5.4518 | 1, 113 | 0.0213 * | |
| Session x within session | 0.2777 | 5, 113 | 0.9245 | |
| RMSSD | Session | 1.1591014 | 5, 113 | 0.1683 |
| Interval within session | 12.959291 | 1, 113 | 0.0005 * | |
| Session x within session | 1.018265 | 5, 113 | 0.4104 |
| F | df | p | ||
|---|---|---|---|---|
| LF | Session | 0.32508 | 5, 113 | 0.8970 |
| Interval within session | 0.23844 | 1, 113 | 0.6263 | |
| Session x within session | 1.67228 | 5, 113 | 0.1470 | |
| HF | Session | 0.51381 | 5, 113 | 0.7653 |
| Interval within session | 3.37757 | 1, 113 | 0.0687 | |
| Session x within session | 1.06261 | 5, 113 | 0.3850 | |
| LF/HF | Session | 0.82692 | 5, 113 | 0.5331 |
| Interval within session | 4.96285 | 1, 113 | 0.0279 * | |
| Session x within session | 3.07045 | 5, 113 | 0.0123 * |
| F | df | p | ||
|---|---|---|---|---|
| Cortisol | Over 6 sessions | 1.37 | 5, 159 | 0.24 |
| Within-session | 25.36 | 1, 159 | <0.0001 * | |
| Interaction | 0.42 | 5, 159 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griffioen, R.E.; van Boxtel, G.J.M.; Verheggen, T.; Enders-Slegers, M.-J.; Van Der Steen, S. Group Changes in Cortisol and Heart Rate Variability of Children with Down Syndrome and Children with Autism Spectrum Disorder during Dog-Assisted Therapy. Children 2023, 10, 1200. https://doi.org/10.3390/children10071200
Griffioen RE, van Boxtel GJM, Verheggen T, Enders-Slegers M-J, Van Der Steen S. Group Changes in Cortisol and Heart Rate Variability of Children with Down Syndrome and Children with Autism Spectrum Disorder during Dog-Assisted Therapy. Children. 2023; 10(7):1200. https://doi.org/10.3390/children10071200
Chicago/Turabian StyleGriffioen, Richard E., Geert J. M. van Boxtel, Theo Verheggen, Marie-Jose Enders-Slegers, and Steffie Van Der Steen. 2023. "Group Changes in Cortisol and Heart Rate Variability of Children with Down Syndrome and Children with Autism Spectrum Disorder during Dog-Assisted Therapy" Children 10, no. 7: 1200. https://doi.org/10.3390/children10071200
APA StyleGriffioen, R. E., van Boxtel, G. J. M., Verheggen, T., Enders-Slegers, M.-J., & Van Der Steen, S. (2023). Group Changes in Cortisol and Heart Rate Variability of Children with Down Syndrome and Children with Autism Spectrum Disorder during Dog-Assisted Therapy. Children, 10(7), 1200. https://doi.org/10.3390/children10071200






