Danish Linguistic Validation and Cultural Adaptation of the LIMB-Q Kids
Abstract
1. Introduction
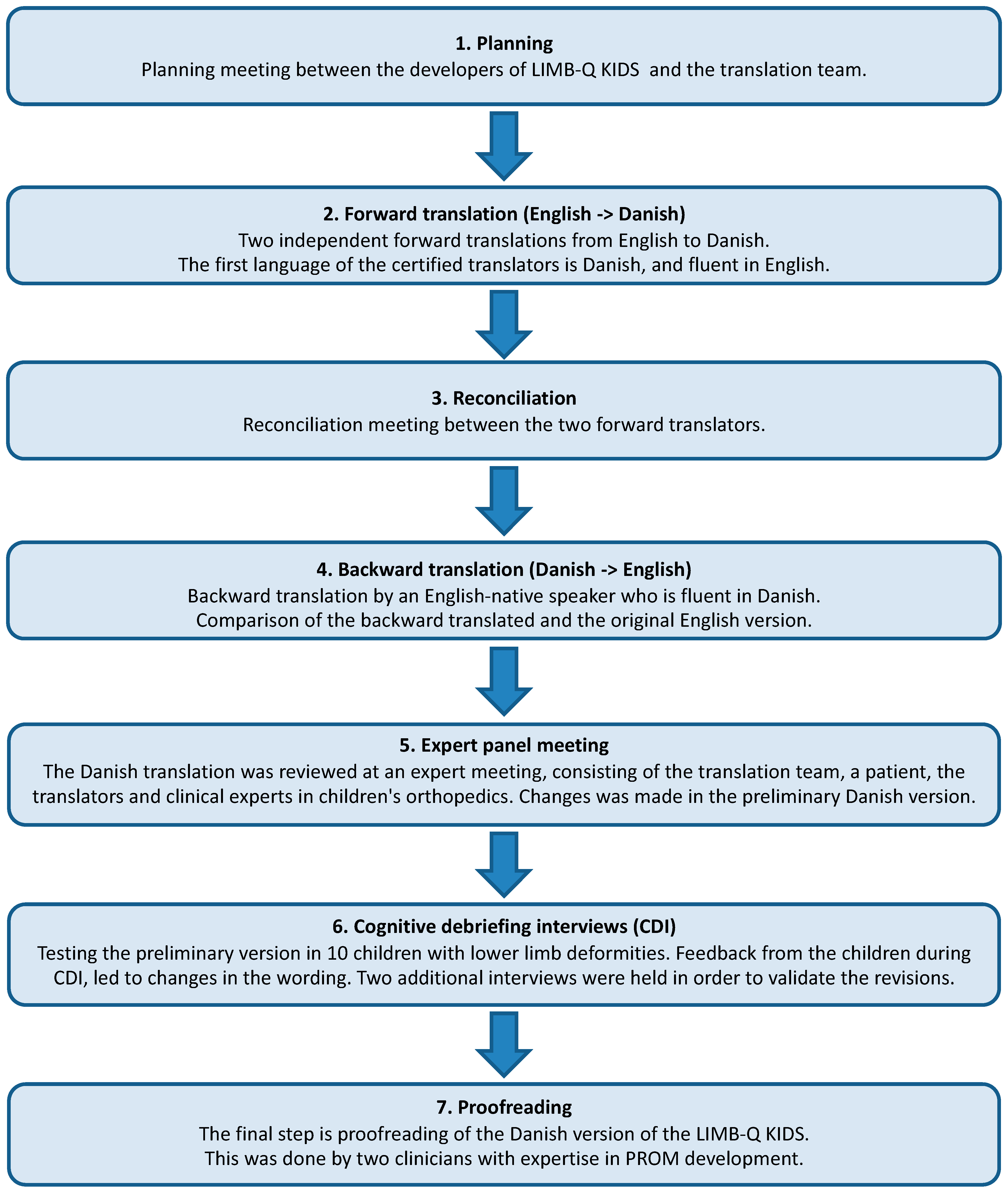
2. Materials and Methods
- 1.
- Planning
- 2.
- Forward translation
- 3.
- Reconciliation
- 4.
- Back translation
- 5.
- Expert panel meeting
- 6.
- Cognitive debriefing interviews (CDI)
- 7.
- Proofreading
3. Results
3.1. Forward Translation
3.2. Backward Translation
3.3. Expert Panel Meeting
3.4. Cognitive Debriefing Interviews
3.5. Proofreading
4. Discussion
4.1. Strengths and Limitations
4.2. Applicability and Development of the LIMB-Q KIDS Module
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amakoutou, K.; Liu, R.W. Current use of patient-reported outcomes in pediatric limb deformity surgery. J. Pediatr. Orthop. B 2021, 30, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Chhina, H.; Klassen, A.; Bade, D.; Kopec, J.; Cooper, A. Establishing content validity of LIMB-Q Kids: A new patient-reported outcome measure for lower limb deformities. Qual. Life Res. 2022, 31, 2805–2818. [Google Scholar] [CrossRef]
- Rerucha, C.M.; Dickison, C.; Baird, D.C. Lower Extremity Abnormalities in Children. Am. Fam. Physician 2017, 96, 226–233. [Google Scholar] [PubMed]
- Dobbe, A.M.; Gibbons, P.J. Common paediatric conditions of the lower limb. J. Paediatr. Child Health 2017, 53, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.E.; Davis, L.E. Leg Length Discrepancy: The Natural History (And What Do We Really Know). J. Pediatr. Orthop. 2019, 39, S10–S13. [Google Scholar] [CrossRef]
- Schirò, G.R.; Sessa, S.; Piccioli, A.; Maccauro, G. Primary amputation vs limb salvage in mangled extremity: A systematic review of the current scoring system. BMC Musculoskelet. Disord. 2015, 16, 372. [Google Scholar] [CrossRef]
- Grzebień, A.; Chabowski, M.; Malinowski, M.; Uchmanowicz, I.; Milan, M.; Janczak, D. Analysis of selected factors determining quality of life in patients after lower limb amputation—A review article. Ann. Surg. 2017, 89, 57–61. [Google Scholar] [CrossRef]
- McGee, R.G. How to Include Patient-Reported Outcome Measures in Clinical Trials. Curr. Osteoporos. Rep. 2020, 18, 480–485. [Google Scholar] [CrossRef]
- Patel, D.A.; Sharda, R.; Hovis, K.L.; Nichols, E.E.; Sathe, N.; Penson, D.F.; Feurer, I.D.; McPheeters, M.L.; Vaezi, M.F.; Francis, D.O. Patient-reported outcome measures in dysphagia: A systematic review of instrument development and validation. Dis. Esophagus 2017, 30, 1–23. [Google Scholar] [CrossRef]
- Phillips, L.; Carsen, S.; Vasireddi, A.; Mulpuri, K. Use of Patient-reported Outcome Measures in Pediatric Orthopaedic Literature. J. Pediatr. Orthop. 2018, 38, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Chhina, H.; Klassen, A.; Kopec, J.; Park, S.; Fortes, C.; Cooper, A. Quality of life of children with lower limb deformities: A systematic review of patient-reported outcomes and development of a preliminary conceptual framework. J. Limb Lengthening Reconstr. 2017, 3, 19. [Google Scholar] [CrossRef]
- Chhina, H.; Klassen, A.F.; Kopec, J.A.; Oliffe, J.; Iobst, C.; Dahan-Oliel, N.; Aggarwal, A.; Nunn, T.; Cooper, A.P. What matters to children with lower limb deformities: An international qualitative study guiding the development of a new patient-reported outcome measure. J. Patient-Rep. Outcomes 2021, 5, 30. [Google Scholar] [CrossRef]
- Wild, D.; Grove, A.; Martin, M.; Eremenco, S.; McElroy, S.; Verjee-Lorenz, A.; Erikson, P. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: Report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 2005, 8, 94–104. [Google Scholar] [CrossRef]
- Team Q-P. Q-Portfolio. 2023. Available online: https://qportfolio.org/ (accessed on 20 June 2023).
- Willis, G. Cognitive Interviewing: A Tool for Improving Questionnaire Design. 2005. Available online: https://methods.sagepub.com/book/cognitive-interviewing (accessed on 20 June 2023).
- Krogsgaard, M.R.; Brodersen, J.; Christensen, K.B.; Siersma, V.; Jensen, J.; Hansen, C.F.; Engebretsen, L.; Visnes, H.; Forssblad, M.; Comins, J.D. How to translate and locally adapt a PROM. Assessment of cross-cultural differential item functioning. Scand. J. Med. Sci. Sports 2021, 31, 999–1008. [Google Scholar] [CrossRef]
- Fry, A.; Mitchell, S.A.; Wiener, L. Considerations for conducting and reporting digitally supported cognitive interviews with children and adults. J. Patient-Rep. Outcomes 2021, 5, 131. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.; Fresen, J.; Gosheger, G.; Chhina, H.; Brune, C.S.; Toporowski, G.; Frommer, A.; Laufer, A.; Cooper, A.; Roedl, R.; et al. LIMB-Q Kids—German Translation and Cultural Adaptation. Children 2022, 9, 1405. [Google Scholar] [CrossRef]
- Kane, L.T.; Namdari, S.; Plummer, O.R.; Beredjiklian, P.; Vaccaro, A.; Abboud, J.A. Use of Computerized Adaptive Testing to Develop More Concise Patient-Reported Outcome Measures. JBJS Open Access 2020, 5, e0052. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, P.D.; Borst, E.W.; Green, S.A.; Marx, R.G.; Fragomen, A.T.; Rozbruch, S.R. Validation of a modified Scoliosis Research Society instrument for patients with limb deformity: The limb deformity-Scoliosis Research Society (LD-SRS) score. J. Limb Lengthening Reconstr. 2016, 2, 86. [Google Scholar] [CrossRef]
- Leggett, H.; Scantlebury, A.; Byrne, A.; Harden, M.; Hewitt, C.; O’carroll, G.; Sharma, H.; McDaid, C.; Adamson, J.; Cocks, K.; et al. Exploring what is important to patients with regards to quality of life after experiencing a lower limb reconstructive procedure: A qualitative evidence synthesis. Health Qual. Life Outcomes 2021, 19, 158. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Iobst, C.A.; Rozbruch, S.R.; Sabharwal, S.; Eismann, E.A. Limb Lengthening and Reconstruction Society AIM index reliably assesses lower limb deformity. Clin. Orthop. Relat. Res. 2013, 471, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.W.H.; Wong, C.K.H.; Cheung, J.P.Y. Comparative study of the use of Paediatric Quality of Life Inventory 4.0 generic core scales in paediatric patients with spine and limb pathologies. Bone Jt. J. 2020, 102-B, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Bakircioglu, S.; Goker, B.; Yilmaz, A.; Aksoy, T.; Aksoy, M.C.; Yilmaz, G. Outcomes of Limb Lengthening with Computer-assisted Hexapod External Fixators in the Treatment of Fibular Hemimelia. J. Pediatr. Orthop. 2023. [Google Scholar] [CrossRef] [PubMed]
- Arguelles, G.R.B.; Shin, M.B.; Lebrun, D.G.M.; Kocher, M.S.M.; Baldwin, K.D.M.; Patel, N.M.M. The Majority of Patient-reported Outcome Measures in Pediatric Orthopaedic Research Are Used without Validation. J. Pediatr. Orthop. 2021, 41, e74–e79. [Google Scholar] [CrossRef] [PubMed]
| Patient | Age (Years) | Gender (M/F) | Diagnosis | Type of Treatment | Stage of Treatment |
|---|---|---|---|---|---|
| First Round of Interviews | |||||
| 1 | 15 | M | HME | Ring fixation tibia (TSF) | Ongoing |
| 2 | 17 | M | Post Traumatic Proximal Tibia | Ring fixation tibia (TLHex) | Ongoing |
| 3 | 11 | F | Genu Valgum | 8-Plates | Planned |
| 4 | 13 | M | PFFD | Intramedullary femoral lengthening + 8-plates | Previous |
| 5 | 15 | M | Tall Stature | Hemiepiphysiodesis | Planned |
| 6 | 13 | F | Epiphysiolysis/Fracture | Intramedullary femoral lengthening | Ongoing |
| 7 | 11 | M | Slipped Caput Femoris Epiphysiolysis | Open repositioning and prophylactic contralateral pinning | Ongoing |
| 8 | 10 | F | Genu Valgum | 8-plates | Finished |
| 9 | 9 | F | McCune Albright Syndrome, Left Femur Fracture in 2019 | Gap-nail | Ongoing |
| 10 | 8 | F | Talus Fracture | Casting | Finished |
| Second Round of Interviews | |||||
| 11 | 10 | M | Genu Valgum | Guided growth | Planned |
| 12 | 8 | F | Distal Tibia Epiphysiolysis | Guided growth, planned Acute correction with plate | Ongoing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jønsson, C.E.; Poulsen, L.; Rölfing, J.D.; Chhina, H.; Cooper, A.; Sørensen, J.A. Danish Linguistic Validation and Cultural Adaptation of the LIMB-Q Kids. Children 2023, 10, 1107. https://doi.org/10.3390/children10071107
Jønsson CE, Poulsen L, Rölfing JD, Chhina H, Cooper A, Sørensen JA. Danish Linguistic Validation and Cultural Adaptation of the LIMB-Q Kids. Children. 2023; 10(7):1107. https://doi.org/10.3390/children10071107
Chicago/Turabian StyleJønsson, Christopher Emil, Lotte Poulsen, Jan Duedal Rölfing, Harpreet Chhina, Anthony Cooper, and Jens Ahm Sørensen. 2023. "Danish Linguistic Validation and Cultural Adaptation of the LIMB-Q Kids" Children 10, no. 7: 1107. https://doi.org/10.3390/children10071107
APA StyleJønsson, C. E., Poulsen, L., Rölfing, J. D., Chhina, H., Cooper, A., & Sørensen, J. A. (2023). Danish Linguistic Validation and Cultural Adaptation of the LIMB-Q Kids. Children, 10(7), 1107. https://doi.org/10.3390/children10071107





