Prospective One-Year Follow-Up of Sensory Processing in Phelan–McDermid Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
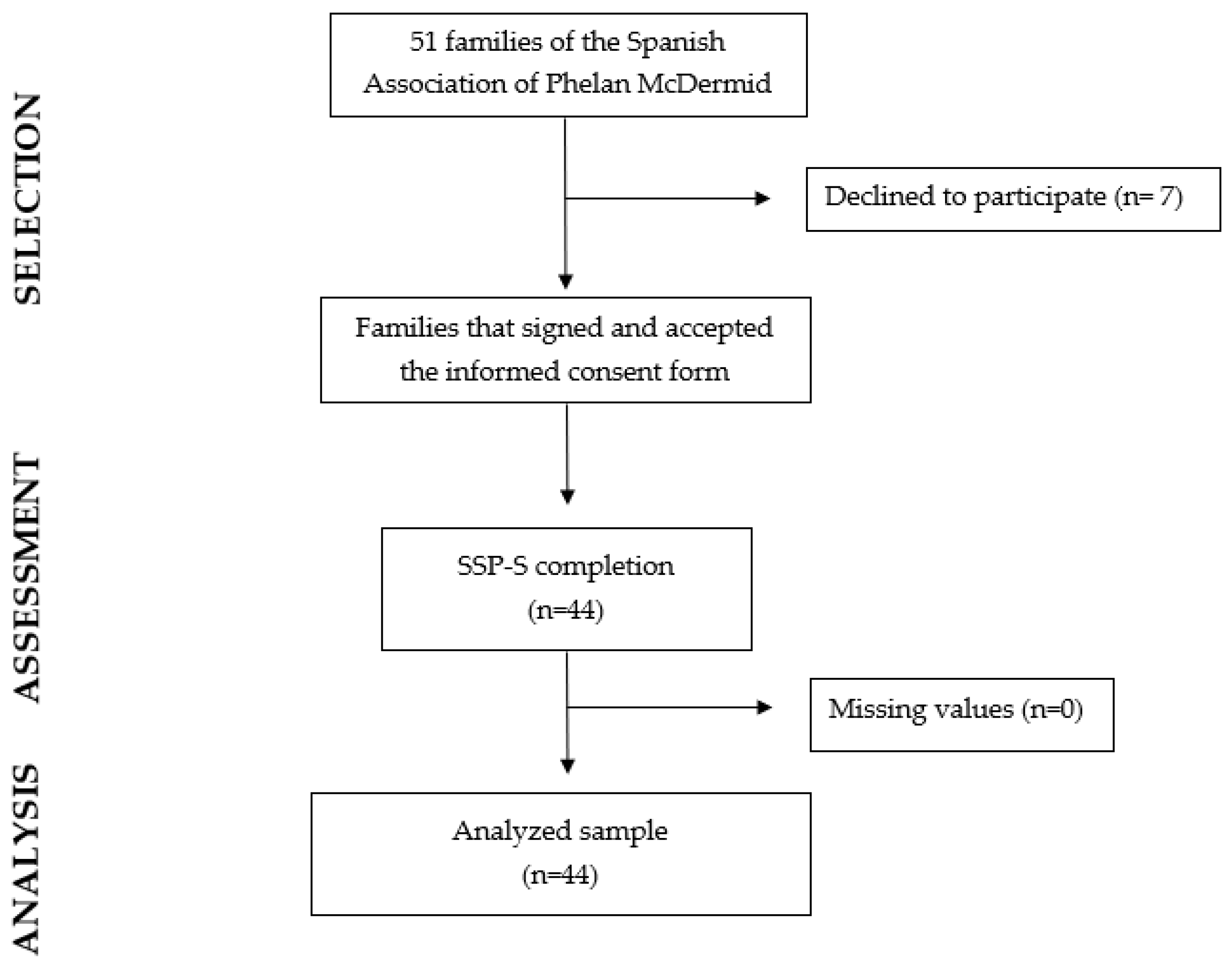
2.2. Participants
2.3. Procedure
2.4. Variables and Data Measurements
2.5. Data Analysis
3. Results
3.1. Socio-Demographic and Genetic Description of the Sample
3.2. Sensory Reactivity Scores according to the PMS Genetic Alteration
3.3. Comparative One-Year Follow-Up Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phelan, K.; Boccuto, L.; Powell, C.M.; Boeckers, T.M.; van Ravenswaaij-Arts, C.; Rogers, R.C.; Sala, C.; Verpelli, C.; Thurm, A.; Bennett, W.E., Jr.; et al. Phelan-McDermid syndrome: A classification system after 30 years of experience. Orphanet J. Rare Dis. 2022, 17, 27–30. [Google Scholar] [CrossRef]
- Ricciardello, A.; Tomaiuolo, P.; Persico, A.M. Genotype-phenotype correlation in Phelan-McDermid syndrome: A comprehensive review of chromosome 22q13 deleted genes. Am. J. Med. Genet. A 2021, 185, 2211–2233. [Google Scholar] [CrossRef]
- Kolevzon, A.; Angarita, B.; Bush, L.; Wang, A.T.; Frank, Y.; Yang, A.; Rapaport, R.; Saland, J.; Srivastava, S.; Farrell, C.; et al. Phelan-McDermid syndrome: A review of the literature and practice parameters for medical assessment and monitoring. J. Neurodev. Disord. 2014, 6, 39–50. [Google Scholar] [CrossRef]
- Kohlenberg, T.M.; Trelles, M.P.; McLarney, B.; Betancur, C.; Thurm, A.; Kolevzon, A. Psychiatric illness and regression in individuals with Phelan-McDermid syndrome. J. Neurodev. Disord. 2020, 12, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Kolevzon, A.; Delaby, E.; Berry-Kravis, E.; Buxbaum, J.D.; Betancur, C. Neuropsychiatric decompensation in adolescents and adults with Phelan-McDermid syndrome: A systematic review of the literature. Mol. Autism 2019, 10, 50–71. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, R.C.; Cohn, E.S.; Burke, J.; Dumont, R.; Miller, A.; Mailloux, Z. Linking sensory factors to participation: Establishing intervention goals with parents for children with autism spectrum disorder. Am. J. Occup. Ther. 2015, 69, 6905185005p1–6905185005p8. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, T.; Miller, L.J.; Schoen, S.; Nielsen, D.M.; Baron-Cohen, S. Sensory over-responsivity in adults with autism spectrum conditions. Autism 2014, 18, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, R.C.; Mailloux, Z.; Ridgway, E.; Berruti, A.S.; Dumont, R.L.; Jones, E.A.; Leiby, B.E.; Sancimino, C.; Yi, M.; Molholm, S. Sensory Phenotypes in Autism: Making a Case for the Inclusion of Sensory Integration Functions. J. Autism Dev. Disord. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.V.; Bilder, D.A.; Wiggins, L.D.; Hughes, M.M.; Davis, J.; Hall-Lande, J.A.; Lee, L.C.; McMahon, W.M.; Bakian, A.V. Sensory features in autism: Findings from a large population-based surveillance system. Autism Res. 2022, 15, 751–760. [Google Scholar] [CrossRef]
- He, J.L.; Williams, Z.J.; Harris, A.; Powell, H.; Schaaf, R.; Tavassoli, T.; Puts, N.A.J. A working taxonomy for describing the sensory differences of autism. Mol. Autism 2023, 14, 15–31. [Google Scholar] [CrossRef]
- Ben-Sasson, A.; Cermak, S.A.; Orsmond, G.I.; Carter, A.S.; Kadlec, M.B.; Dunn, W. Extreme sensory modulation behaviors in toddlers with autism. Am. J. Occup. Ther. 2007, 61, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, A.; Hen, L.; Fluss, R.; Cermak, S.A.; Engel-Yeger, B.; Gal, E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ismael, N.; Lawson, L.M.; Hartwell, J. Relationship between Sensory Processing and Participation in Daily Occupations for Children with Autism Spectrum Disorder: A Systematic Review of Studies That Used Dunn’s Sensory Processing Framework. Am. J. Occup. Ther. 2018, 72, 7203205030p1–7203205030p9. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.J.; Hinkley, L.B.; Hill, S.S.; Nagarajan, S.S. Sensory processing in autism: A review of neurophysiologic findings. Pediatr. Res. 2011, 69, 48R–54R. [Google Scholar] [CrossRef]
- Watson, L.R.; Patten, E.; Baranek, G.T.; Poe, M.; Boyd, B.A.; Freuler, A.; Lorenzi, J. Differential associations between sensory response patterns and language, social, and communication measures in children with autism or other developmental disabilities. J. Speech Lang. Hear. Res. 2011, 54, 1562–1576. [Google Scholar] [CrossRef]
- Williams, K.L.; Kirby, A.V.; Watson, L.R.; Sideris, J.; Bulluck, J.; Baranek, G.T. Sensory features as predictors of adaptive behaviors: A comparative longitudinal study of children with autism spectrum disorder and other developmental disabilities. Res. Dev. Disabil. 2018, 81, 103–112. [Google Scholar] [CrossRef]
- Liss, M.; Saulnier, C.; Fein, D.; Kinsbourne, M. Sensory and attention abnormalities in autistic spectrum disorders. Autism 2006, 10, 155–172. [Google Scholar] [CrossRef]
- Battaglia, A. Sensory impairment in mental retardation: A potential role for NGF. Arch. Ital. Biol. 2011, 149, 193–203. [Google Scholar] [CrossRef]
- Boyd, B.; Baranek, G.; Sideris, J.; Poe, M.; Watson, L.; Patten, E.; Miller, H. Sensory features and repetitive behaviors in children with autism and developmental delays. Autism Res. 2010, 3, 78–87. [Google Scholar] [CrossRef]
- Tavassoli, T.; Miller, L.J.; Schoen, S.A.; Brout, J.J.; Sullivan, J.; Baron-Cohen, S. Sensory reactivity, empathizing and systemizing in autism spectrum conditions and sensory processing disorder. Dev. Cogn. Neurosci. 2017, 29, 72–77. [Google Scholar] [CrossRef]
- Tavassoli, T.; Layton, C.; Levy, T.; Rowe, M.; George-Jones, J.; Zweifach, J.; Lurie, S.; Buxbaum, J.D.; Kolevzon, A.; Siper, P.M. Sensory reactivity phenotype in Phelan–McDermid syndrome is distinct from idiopathic ASD. Genes 2021, 12, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Mieses, A.M.; Tavassoli, T.; Li, E.; Soorya, L.; Lurie, S.; Wang, A.T.; Siper, P.M.; Kolevzon, A. Brief report: Sensory reactivity in children with Phelan-McDermid syndrome. J. Autism Dev. Disord. 2016, 46, 2508–2513. [Google Scholar] [CrossRef] [PubMed]
- Serrada-Tejeda, S.; Cuadrado, M.L.; Martínez-Piédrola, R.M.; Máximo-Bocanegra, N.; Sánchez-Herrera-Baeza, P.; Camacho-Montaño, L.R.; Pérez-de-Heredia-Torres, M. Sensory processing and adaptive behavior in Phelan-McDermid syndrome: A cross-sectional study. Eur. J. Pediatr. 2022, 181, 3141–3152. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.; Hepburn, S.; Young, G.S.; Rogers, S.J. Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: A longitudinal study. Autism 2016, 20, 572–579. [Google Scholar] [CrossRef]
- Perez Repetto, L.; Jasmin, E.; Fombonne, E.; Gisel, E.; Couture, M. Longitudinal study of sensory features in children with autism spectrum disorder. Autism Res. Treat. 2017, 2017, 1934701–1934708. [Google Scholar] [CrossRef]
- Baranek, G.T.; Carlson, M.; Sideris, J.; Kirby, A.V.; Watson, L.R.; Williams, K.L.; Bulluck, J. Longitudinal assessment of stability of sensory features in children with autism spectrum disorder or other developmental disabilities. Autism Res. 2019, 12, 100–111. [Google Scholar] [CrossRef]
- Dwyer, P.; Saron, C.D.; Rivera, S.M. Identification of longitudinal sensory subtypes in typical development and autism spectrum development using growth mixture modelling. Res. Autism Spectr. Disord. 2020, 78, 101645–101677. [Google Scholar] [CrossRef]
- Phelan, M.C.; Rogers, R.C.; Saul, R.A.; Stapleton, G.A.; Sweet, K.; McDermid, H.; Shaw, S.R.; Claytor, J.; Willis, J.; Kelly, D.P. 22q13 deletion syndrome. Am. J. Med. Genet. 2001, 101, 91–99. [Google Scholar] [CrossRef]
- Soorya, L.; Kolevzon, A.; Zweifach, J.; Lim, T.; Dobry, Y.; Schwartz, L.; Frank, Y.; Wang, A.T.; Cai, G.; Parkhomenko, E.; et al. Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol. Autism 2013, 4, 18–34. [Google Scholar] [CrossRef]
- Zwanenburg, R.J.; Ruiter, S.A.; van den Heuvel, E.R.; Flapper, B.C.; Van Ravenswaaij-Arts, C.M. Developmental phenotype in Phelan-McDermid (22q13.3 deletion) syndrome: A systematic and prospective study in 34 children. J. Neurodev. Disord. 2016, 8, 16–27. [Google Scholar] [CrossRef]
- Denayer, A.; Van Esch, H.; De Ravel, T.; Frijns, J.P.; Van Buggenhout, G.; Vogels, A.; Devriendt, K.; Geutjens, J.; Thiry, P.; Swillen, A. Neuropsychopathology in 7 patients with the 22q13 deletion syndrome: Presence of bipolar disorder and progressive loss of skills. Mol. Syndromol. 2012, 3, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Reierson, G.; Bernstein, J.; Froehlich-Santino, W.; Urban, A.; Purmann, C.; Berquist, S.; Jordan, J.; O’Hara, R.; Hallmayer, J. Characterizing regression in Phelan McDermid Syndrome (22q13 deletion syndrome). J. Psychiatr. Res. 2017, 91, 139–144. [Google Scholar] [CrossRef]
- Dille, Y.; Lagae, L.; Swillen, A.; Buggenhout, G.V. Neurodevelopmental profile and stages of regression in Phelan–McDermid Syndrome. Dev. Med. Child Neurol. 2022, 65, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Philippe, A.; Boddaert, N.; Vaivre-Douret, L.; Robel, L.; Danon-Boileau, L.; Malan, V.; de Blois, M.C.; Héron, D.; Colleaux, L.; Golse, B.; et al. Neurobehavioral profile and brain imaging study of the 22q13.3 deletion syndrome in childhood. Pediatrics 2008, 122, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Estes, A.; Zwaigenbaum, L.; Gu, H.; St. John, T.; Paterson, S.; Elison, J.T.; Hazlett, H.; Botteron, K.; Dager, S.R.; Schultz, R.T.; et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J. Neurodev. Disord. 2015, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- WMA. Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects; World Medical Association: Ferney-Voltaire, France, 2017. [Google Scholar]
- EU General Data Protection Regulation (GDPR). Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of such Data, and Repealing Directive 95/46/EC (General Data Protection Regulation), OJ 2016 L 119/1. Available online: https://www.sanidad.gob.es/en/proteccionDatosPersonales/home.htm (accessed on 1 June 2020).
- Ley Orgánica 3/2018, de 5 de Diciembre, de Protección de Datos Personales y Garantía de los Derechos Digitales (Boletín Oficial del Estado, Número 294, de 6 de Diciembre de 2018). Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2018-16673 (accessed on 1 June 2020).
- Beaudry-Bellefeuille, I.; Lane, S.J. Examining Sensory Overresponsiveness in Preschool Children with Retentive Fecal Incontinence. Am. J. Occup. Ther. 2017, 71, 7105220020p1–7105220020p8. [Google Scholar] [CrossRef] [PubMed]
- Román-Oyola, R.; Reynolds, S.E. Validating the Response Process of the Spanish Version of the Short Sensory Profile: A Pilot Study Using Cognitive Interviews. J. Occup. Ther. Sch. Early Interv. 2010, 3, 197–206. [Google Scholar] [CrossRef]
- Dunn, W. The Sensory Profile: User’s Manual; Psychological Corporation: San Antonio, TX, USA, 1999. [Google Scholar]
- Droogmans, G.; Swillen, A.; Van Buggenhout, G. Deep phenotyping of development, communication and behavior in Phelan-McDermid syndrome. Mol. Syndromol. 2020, 10, 294–305. [Google Scholar] [CrossRef]
- Lyons-Warren, A.M.; McCormack, M.C.; Holder, J.L., Jr. Sensory Processing Phenotypes in Phelan-McDermid Syndrome and SYNGAP1-Related Intellectual Disability. Brain Sci. 2022, 12, 137. [Google Scholar] [CrossRef]
- DeLorey, T.M.; Sahbaie, P.; Hashemi, E.; Li, W.W.; Salehi, A.; Clark, D.J. Somatosensory and sensorimotor consequences associated with the heterozygous disruption of the autism candidate gene, Gabrb3. Behav. Brain Res. 2011, 216, 36–45. [Google Scholar] [CrossRef]
- Sarasua, S.M.; Boccuto, L.; Sharp, J.L.; Dwivedi, A.; Chen, C.F.; Rollins, J.D.; Rogers, R.C.; Phelan, K.; DuPont, B.R. Clinical and genomic evaluation of 201 patients with Phelan-McDermid syndrome. Hum. Genet. 2014, 133, 847–859. [Google Scholar] [CrossRef]
- Harris, K.P.; Akbergenova, Y.; Cho, R.W.; Baas-Thomas, M.S.; Littleton, J.T. Shank Modulates Postsynaptic Wnt Signaling to Regulate Synaptic Development. J. Neurosci. 2016, 36, 5820–5832. [Google Scholar] [CrossRef] [PubMed]
- Puts, N.A.; Edden, R.A.; Evans, C.J.; McGlone, F.; McGonigle, D.J. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J. Neurosci. 2011, 31, 16556–16560. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Sapey-Triomphe, L.A.; Lamberton, F.; Sonié, S.; Mattout, J.; Schmitz, C. Tactile hypersensitivity and GABA concentration in the sensorimotor cortex of adults with autism. Autism Res. 2019, 12, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, T.; Auyeung, B.; Murphy, L.C.; Baron-Cohen, S.; Chakrabarti, B. Variation in the autism candidate gene GABRB3 modulates tactile sensitivity in typically developing children. Mol. Autism 2012, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Walinga, M.; Jesse, S.; Alhambra, N.; European Phelan-McDermid syndrome consortium; Van Buggenhout, G. Consensus recommendations on altered sensory functioning in Phelan-McDermid syndrome. Eur. J. Med. Genet. 2023, 66, 104726. [Google Scholar] [CrossRef] [PubMed]
| Age (years), mean (SD) | 12.6 (7.80) | ||
| Age range (years), n (%) | |||
| From 3 to 5 | 3 (7%) | ||
| From 6 to 11 | 25 (57%) | ||
| From 12 to 17 | 8 (18%) | ||
| From 18 to 24 | 4 (9%) | ||
| from25 to 40 | 4 (9%) | ||
| Gender. n (%) | |||
| Male | 22 (50%) | ||
| Female | 22 (50%) | ||
| Genetic alteration, n (%) | |||
| Deletion | 38 (86%) | ||
| Deletion size interval | 52 kb–8.53 Mb | ||
| Point mutation | 6 (14%) | ||
| Caregiver, n (%) | |||
| Mother | 22 (50%) | ||
| Father | 1 (2%) | ||
| Both parents | 20 (46%) | ||
| Another person | 1 (2%) | ||
| Rehabilitation services, n (%) | |||
| Physical therapy | 23 (52.3%) | ||
| Speech and language therapy | 34 (77.3%) | ||
| Psychology | 19 (43.2%) | ||
| Occupational therapy | 10 (22.7%) | ||
| SSP Section/Subscale (items) | Classification | ||
| Definite Difference | Probable Difference | Typical Performance | |
| Tactile Sensitivity (1–7) | 7–26 | 27–29 | 30–35 |
| Taste/Smell Sensitivity (8–11) | 4–11 | 12–14 | 15–20 |
| Movement Sensitivity (12–14) | 3–10 | 11–12 | 13–15 |
| Underresponsive/Seeks Sensation (15–21) | 7–23 | 24–26 | 27–35 |
| Auditory Filtering (22–27) | 6–19 | 20–22 | 23–30 |
| Low/Weak Energy (28–33) | 6–23 | 24–25 | 26–30 |
| Visual/Auditory Sensitivity (34–38) | 5–15 | 16–18 | 19–25 |
| Total score (1–38) | 38–141 | 142–154 | 155–190 |
| SHANK3 Deletion (n = 38) | SHANK3 Mutation (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|
| Score Median (IQR) | DD N (%) | PD N (%) | TP N (%) | Score Median (IQR) | DD N (%) | PD N (%) | TP N (%) | |
| Total score | 136 (77–173) | 24 (62.2%) | 5 (13.5%) | 9 (24.3%) | 147.50 (116–169) | 2 (33%) | 2 (33%) | 2 (33%) |
| Tactile Sensitivity | 28 (18–35) | 16 (40.5%) | 5 (13.5%) | 17 (45.9%) | 30 (21–32) | 2 (33%) | --- | 4 (66%) |
| Taste/Smell Sensitivity | 20 (4–21) | 1 (2.7%) | 3 (5.4%) | 34 (91.9%) | 20 (16–20) | --- | --- | 6 (100%) |
| Movement Sensitivity | 13 (5–15) | 10 (24.3%) | 5 (13.5%) | 23 (62.5%) | 13 (8–15) | 2 (33%) | --- | 4 (66%) |
| Underresponsive/Seeks Sensation | 21 (8–32) | 26 (67.6%) | 5 (13.5%) | 7 (18.9%) | 25 (17–32) | 2 (33%) | 2 (33%) | 2 (33%) |
| Auditory Filtering | 19 87–27) | 21 (54.1%) | 11 (29.7%) | 6 (16.2%) | 21.50 (17–27) | 3 (50%) | --- | 3 (50%) |
| Low/ Weak Energy | 17 (6–30) | 28 (73%) | 1 (2.7%) | 9 (24.3%) | 21 (8–33) | 3 (50%) | 1 (16%) | 2 (33%) |
| Visual/Auditory Sensitivity | 20 (8–25) | 6 (16.2%) | 5 (13.5%) | 27 (70.3%) | 20 (16–23) | --- | 2 (33%) | 4 (66%) |
| Measure, Mean (SD) | Mean. Dif. | Student-t Test | d | |||
|---|---|---|---|---|---|---|
| Pre | Post | t (43) | p-Value | |||
| Total score (1–38) | 133.86 (19.65) | 137.84 (20.96) | −3.98 | −1.76 | 0.086 | −0.27 |
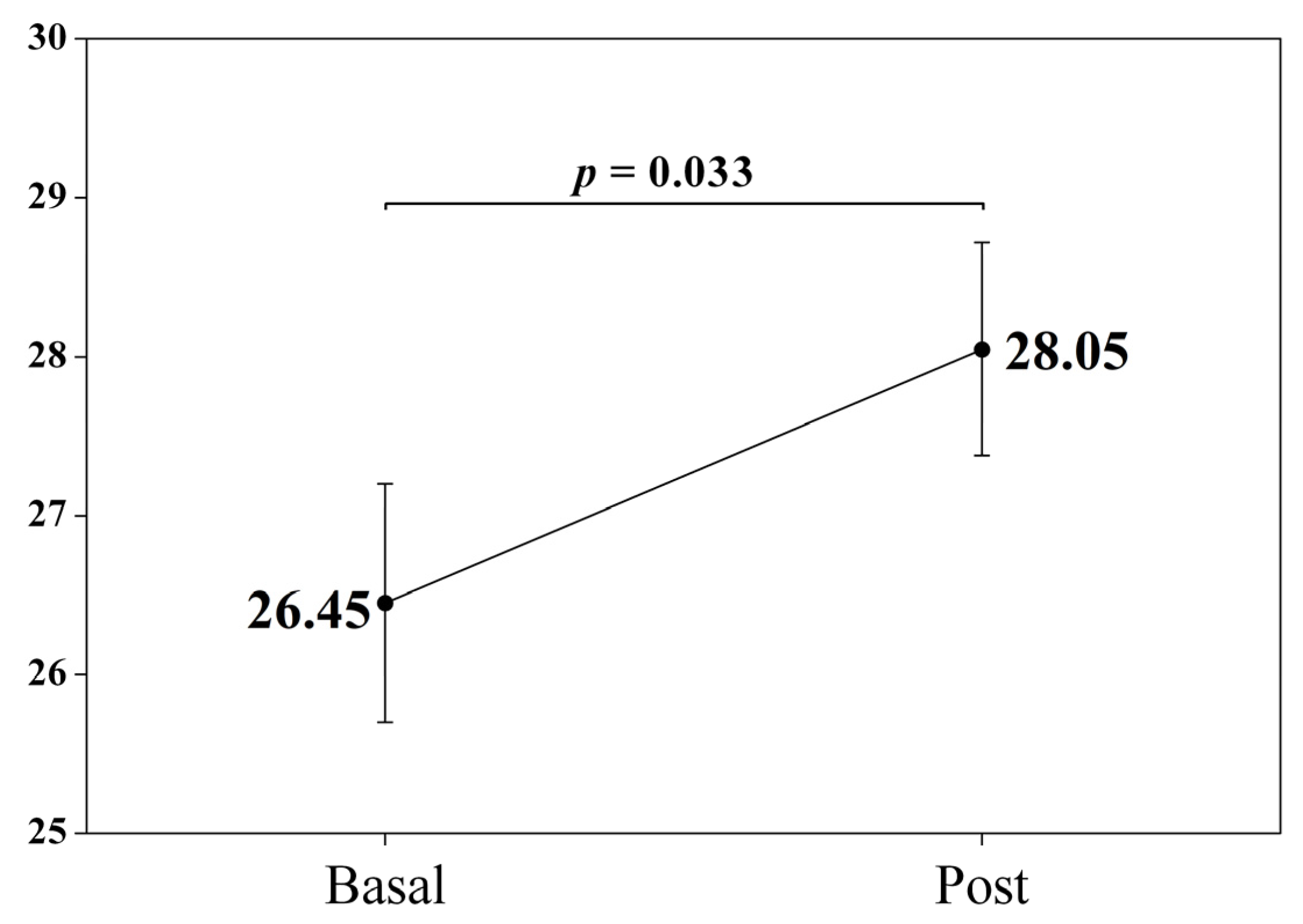
| Tactile Sensitivity (1–7) | 26.45 (4.96) | 28.05 (4.43) | −1.59 | −2.20 | 0.033 * | −0.33 |
| Taste/Smell Sensitivity (8–11) | 18.20 (2.95) | 18.48 (3.00) | −0.27 | −0.88 | 0.382 | −0.13 |
| Movement Sensitivity (12–14) | 12.39 (3.01) | 12.55 (2.61) | −0.16 | −0.44 | 0.66 | −0.07 |
| Underresponsive/Seeks Sensation (15–21) | 20.93 (6.63) | 21.16 (6.02) | −0.23 | −0.32 | 0.749 | −0.05 |
| Auditory Filtering (22–27) | 18.64 (4.90) | 19.20 (4.61) | −0.57 | −0.93 | 0.359 | −0.14 |
| Low/Weak Energy (28–33) | 18.41 (6.86) | 18.98 (7.12) | −0.57 | −0.91 | 0.369 | −0.15 |
| Visual/Auditory Sensitivity (34–38) | 19.43 (3.49) | 19.43 (3.78) | 0.00 | 0.00 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrada-Tejeda, S.; Sánchez-Herrera-Baeza, P.; Martínez-Piédrola, R.M.; Máximo-Bocanegra, N.; Trugeda-Pedrajo, N.; Rodríguez-Pérez, M.P.; Fernández-Gómez, G.; Pérez-de-Heredia-Torres, M. Prospective One-Year Follow-Up of Sensory Processing in Phelan–McDermid Syndrome. Children 2023, 10, 1086. https://doi.org/10.3390/children10061086
Serrada-Tejeda S, Sánchez-Herrera-Baeza P, Martínez-Piédrola RM, Máximo-Bocanegra N, Trugeda-Pedrajo N, Rodríguez-Pérez MP, Fernández-Gómez G, Pérez-de-Heredia-Torres M. Prospective One-Year Follow-Up of Sensory Processing in Phelan–McDermid Syndrome. Children. 2023; 10(6):1086. https://doi.org/10.3390/children10061086
Chicago/Turabian StyleSerrada-Tejeda, Sergio, Patricia Sánchez-Herrera-Baeza, Rosa M. Martínez-Piédrola, Nuria Máximo-Bocanegra, Nuria Trugeda-Pedrajo, M.ª Pilar Rodríguez-Pérez, Gemma Fernández-Gómez, and Marta Pérez-de-Heredia-Torres. 2023. "Prospective One-Year Follow-Up of Sensory Processing in Phelan–McDermid Syndrome" Children 10, no. 6: 1086. https://doi.org/10.3390/children10061086
APA StyleSerrada-Tejeda, S., Sánchez-Herrera-Baeza, P., Martínez-Piédrola, R. M., Máximo-Bocanegra, N., Trugeda-Pedrajo, N., Rodríguez-Pérez, M. P., Fernández-Gómez, G., & Pérez-de-Heredia-Torres, M. (2023). Prospective One-Year Follow-Up of Sensory Processing in Phelan–McDermid Syndrome. Children, 10(6), 1086. https://doi.org/10.3390/children10061086