Changes in Cranial Shape and Developmental Quotient at 6 Months of Age in Preterm Infants
Abstract
1. Introduction
2. Materials/Subjects
2.1. Ethics
2.2. Participants
2.3. D Data Collection and Analysis
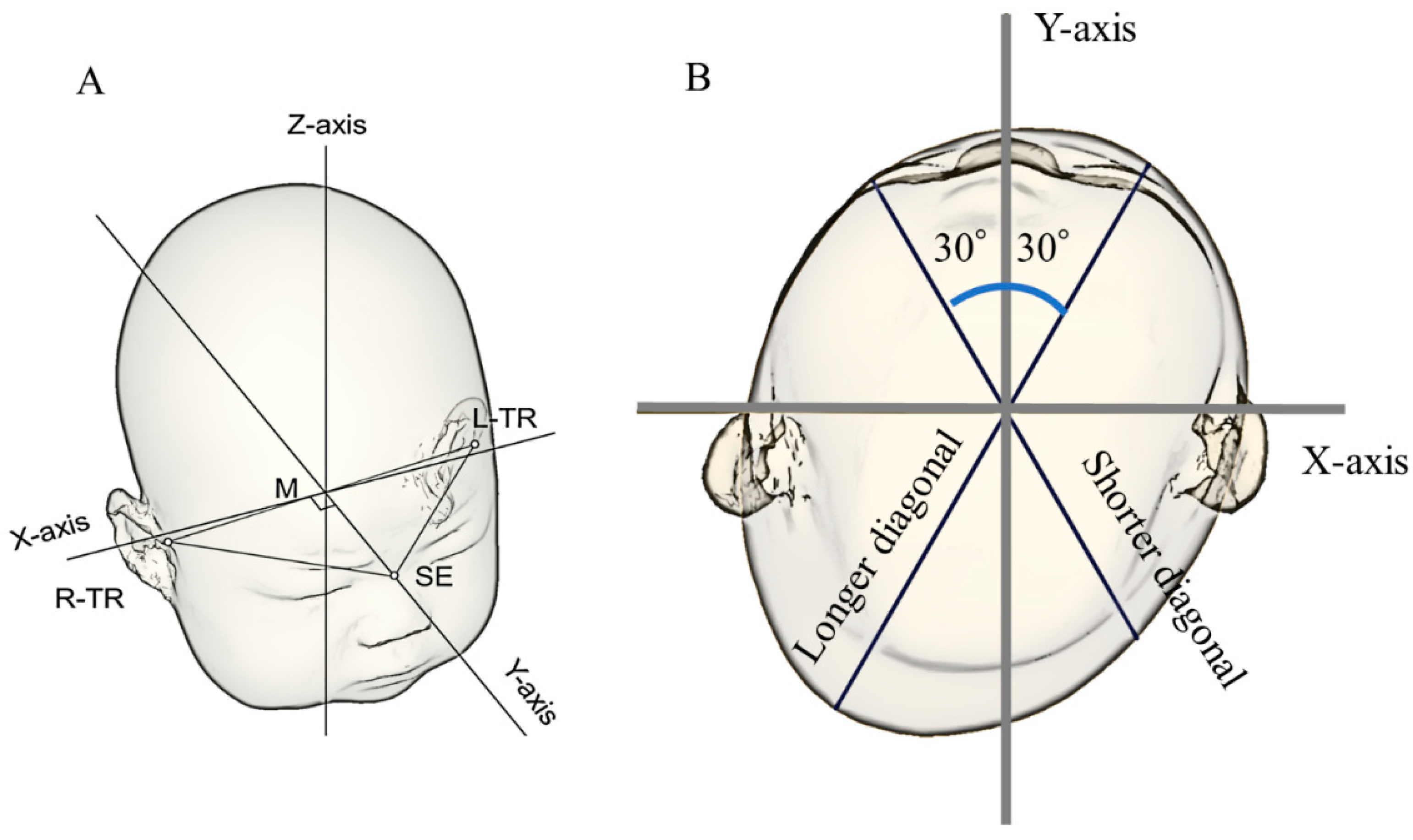
2.4. Reference Plane
2.5. Measurement Plane and Symmetry-Related Parameters
2.6. Study 1: Cranial Shape in Preterm Infants over Time
2.7. Study 2: Sex Differences in Cranial Shape in Preterm Infants
2.8. Study 3: Examination of the Relationship between Development and Cranial Shape at T3
3. Results
3.1. Characteristics of the Participants
3.2. Study 1: Cranial Shape in Preterm Infants over Time
3.2.1. Changes in the CI and Prevalence of Dolichocephaly and Brachycephaly
3.2.2. Changes in the CVAI and Prevalence of DP
3.3. Study 2: Sex Differences in Cranial Shape in Preterm Infants
3.4. Study 3: Relationship between Development and Cranial Shape at T3
4. Discussion
4.1. Dolichocephaly
4.2. Plagiocephaly
4.3. Relationship between the DQ and Cranial Shape
4.4. Cranial Shape-Correcting Devices
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baum, J.D.; Searls, D. Head shape and size of pre-term low-birthweight infants. Dev. Med. Child. Neurol. 2008, 13, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Dieks, J.K.; Jünemann, L.; Hensel, K.O.; Bergmann, C.; Schmidt, S.; Quast, A.; Horn, S.; Sigler, M.; Meyer-Marcotty, P.; Santander, P. Stereophotogrammetry can feasibly assess ‘physiological’ longitudinal three-dimensional head development of very preterm infants from birth to term. Sci. Rep. 2022, 12, 8940. [Google Scholar] [CrossRef] [PubMed]
- Ifflaender, S.; Rüdiger, M.; Konstantelos, D.; Wahls, K.; Burkhardt, W. Prevalence of head deformities in preterm infants at term equivalent age. Early Hum. Dev. 2013, 89, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- McCarty, D.B.; Peat, J.R.; Malcolm, W.F.; Smith, P.B.; Fisher, K.; Goldstein, R.F. Dolichocephaly in preterm infants: Prevalence, risk factors, and early motor outcomes. Am. J. Perinatol. 2017, 34, 372–378. [Google Scholar] [CrossRef]
- Elliman, A.M.; Bryan, E.M.; Elliman, A.D.; Starte, D. Narrow heads of preterm infants—Do they matter? Dev. Med. Child. Neurol. 1986, 28, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Nuysink, J.; Eijsermans, M.J.; van Haastert, I.C.; Koopman-Esseboom, C.; Helders, P.J.; de Vries, L.S.; van der Net, J. Clinical course of asymmetric motor performance and deformational plagiocephaly in very preterm infants. J. Pediatr. 2013, 163, 658–665. [Google Scholar] [CrossRef]
- Ifflaender, S.; Rüdiger, M.; Konstantelos, D.; Lange, U.; Burkhardt, W. Individual course of cranial symmetry and proportion in preterm infants up to 6 months of corrected age. Early Hum. Dev. 2014, 90, 511–515. [Google Scholar] [CrossRef]
- Nuysink, J.; van Haastert, I.C.; Eijsermans, M.J.; Koopman-Esseboom, C.; van der Net, J.; de Vries, L.S.; Helders, P.J. Prevalence and predictors of idiopathic asymmetry in infants born preterm. Early Hum. Dev. 2012, 88, 387–392. [Google Scholar] [CrossRef]
- Launonen, A.M.; Aarnivala, H.; Kyteas, P.; Vuollo, V.; Heikkinen, T.; Kau, C.H.; Pirttiniemi, P.; Harila, V.; Valkama, A.M. A 3D Follow-up study of cranial asymmetry from early infancy to toddler age after preterm versus term birth. J. Clin. Med. 2019, 8, 1665. [Google Scholar] [CrossRef]
- Collett, B.R.; Gray, K.E.; Starr, J.R.; Heike, C.L.; Cunningham, M.L.; Speltz, M.L. Development at age 36 months in children with deformational plagiocephaly. Pediatrics 2013, 131, e109–e115. [Google Scholar] [CrossRef]
- Speltz, M.L.; Collett, B.R.; Stott-Miller, M.; Starr, J.R.; Heike, C.; Wolfram-Aduan, A.M.; King, D.; Cunningham, M.L. Case-control study of neurodevelopment in deformational plagiocephaly. Pediatrics 2010, 125, e537–e542. [Google Scholar] [CrossRef] [PubMed]
- Kordestani, R.K.; Patel, S.; Bard, D.E.; Gurwitch, R.; Panchal, J. Neurodevelopmental delays in children with deformational plagiocephaly. Plast. Reconstr. Surg. 2006, 117, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Miyabayashi, H.; Nagano, N.; Kato, R.; Hashimoto, S.; Saito, K.; Noto, T.; Ohashi, S.; Masunaga, K.; Morioka, I. Cranial shapes of Japanese preterm infants at one month of age using a three-dimensional scanner. Brain Dev. 2022, 44, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Miyabayashi, H.; Nagano, N.; Hashimoto, S.; Saito, K.; Kato, R.; Noto, T.; Sasano, M.; Sumi, K.; Yoshino, A.; Morioka, I. Evaluating cranial growth in Japanese infants using a three-dimensional scanner: Relationship between growth-related parameters and deformational plagiocephaly. Neurol. Med. Chir. Tokyo 2022, 62, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Miyabayashi, H.; Nagano, N.; Kato, R.; Noto, T.; Hashimoto, S.; Saito, K.; Morioka, I. Cranial shape in infants aged one month can predict the severity of deformational plagiocephaly at the age of six months. J. Clin. Med. 2022, 11, 1797. [Google Scholar] [CrossRef] [PubMed]
- Ifflaender, S.; Rüdiger, M.; Koch, A.; Burkhardt, W. Three-dimensional digital capture of head size in neonates—A method evaluation. PLoS ONE 2013, 8, e61274. [Google Scholar] [CrossRef]
- Holowka, M.A.; Reisner, A.; Giavedoni, B.; Lombardo, J.R.; Coulter, C. Plagiocephaly severity scale to aid in clinical treatment recommendations. J. Craniofac. Surg. 2017, 28, 717–722. [Google Scholar] [CrossRef]
- Plank, L.H.; Giavedoni, B.; Lombardo, J.R.; Geil, M.D.; Reisner, A. Comparison of infant head shape changes in deformational plagiocephaly following treatment with a cranial remolding orthosis using a noninvasive laser shape digitizer. J. Craniofac. Surg. 2006, 17, 1084–1091. [Google Scholar] [CrossRef]
- Loveday, B.P.; de Chalain, T.B. Active counterpositioning or orthotic device to treat positional plagiocephaly? J. Craniofac. Surg. 2001, 12, 308–313. [Google Scholar] [CrossRef]
- Koizumi, T.; Komuro, Y.; Hashizume, K.; Yanai, A. Cephalic index of Japanese children with normal brain development. J. Craniofac. Surg. 2010, 21, 1434–1437. [Google Scholar] [CrossRef]
- Takamatsu, A.; Hikosaka, M.; Kaneko, T.; Mikami, M.; Kaneko, A. Evaluation of the molding helmet therapy for Japanese infants with deformational plagiocephaly. JMA J. 2021, 4, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Enjoji, M.; Yanai, N. Analytic test for development in infancy and childhood. Pediatr. Int. 1961, 4, 2–6. [Google Scholar] [CrossRef]
- Yoshida, H.; Takahashi, H.; Kanda, Y.; Kitaoka, K.; Hara, M. Long-term outcomes of cochlear implantation in children with congenital cytomegalovirus infection. Otol. Neurotol. 2017, 38, e190–e194. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- De Bock, F.; Braun, V.; Renz-Polster, H. Deformational plagiocephaly in normal infants: A systematic review of causes and hypotheses. Arch. Dis. Child. 2017, 102, 535–542. [Google Scholar] [CrossRef]
- Wilbrand, J.F.; Seidl, M.; Wilbrand, M.; Streckbein, P.; Böttger, S.; Pons-Kuehnemann, J.; Hahn, A.; Howaldt, H.-P. A prospective randomized trial on preventative methods for positional head deformity: Physiotherapy versus a positioning pillow. J. Pediatr. 2013, 162, 1216–1221.e1. [Google Scholar] [CrossRef]
- Aihara, Y.; Komatsu, K.; Dairoku, H.; Kubo, O.; Hori, T.; Okada, Y. Cranial molding helmet therapy and establishment of practical criteria for management in Asian infant positional head deformity. Childs Nerv. Syst. 2014, 30, 1499–1509. [Google Scholar] [CrossRef]
- Uchio, Y.; Shima, N.; Nakamura, K.; Ikai, T.; Nitta, O. Effects of continued positioning pillow use until a corrected age of six months on cranial deformation and neurodevelopment in preterm infants: A prospective case-control study. Early Hum. Dev. 2020, 148, 105137. [Google Scholar] [CrossRef]
- Food and Drug Administration. Do Not Use Infant Head Shaping Pillows to Prevent or Treat Any Medical Condition: FDA Safety Communication. Available online: https://www.fda.gov/medical-devices/safety-communications/do-not-use-infant-head-shaping-pillows-prevent-or-treat-any-medical-condition-fda-safety (accessed on 12 April 2023).

| Preterm Infants | Full-Term Infants | ||
|---|---|---|---|
| N | 26 | 88 | |
| Male | 13 (50) | 45 (51.1) | |
| Maternal age, years | 34.1 ± 4.8 | 34.0 ± 5.4 | |
| First birth | 9 (34.6) | 42 (47.7) | |
| Delivery method | † | ||
| Vaginal | 5 (19.2) | 44 (50) | |
| Cesarean section | 21 (80.8) | 34 (38.6) | |
| Vacuum or forceps | 0 (0) | 10 (11.4) | |
| Cephalic fetal presentation | 21 (80.8) | 82 (93.2) | |
| Multiple pregnancies | 5 (19.2) | 4 (4.5) | † |
| Gestational age, weeks | 34.7 ± 1.9 | 38.6 ± 1.3 | † |
| Birth weight, g | 2114 ± 486 | 3019 ± 320 | † |
| Birth head circumference, cm | 31.0 ± 1.9 | 33.7 ± 1.4 | † |
| Mechanical ventilation | 7 (26.9) | 0 (0) | † |
| p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|
| A | T1 | T2 | T3 | Whole | T1–T2 | T2–T3 | T1–T3 | |
| Cephalic index, % | Preterm infants | 77.2 ± 6.1 | 82.9 ± 7.1 | 85.4 ± 6.1 | <0.01 | <0.01 | <0.01 | <0.01 |
| Full-term infants | 84.7 ± 4.5 | 88.1 ± 5.6 | 89.0 ± 5.8 | <0.01 | <0.01 | <0.01 | <0.01 | |
| p-value | <0.01 | <0.01 | <0.01 | |||||
| B | T1 | T2 | T3 | Whole | T1–T2 | T2–T3 | T1–T3 | |
| Prevalence of dolichocephaly, N (%) | Preterm infants | 19 (73.1) | 9 (34.6) | 4 (15.4) | <0.01 | 0.01 | 0.22 | <0.01 |
| Full-term infants | 10 (11.4) | 5 (5.7) | 4 (4.5) | 0.04 | 0.55 | 1.00 | 0.34 | |
| p-value | <0.01 | <0.01 | 0.08 | |||||
| C | T1 | T2 | T3 | Whole | T1–T2 | T2–T3 | T1–T3 | |
| Prevalence of brachycephaly, N (%) | Preterm infants | 0 (0) | 2 (7.7) | 3 (11.5) | 0.17 | - | 1.0 | - |
| Full-term infants | 1 (1.1) | 16 (18.2) | 19 (21.6) | <0.01 | <0.01 | 1.0 | <0.01 | |
| p-value | 1.0 | 0.24 | 0.40 |
| p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|
| A | T1 | T2 | T3 | Whole | T1–T2 | T2–T3 | T1–T3 | |
| CVAI, % | Preterm infants | 4.8 (2.3–5.9) | 5.7 (2.5–9.1) | 3.3 (2.8–8.5) | 0.23 | 0.15 | 0.17 | 0.55 |
| Full-term infants | 5.0 (3.0–7.0) | 5.8 (3.0–7.9) | 4.6 (2.5–6.8) | <0.01 | 0.13 | <0.01 | 0.38 | |
| p-value | 0.24 | 0.77 | 0.91 | |||||
| B | T1 | T2 | T3 | Whole | T1–T2 | T2–T3 | T1–T3 | |
| Prevalence of DP, N (%) | Preterm infants | 13 (50.0) | 14 (53.8) | 9 (34.6) | 0.17 | 1.00 | 0.22 | 1.00 |
| Full-term infants | 43 (48.9) | 49 (55.7) | 38 (43.2) | 0.03 | 0.63 | 0.03 | 1.00 | |
| p-value | 1.00 | 1.00 | 0.50 |
| T1 | T2 | T3 | ||
|---|---|---|---|---|
| CI | Male | 77.6 ± 6.6 | 82.3 ± 7.7 | 85.1 ± 7.2 |
| Female | 76.8 ± 5.8 | 83.5 ± 6.7 | 85.6 ± 5.1 | |
| p-value | 0.76 | 0.67 | 0.85 | |
| CVAI | Male | 5.2 (3.2–6.3) | 5.3 (2.3–10.0) | 3.2 (2.9–10.0) |
| Female | 3.5 (2.0–5.5) | 6.0 (3.3–7.3) | 3.5 (2.8–5.9) | |
| p-value | 0.40 | 0.94 | 0.80 |
| A: CI | |||
|---|---|---|---|
| Correlation coefficient | 95% confidence interval | p-value | |
| CI at T1 | 0.267 | −0.134–0.593 | 0.19 |
| CI at T2 | 0.224 | −0.178–0.563 | 0.27 |
| CI at T3 | 0.241 | −0.164–0.573 | 0.24 |
| B: CVAI | |||
| Rank correlation coefficient | 95% confidence interval | p-value | |
| CVAI at T1 | −0.271 | −0.596–0.130 | 0.18 |
| CVAI at T2 | −0.093 | −0.464–0.305 | 0.65 |
| CVAI at T3 | −0.010 | −0.396–0.379 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanomori, A.; Miyabayashi, H.; Tanaka, Y.; Maedomari, T.; Mukai, C.; Saito, K.; Okahashi, A.; Nagano, N.; Morioka, I. Changes in Cranial Shape and Developmental Quotient at 6 Months of Age in Preterm Infants. Children 2023, 10, 855. https://doi.org/10.3390/children10050855
Nakanomori A, Miyabayashi H, Tanaka Y, Maedomari T, Mukai C, Saito K, Okahashi A, Nagano N, Morioka I. Changes in Cranial Shape and Developmental Quotient at 6 Months of Age in Preterm Infants. Children. 2023; 10(5):855. https://doi.org/10.3390/children10050855
Chicago/Turabian StyleNakanomori, Aya, Hiroshi Miyabayashi, Yukari Tanaka, Taishin Maedomari, Chihiro Mukai, Katsuya Saito, Aya Okahashi, Nobuhiko Nagano, and Ichiro Morioka. 2023. "Changes in Cranial Shape and Developmental Quotient at 6 Months of Age in Preterm Infants" Children 10, no. 5: 855. https://doi.org/10.3390/children10050855
APA StyleNakanomori, A., Miyabayashi, H., Tanaka, Y., Maedomari, T., Mukai, C., Saito, K., Okahashi, A., Nagano, N., & Morioka, I. (2023). Changes in Cranial Shape and Developmental Quotient at 6 Months of Age in Preterm Infants. Children, 10(5), 855. https://doi.org/10.3390/children10050855






