Evaluation of a Pre-Filled Table and a Flowchart-Based Algorithm as Cognitive Aids to Reduce Deviations in Dose Calculation for Intraoperative Red Blood Cell Transfusions in Children—An International Web-Based Simulation
Abstract
1. Introduction
2. Materials and Methods
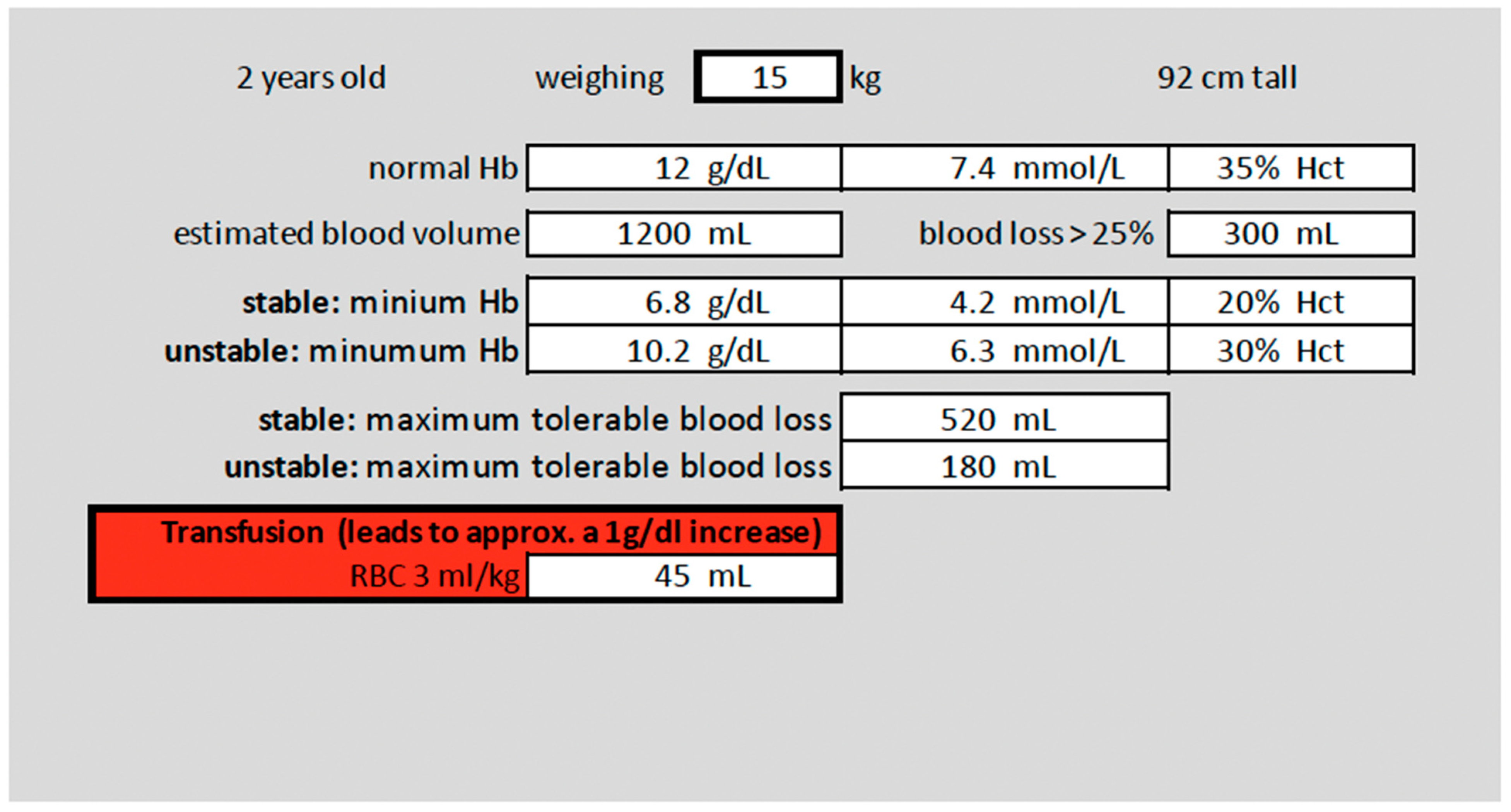
2.1. Normal Value Definitions and Calculations
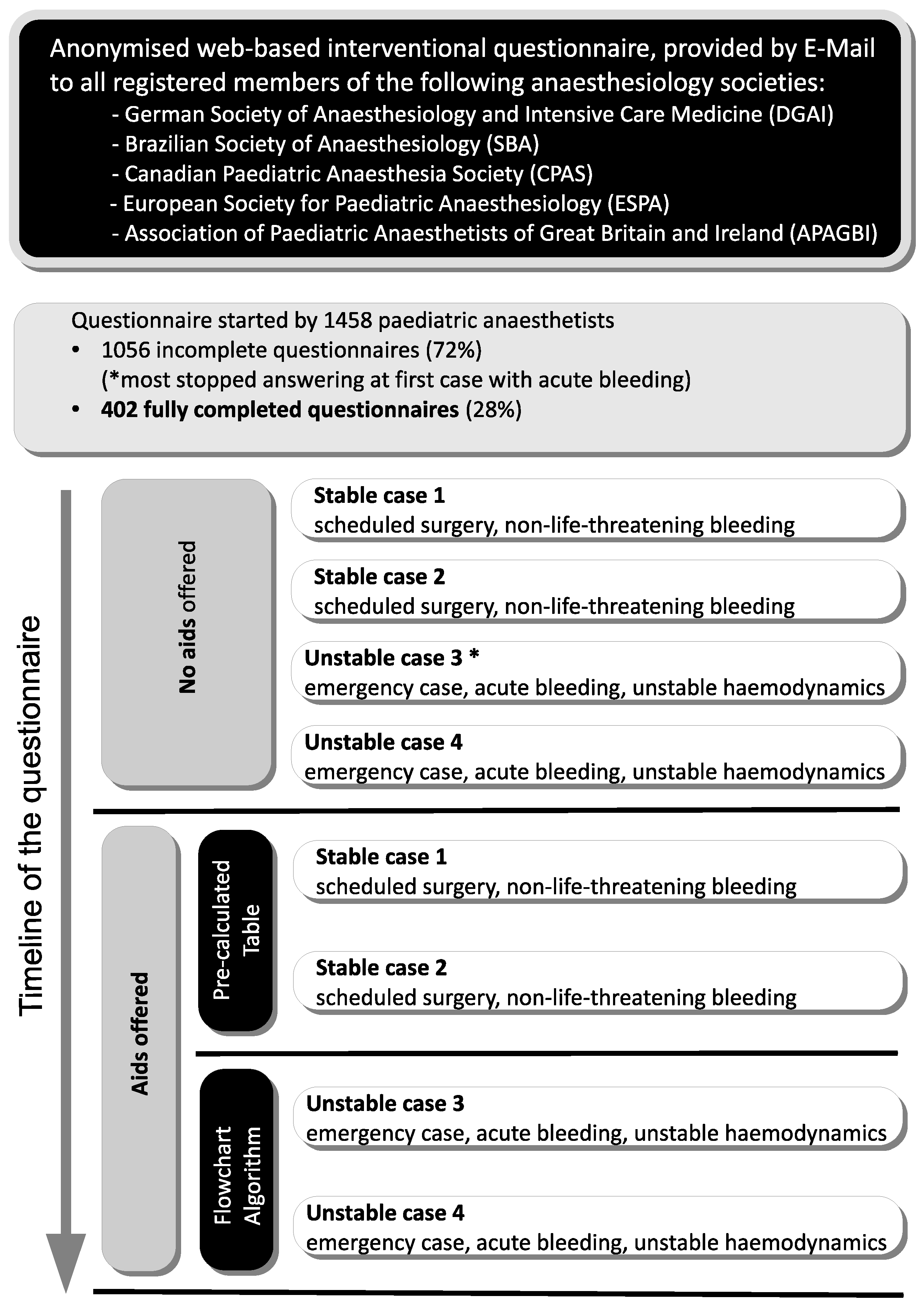
2.2. Recruitment of Participants, Evaluation of Data, and Statistical Analysis
2.3. Endpoints
3. Results
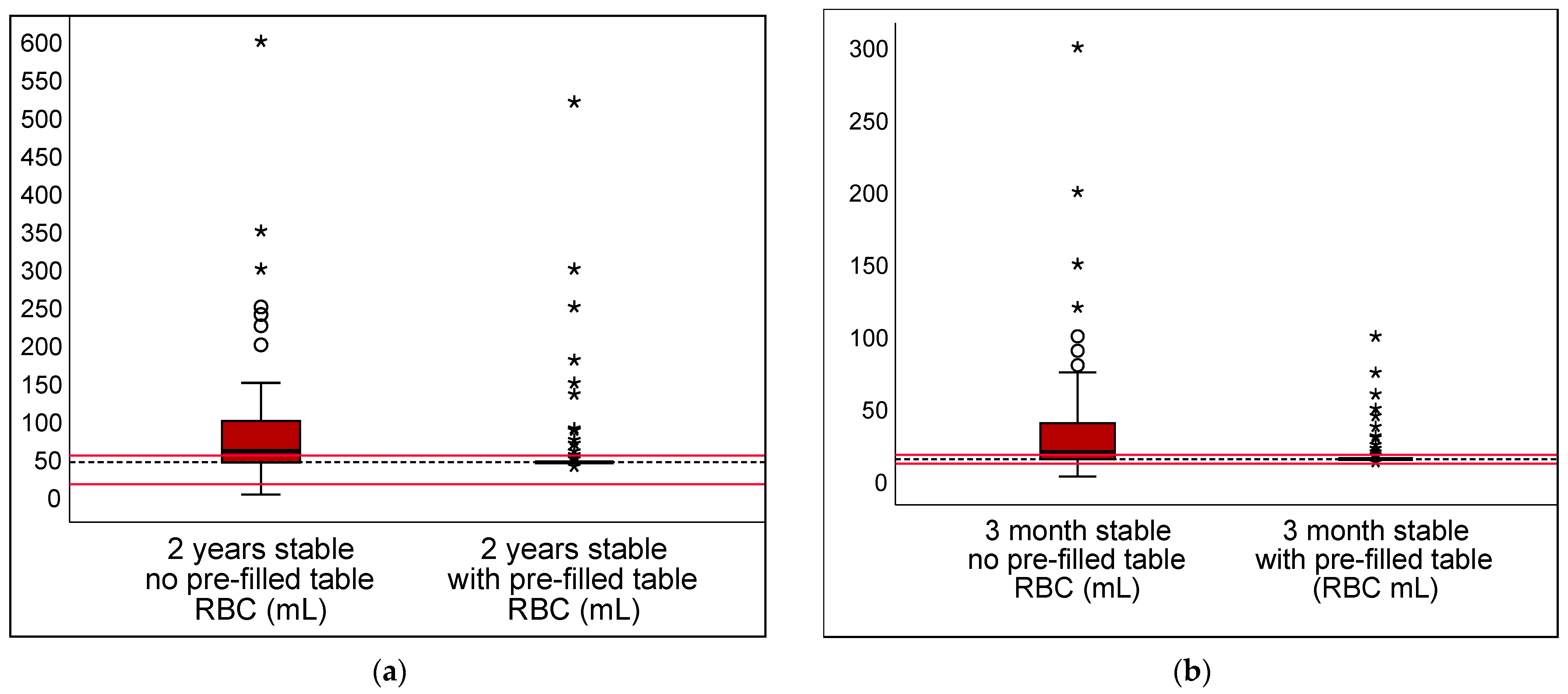
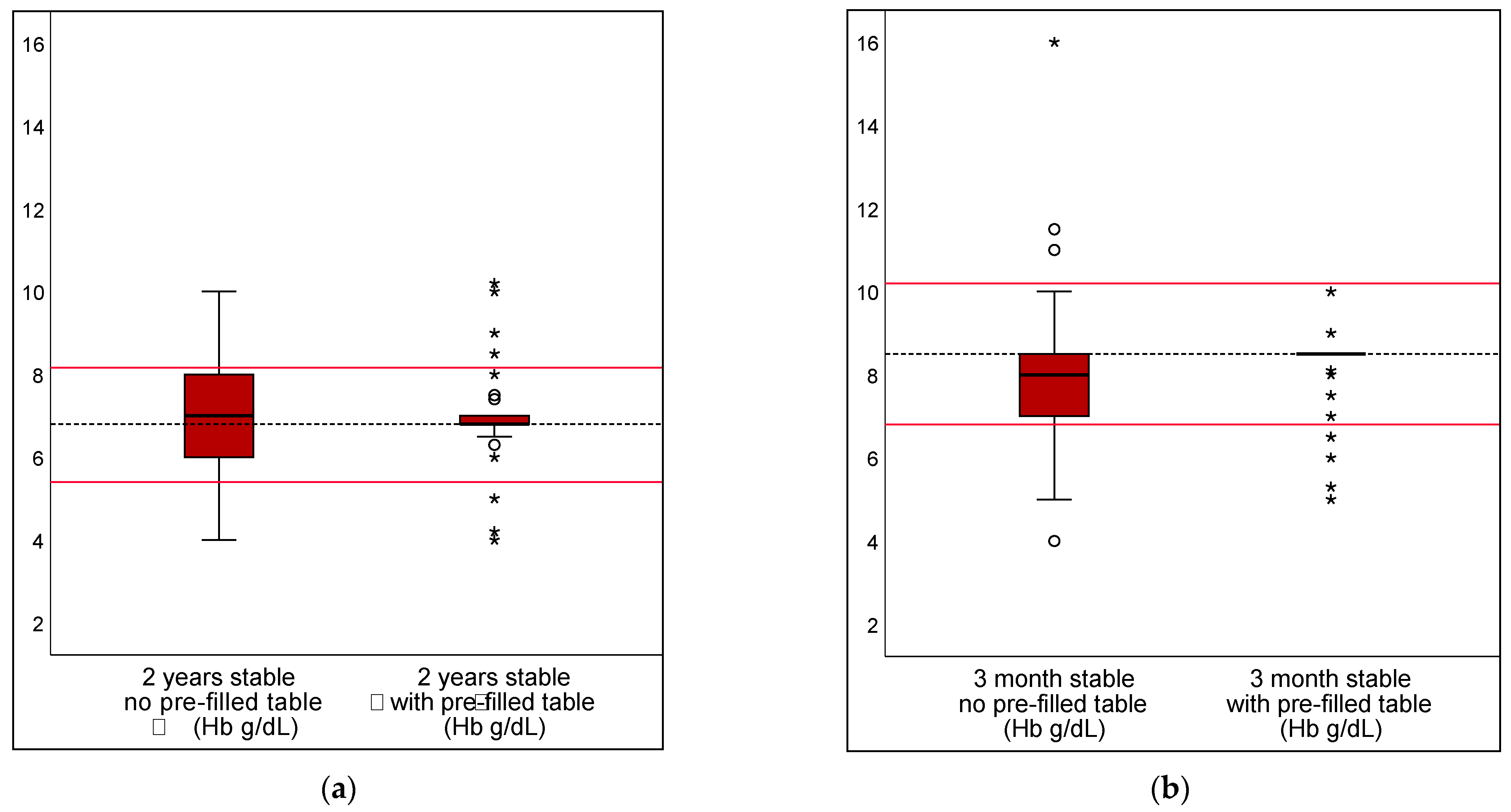
3.1. Pre-Filled Table in Stable Scenarios
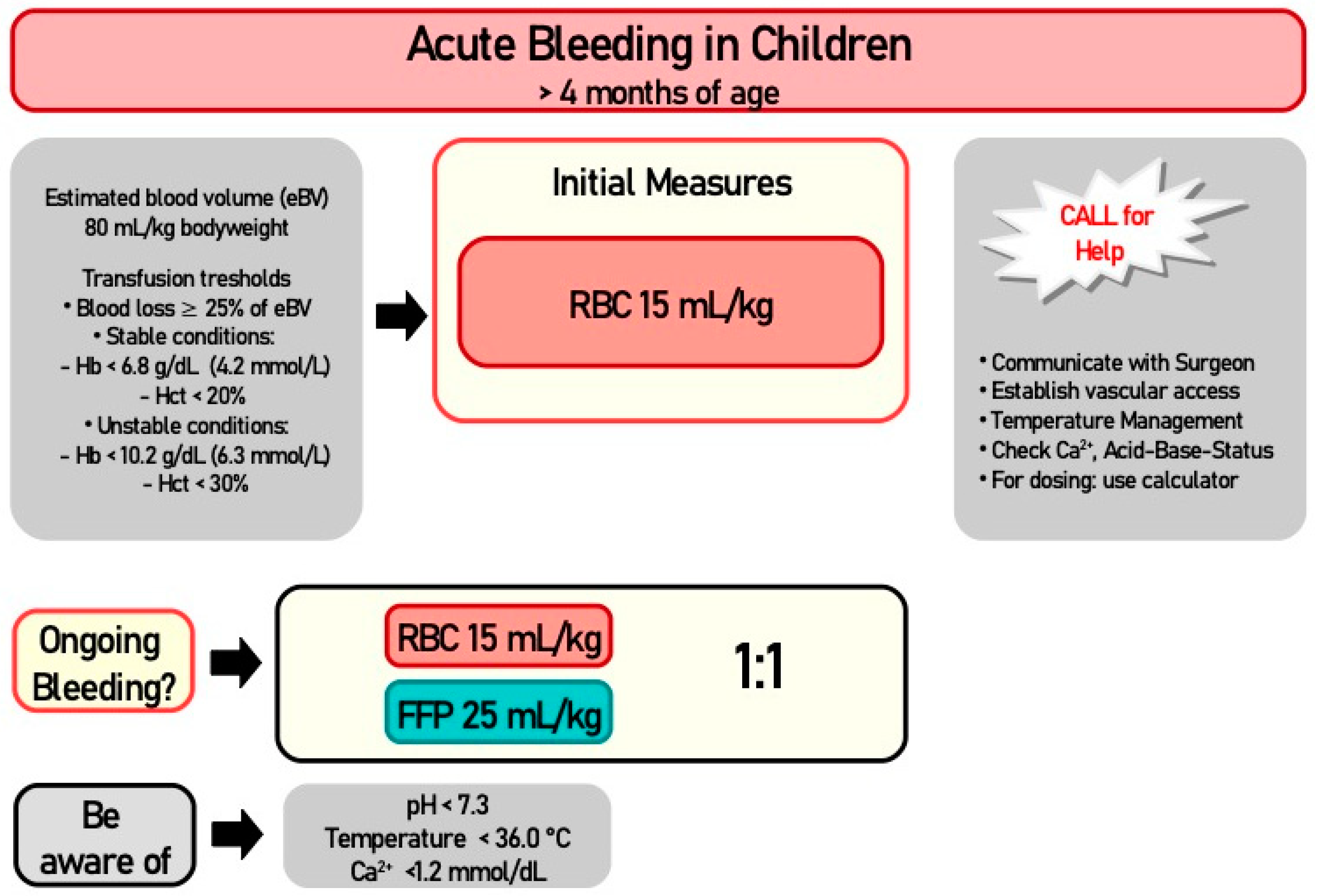
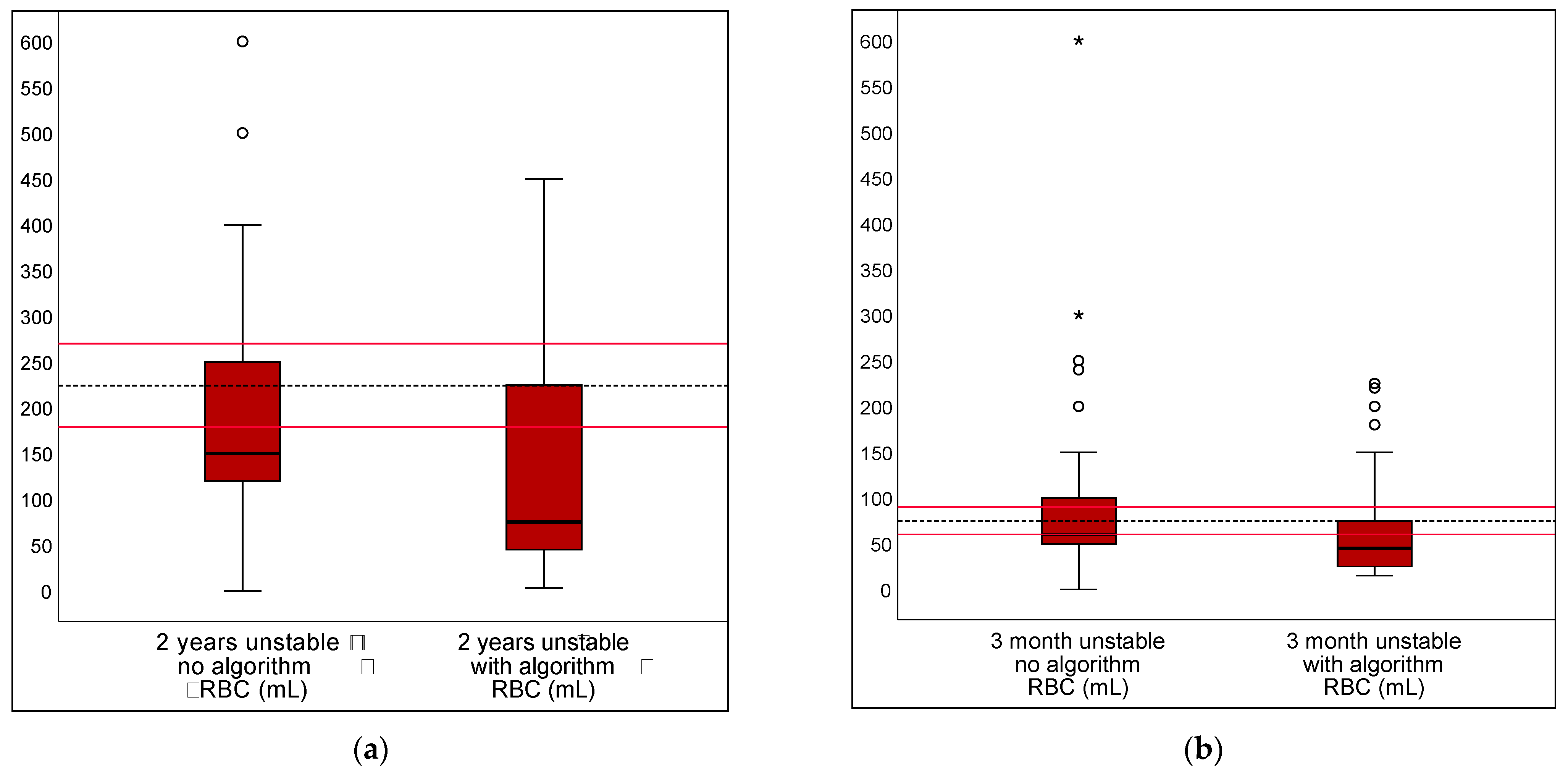
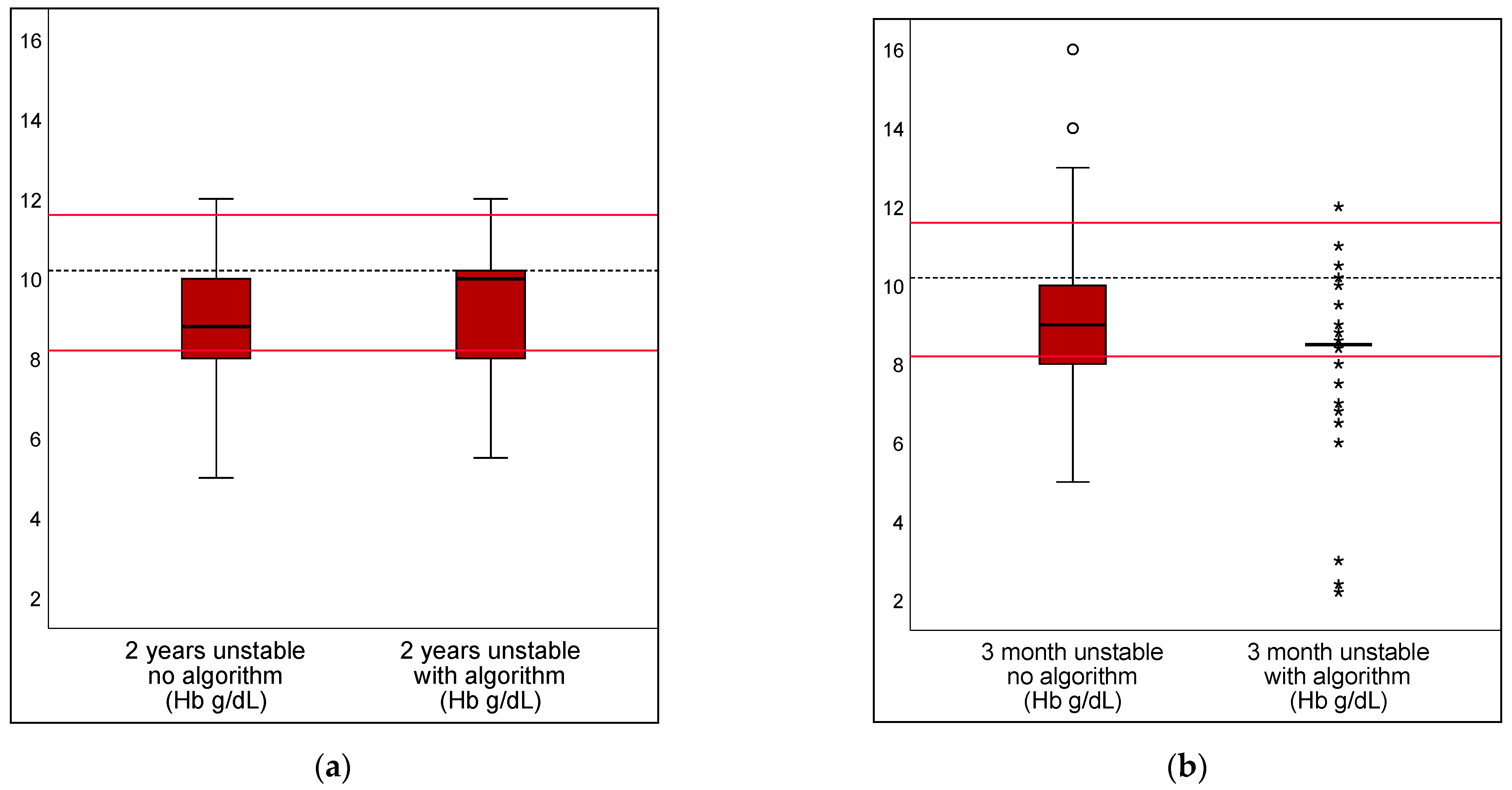
3.2. Algorithm in Unstable Scenarios
3.3. Experience
3.4. Transfusion Thresholds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhananker, S.M.; Ramamoorthy, C.; Geiduschek, J.M.; Posner, K.L.; Domino, K.B.; Haberkern, C.M.; Campos, J.S.; Morray, J.P. Anesthesia-related cardiac arrest in children: Update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth. Analg. 2007, 105, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Corpman, D.; Bakhtary, S.; Manuel, S.P. Overtransfusion after unexpected intraoperative hemorrhage: A retrospective study. J. Clin. Anesth. 2020, 62, 109720. [Google Scholar] [CrossRef] [PubMed]
- Piekarski, F.; Neef, V.; Meybohm, P.; Rolle, U.; Schneider, W.; Zacharowski, K.; Schmitt, E. Independent Risk Factors for RBC Transfusion in Children Undergoing Surgery. Analysis of 14,248 Cases at a German University Hospital. Children 2021, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Kozek-Langenecker, S.A.; Ahmed, A.B.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D.; et al. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology: First update 2016. Eur. J. Anaesthesiol. 2017, 34, 332–395. [Google Scholar] [CrossRef] [PubMed]
- Karam, O.; Russell, R.T.; Stricker, P.; Vogel, A.M.; Bateman, S.T.; Valentine, S.L.; Spinella, P.C.; Pediatric Critical Care Transfusion and Anemia Expertise, Initiative (TAXI); Pediatric Critical Care Blood Research, Network. Recommendations on RBC Transfusion in Critically Ill Children with Nonlife-Threatening Bleeding or Hemorrhagic Shock from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr. Crit. Care Med. 2018, 19, S127–S132. [Google Scholar] [CrossRef] [PubMed]
- Goobie, S.M.; Gallagher, T.; Gross, I.; Shander, A. Society for the advancement of blood management administrative and clinical standards for patient blood management programs. 4th edition (pediatric version). Pediatr. Anesth. 2019, 29, 231–236. [Google Scholar] [CrossRef] [PubMed]
- German Medical Association. Cross-Sectional Guidelines for Therapy with Blood Components and Plasma Derivatives; German Medical Association: Berlin, Germany, 2014. [Google Scholar]
- Serious Hazards of Transfusion (SHOT) Steering Group. The 2020 Annual SHOT Report; SHOT: Manchester, UK, 2021. [Google Scholar]
- Whitney, G.; Daves, S.; Hughes, A.; Watkins, S.; Woods, M.; Kreger, M.; Marincola, P.; Chocron, I.; Donahue, B. Implementation of a transfusion algorithm to reduce blood product utilization in pediatric cardiac surgery. Pediatr. Anesth. 2013, 23, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, J.; Laschat, M.; Wappler, F. Medication errors in pediatric emergencies: A systematic analysis. Dtsch. Ärzteblatt Int. 2012, 109, 609–616. [Google Scholar] [CrossRef]
- Kaufmann, J.; Engelhardt, T.; Steinwegs, I.; Hinkelbein, J.; Piekarski, F.; Laschat, M.; Boehmer, A.; Hellmich, M.; Wappler, F. The influence of education and experience on paediatric emergency drug dosing errors—An interventional questionnaire study using a tabular aid. Anasthesiol. Intensivmed. 2019, 60, 164–170. [Google Scholar]
- Kaufmann, J.; Uhl, S.; Singer, E.; Eifinger, F.; Klein, T.; Lechleuthner, A.; Engelhardt, T.; Wappler, F.; Bohmer, A. Improving Pediatric Drug Safety in Prehospital Emergency Care-10 Years on. J. Patient Saf. 2021, 17, e1241–e1246. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, B.R.; Sterne, J.A. Essential Medical Statistics, 2nd ed.; Blackwell Scientific: Malden, MA, USA, 2003. [Google Scholar]
- Van de Voorde, P.; Turner, N.M.; Djakow, J.; de Lucas, N.; Martinez-Mejias, A.; Biarent, D.; Bingham, R.; Brissaud, O.; Hoffmann, F.; Johannesdottir, G.B.; et al. European Resuscitation Council Guidelines 2021: Paediatric Life Support. Resuscitation 2021, 161, 327–387. [Google Scholar] [CrossRef] [PubMed]
- German Medical Association. Cross-Sectional Guidelines for Therapy with Blood Components and Plasma Derivatives—Amended Edition 2020; German Medical Association: Berlin, Germany, 2020. [Google Scholar]
- Kietaibl, S.; Ahmed, A.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D.; et al. Management of severe peri-operative bleeding: Guidelines from the European Society of Anaesthesiology and Intensive Care: Second update 2022. Eur. J. Anaesthesiol. 2023, 40, 226–304. [Google Scholar] [CrossRef] [PubMed]
- New, H.V.; Berryman, J.; Bolton-Maggs, P.H.; Cantwell, C.; Chalmers, E.A.; Davies, T.; Gottstein, R.; Kelleher, A.; Kumar, S.; Morley, S.L.; et al. Guidelines on transfusion for fetuses, neonates and older children. Br. J. Haematol. 2016, 175, 784–828. [Google Scholar] [CrossRef]
- Neonatal and Paediatrics Steering Committee; Neonatal and Paediatrics Expert Working Group. Patient Blood Management Guidelines: Module 6: Neonatal and Paediatrics; Charles Sturt University: Bathurst, Australia, 2016. [Google Scholar]
- Kaufmann, J.; Wolf, A.R.; Becke, K.; Laschat, M.; Wappler, F.; Engelhardt, T. Drug safety in paediatric anaesthesia. Br. J. Anaesth. 2017, 119, 1248. [Google Scholar] [CrossRef]
- Kaufmann, J.; Roth, B.; Engelhardt, T.; Lechleuthner, A.; Laschat, M.; Hadamitzky, C.; Wappler, F.; Hellmich, M. Development and Prospective Federal State-Wide Evaluation of a Device for Height-Based Dose Recommendations in Prehospital Pediatric Emergencies: A Simple Tool to Prevent Most Severe Drug Errors. Prehosp. Emerg. Care 2018, 22, 252–259. [Google Scholar] [CrossRef] [PubMed]
| Short Name | Case | Preexisting Conditions | Calculations: Values Used as Correct |
|---|---|---|---|
| 2 years, stable | 2-year-old female, 15 kg body weight, scheduled surgery: partial spleen resection | Bronchial asthma, ASA Classification 2, stable hemodynamics, preoperative Hb-level 12 g/dL |
|
| 3 months, stable | 3-month-old male, 5 kg body weight, scheduled surgery: pull-through procedure (Hirschsprung’s disease) | No pre-existing cardiovascular conditions, born at term, stable hemodynamics, preoperative Hb-level 13 g/dL |
|
| 2 years, unstable | 2-year-old male, 15 kg body weight, emergency neurosurgical treatment of traumatic epidural hemorrhage, relevant bleeding situation and continuous blood loss after craniotomy | No pre-existing conditions known; currently impaired cardiovascular conditions due to acute bleeding |
|
| 3 months, unstable | 3-month-old male, 5 kg body weight, scheduled surgery: hemi-hepatectomy (congenital hepatoblastoma), relevant blood loss due to complications | No pre-existing cardiovascular conditions, born at term; currently impaired cardiovascular conditions due to acute bleeding |
|
| Deviation Grade | Unaided | Aided | Diff | 95% CI | p |
|---|---|---|---|---|---|
| 2 years, 15 kg, preop Hb 12 g/dL; splenectomy, stable conditions | |||||
| Hb transfusion threshold; correct value set as 6.8 g/dL | |||||
| DRV120 | 13.4% | 6.2% | 7.2% | 2.9–11.5% | 0.0011 |
| DRV300 | 0.2% | 0.0% | 0.2% | −0.2–0.7% | 0.3173 |
| DRV1000 | 1.7% | 0.0% | 0.2% | −0.2–0.7% | 0.3173 |
| RBC transfusion volume; correct value set as 45 mL | |||||
| DRV120 | 66.7% | 6.5% | 60.2% | 51.8–68.6% | <0.001 |
| DRV300 | 18.9% | 1.7% | 17.2% | 12.7–21.6% | <0.001 |
| DRV1000 | 1.0% | 0.5% | 0.5% | −0,7–1,7% | 0.4142 |
| MABL; correct value set as 520 mL | |||||
| DRV120 | 72.4% | 27.9% | 44.5% | 34.7–54.3% | <0.001 |
| DRV300 | 11.4% | 1.7% | 9.7% | 6.2–1.3% | <0.001 |
| DRV1000 | 1.7% | 0.0% | 1.7% | 0.5–3.0% | 0.0082 |
| Deviation grade | unaided | aided | Diff | 95% CI | p |
| 3 months, 5 kg, preop Hb 13 g/dL; rectal pull-through, stable conditions | |||||
| Hb transfusion threshold; correct value set as 8.5 g/dL | |||||
| DRV120 | 19.9% | 4.7% | 15.2% | 10.3–20.0% | <0.001 |
| DRV300 | 0.2% | 0.7% | −0.5% | −1.5–0.5% | 0.3173 |
| DRV1000 | 0.0% | 0.2% | −0.2% | −0.7–0.2% | 0.3173 |
| RBC transfusion volume; correct value set as 15 mL | |||||
| DRV120 | 69.7% | 9.5% | 60.2% | 51.5–68.9% | <0.001 |
| DRV300 | 21.4% | 1.2% | 20.1% | 15.5–24.8% | <0.001 |
| DRV1000 | 1.2% | 0 | 1.2% | 0.2–2.3% | 0.0253 |
| MABL; correct value set as 138 mL | |||||
| DRV120 | 73.9% | 24.6% | 49.3% | 39.6–59.0% | <0.001 |
| DRV300 | 7.2% | 0.2% | 7.0% | 4.3–9.6% | <0.001 |
| DRV1000 | 0.5% | 0.2% | 0.2% | −0.6–1.1% | 0.5637 |
| Deviation grade | unaided | aided | Diff | 95% CI | p |
| 2 years, 15 kg; bleeding after major surgery, unstable hemodynamic condition | |||||
| Hb transfusion threshold; correct value set as 10.2 g/dL | |||||
| DRV120 | 49.8% | 27.1% | 22.6% | 14.1–31.2% | <0.001 |
| DRV300 | 0.5% | 0.5% | 0.0% | −1.0–1.0% | 10000 |
| DRV1000 | 0.0% | 0.5% | −0.5% | −1.2–0.2% | 0.1573 |
| RBC transfusion volume; correct value set as 225 mL | |||||
| DRV120 | 82.6% | 61.7% | 20.9% | 9.2–32.6% | <0.001 |
| DRV300 | 10.4% | 47.3% | −36.8% | −44.2–−29.4% | <0.001 |
| DRV1000 | 2.0% | 3.0% | −1.0% | −3.2–1.2% | 0.3711 |
| Deviation grade | unaided | aided | Diff | 95% CI | p |
| 3 months, 5 kg; bleeding after major surgery, unstable hemodynamic condition | |||||
| Hb transfusion threshold; correct value set as 10.2 g/dL | |||||
| DRV120 | 43.8% | 21.9% | 21.9% | 14.0–26.9% | <0.001 |
| DRV300 | 0.5% | 2.0% | −1.5% | −3.0–0.0% | 0.0578 |
| DRV1000 | 0.5% | 1.0% | −0.5% | −1.7–0.7% | 0.4142 |
| RBC transfusion volume; correct value set as 75 mL | |||||
| DRV120 | 82.1% | 67.2% | 14.9% | 3.0–26.9% | 0.0143 |
| DRV300 | 8.7% | 24.6% | −15.9% | −21.6%–−10.3% | <0.001 |
| DRV1000 | 0.7% | 0.0% | 0.7% | −0.1–1.6% | 0.0833 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piekarski, F.; Noone, S.; Engelhardt, T.; Hellmich, M.; Wittenmeier, E.; Quintao, V.; Arnold, P.; Goobie, S.M.; Zacharowski, K.; Kaufmann, J. Evaluation of a Pre-Filled Table and a Flowchart-Based Algorithm as Cognitive Aids to Reduce Deviations in Dose Calculation for Intraoperative Red Blood Cell Transfusions in Children—An International Web-Based Simulation. Children 2023, 10, 815. https://doi.org/10.3390/children10050815
Piekarski F, Noone S, Engelhardt T, Hellmich M, Wittenmeier E, Quintao V, Arnold P, Goobie SM, Zacharowski K, Kaufmann J. Evaluation of a Pre-Filled Table and a Flowchart-Based Algorithm as Cognitive Aids to Reduce Deviations in Dose Calculation for Intraoperative Red Blood Cell Transfusions in Children—An International Web-Based Simulation. Children. 2023; 10(5):815. https://doi.org/10.3390/children10050815
Chicago/Turabian StylePiekarski, Florian, Stephanie Noone, Thomas Engelhardt, Martin Hellmich, Eva Wittenmeier, Vinicius Quintao, Philip Arnold, Susan M. Goobie, Kai Zacharowski, and Jost Kaufmann. 2023. "Evaluation of a Pre-Filled Table and a Flowchart-Based Algorithm as Cognitive Aids to Reduce Deviations in Dose Calculation for Intraoperative Red Blood Cell Transfusions in Children—An International Web-Based Simulation" Children 10, no. 5: 815. https://doi.org/10.3390/children10050815
APA StylePiekarski, F., Noone, S., Engelhardt, T., Hellmich, M., Wittenmeier, E., Quintao, V., Arnold, P., Goobie, S. M., Zacharowski, K., & Kaufmann, J. (2023). Evaluation of a Pre-Filled Table and a Flowchart-Based Algorithm as Cognitive Aids to Reduce Deviations in Dose Calculation for Intraoperative Red Blood Cell Transfusions in Children—An International Web-Based Simulation. Children, 10(5), 815. https://doi.org/10.3390/children10050815








