Serum YKL-40 as a Potential Biomarker for Sepsis in Term Neonates—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Data Collection—Procedure
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Septic Neonates and Controls
3.2. Inflammatory Markers and Serum YKL-40 Levels in Septic Neonates and Controls
3.3. Correlations between YKL-40 Levels and Inflammatory Markers upon Admission
3.4. Diagnostic Value of YKL-40 Levels upon Admission
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef]
- Fleischmann-Struzek, C.; Goldfarb, D.M.; Schlattmann, P.; Schlapbach, L.J.; Reinhart, K.; Kissoon, N. The global burden of paediatric and neonatal sepsis: A systematic review. Lancet Respir. Med. 2018, 6, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Shane, A.L.; Stoll, B.J. Neonatal sepsis: Progress towards improved outcomes. J. Infect. 2014, 68 (Suppl. 1), S24–S32. [Google Scholar] [CrossRef]
- Molloy, E.J.; Wynn, J.L.; Bliss, J.; Koenig, J.M.; Keij, F.M.; McGovern, M.; Kuester, H.; Turner, M.A.; Giannoni, E.; Mazela, J.; et al. Neonatal sepsis: Need for consensus definition, collaboration and core outcomes. Pediatr. Res. 2020, 88, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Puopolo, K.M.; Hansen, N.I.; Lorch, S.A.; DeMauro, S.B.; Greenberg, R.G.; Cotten, C.M.; Sanchez, P.J.; Bell, E.F.; Eichenwald, E.C.; et al. Neurodevelopmental outcomes following neonatal late-onset sepsis and blood culture-negative conditions. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 467–473. [Google Scholar] [CrossRef]
- Celik, I.H.; Hanna, M.; Canpolat, F.E.; Pammi, M. Diagnosis of neonatal sepsis: The past, present and future. Pediatr. Res. 2022, 91, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Cantey, J.B.; Lee, J.H. Biomarkers for the Diagnosis of Neonatal Sepsis. Clin. Perinatol. 2021, 48, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Eschborn, S.; Weitkamp, J.H. Procalcitonin versus C-reactive protein: Review of kinetics and performance for diagnosis of neonatal sepsis. J. Perinatol. 2019, 39, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.H.; Song, J.H.; Park, I.S.; Kim, H.; Park, S.; Oh, M.H. Reference Intervals of Serum Procalcitonin Are Affected by Postnatal Age in Very Low Birth Weight Infants during the First 60 Days after Birth. Neonatology 2015, 108, 60–64. [Google Scholar] [CrossRef]
- Vouloumanou, E.K.; Plessa, E.; Karageorgopoulos, D.E.; Mantadakis, E.; Falagas, M.E. Serum procalcitonin as a diagnostic marker for neonatal sepsis: A systematic review and meta-analysis. Intensive Care Med. 2011, 37, 747–762. [Google Scholar] [CrossRef]
- Pontrelli, G.; De Crescenzo, F.; Buzzetti, R.; Jenkner, A.; Balduzzi, S.; Calò Carducci, F.; Amodio, D.; De Luca, M.; Chiurchiù, S.; Davies, E.H.; et al. Accuracy of serum procalcitonin for the diagnosis of sepsis in neonates and children with systemic inflammatory syndrome: A meta-analysis. BMC Infect. Dis. 2017, 17, 302. [Google Scholar] [CrossRef] [PubMed]
- Stocker, M.; van Herk, W.; El Helou, S.; Dutta, S.; Fontana, M.S.; Schuerman, F.A.B.A.; van den Tooren-de Groot, R.K.; Wieringa, J.W.; Janota, J.; van der Meer-Kappelle, L.H.; et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: A multicentre, randomised controlled trial (NeoPIns). Lancet 2017, 390, 871–881. [Google Scholar] [CrossRef]
- Chiesa, C.; Pacifico, L.; Natale, F.; Hofer, N.; Osborn, J.F.; Resch, B. Fetal and early neonatal interleukin-6 response. Cytokine 2015, 76, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shahkar, L.; Keshtkar, A.; Mirfazeli, A.; Ahani, A.; Roshandel, G. The role of IL-6 for predicting neonatal sepsis: A systematic review and meta-analysis. Iran J. Pediatr. 2011, 21, 411–417. [Google Scholar] [PubMed]
- Eichberger, J.; Resch, B. Reliability of Interleukin-6 Alone and in Combination for Diagnosis of Early Onset Neonatal Sepsis: Systematic Review. Front. Pediatr. 2022, 10, 840778. [Google Scholar] [CrossRef]
- Zhou, M.; Cheng, S.; Yu, J.; Lu, Q. Interleukin-8 for diagnosis of neonatal sepsis: A meta-analysis. PLoS ONE 2015, 10, e0127170. [Google Scholar] [CrossRef]
- Poggi, C.; Lucenteforte, E.; Petri, D.; De Masi, S.; Dani, C. Presepsin for the Diagnosis of Neonatal Early-Onset Sepsis: A Systematic Review and Meta-analysis. JAMA Pediatr. 2022, 176, 750–758. [Google Scholar] [CrossRef]
- Parri, N.; Trippella, G.; Lisi, C.; De Martino, M.; Galli, L.; Chiappini, E. Accuracy of presepsin in neonatal sepsis: Systematic review and meta-analysis. Expert. Rev. Anti Infect. Ther. 2019, 17, 223–232. [Google Scholar] [CrossRef]
- Bourika, V.; Hantzi, E.; Michos, A.; Margeli, A.; Papassotiriou, I.; Siahanidou, T. Clinical Value of Serum Amyloid-A Protein, High-density Lipoprotein Cholesterol and Apolipoprotein-A1 in the Diagnosis and Follow-up of Neonatal Sepsis. Pediatr. Infect. Dis. J. 2020, 39, 749–755. [Google Scholar] [CrossRef]
- Chang, C.; Gao, Q.; Deng, G.; Luo, K.; Zhu, H. Diagnostic and prognostic predictive values of triggering receptor expressed on myeloid cell-1 expression in neonatal sepsis: A meta-analysis and systematic review. Front. Pediatr. 2022, 10, 929665. [Google Scholar] [CrossRef]
- Di Francesco, A.M.; Verrecchia, E.; Manna, S.; Urbani, A.; Manna, R. The chitinases as biomarkers in immune-mediate diseases. Clin. Chem. Lab. Med. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Zhao, T.; Su, Z.; Li, Y.; Zhang, X.; You, Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal. Transduct. Target. Ther. 2020, 5, 201. [Google Scholar] [CrossRef] [PubMed]
- Fusetti, F.; Pijning, T.; Kalk, K.H.; Bos, E.; Dijkstra, B.W. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J. Biol. Chem. 2003, 278, 37753–37760. [Google Scholar] [CrossRef]
- Houston, D.R.; Recklies, A.D.; Krupa, J.C.; van Aalten, D.M. Structure and ligand-induced conformational change of the 39-kDa glycoprotein from human articular chondrocytes. J. Biol. Chem. 2003, 278, 30206–30212. [Google Scholar] [CrossRef]
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.J.; He, C.H.; Takyar, S.; Elias, J.A. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I.M.; Hall, I.E.; Kale, S.; Lee, S.; He, C.H.; Lee, Y.; Chupp, G.L.; Moeckel, G.W.; Lee, C.G.; Elias, J.A.; et al. Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J. Am. Soc. Nephrol. 2013, 24, 309–319. [Google Scholar] [CrossRef]
- Johansen, J.S.; Krabbe, K.S.; Møller, K.; Pedersen, B.K. Circulating YKL-40 levels during human endotoxaemia. Clin. Exp. Immunol. 2005, 140, 343–348. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Liu, W.; He, C.H.; Jacoby, A.; Gornitzky, A.; Ma, B.; Flavell, R.; Lee, C.G.; Elias, J.A. Chitinase 3-like-1 promotes Streptococcus pneumoniae killing and augments host tolerance to lung antibacterial responses. Cell Host Microbe 2012, 12, 34–46. [Google Scholar] [CrossRef]
- Marion, C.R.; Wang, J.; Sharma, L.; Losier, A.; Lui, W.; Andrews, N.; Elias, J.A.; Kazmierczak, B.I.; Roy, C.R.; Dela Cruz, C.S. Chitinase 3-Like 1 (Chil1) Regulates Survival and Macrophage-Mediated Interleukin-1β and Tumor Necrosis Factor Alpha during Pseudomonas aeruginosa Pneumonia. Infect. Immun. 2016, 84, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- Coffman, F.D. Chitinase 3-Like-1 (CHI3L1): A putative disease marker at the interface of proteomics and glycomics. Crit. Rev. Clin. Lab. Sci. 2008, 45, 531–562. [Google Scholar] [CrossRef]
- Prakash, M.; Bodas, M.; Prakash, D.; Nawani, N.; Khetmalas, M.; Mandal, A.; Eriksson, C. Diverse pathological implications of YKL-40: Answers may lie in ‘outside-in’ signaling. Cell Signal. 2013, 25, 1567–1573. [Google Scholar] [CrossRef]
- Spoorenberg, S.M.C.; Vestjens, S.M.T.; Voorn, G.P.; van Moorsel, C.H.M.; Meek, B.; Zanen, P.; Rijkers, G.T.; Bos, W.J.W.; Grutters, J.C.; Ovidius study group. Course of SP-D, YKL-40, CCL18 and CA 15-3 in adult patients hospitalised with community-acquired pneumonia and their association with disease severity and aetiology: A post-hoc analysis. PLoS ONE 2018, 13, e0190575. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Hsiao, P.C.; Tsai, H.T.; Yeh, C.B.; Yang, S.F. Usefulness of plasma YKL-40 in management of community-acquired pneumonia severity in patients. Int. J. Mol. Sci. 2013, 14, 22817–22825. [Google Scholar] [CrossRef]
- Østergaard, C.; Johansen, J.S.; Benfield, T.; Price, P.A.; Lundgren, J.D. YKL-40 is elevated in cerebrospinal fluid from patients with purulent meningitis. Clin. Diagn. Lab. Immunol. 2002, 9, 598–604. [Google Scholar] [CrossRef]
- Hattori, N.; Oda, S.; Sadahiro, T.; Nakamura, M.; Abe, R.; Shinozaki, K.; Nomura, F.; Tomonaga, T.; Matsushita, K.; Kodera, Y.; et al. YKL-40 identified by proteomic analysis as a biomarker of sepsis. Shock 2009, 32, 393–400. [Google Scholar] [CrossRef]
- Kornblit, B.; Hellemann, D.; Munthe-Fog, L.; Bonde, J.; Strøm, J.J.; Madsen, H.O.; Johansen, J.S.; Garred, P. Plasma YKL-40 and CHI3L1 in systemic inflammation and sepsis-experience from two prospective cohorts. Immunobiology 2013, 218, 1227–1234. [Google Scholar] [CrossRef]
- Kjaergaard, A.D.; Helby, J.; Johansen, J.S.; Nordestgaard, B.G.; Bojesen, S.E. Elevated plasma YKL-40 and risk of infectious disease: A prospective study of 94665 individuals from the general population. Clin. Microbiol. Infect. 2020, 26, e1–e1411. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Wang, X.W.; Qie, L.P. Disease indicators for sepsis and analysis of sepsis treatment in children using the continuous blood purification technique. Genet. Mol. Res. 2015, 14, 5685–5693. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.; Giroir, B.; Randolph, A.; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Chung, M.H.; Bin, J.H.; Cho, K.S.; Lee, J.; Suh, J.S. Urinary YKL-40 as a Candidate Biomarker for Febrile Urinary Tract Infection in Young Children. Ann. Lab. Med. 2018, 38, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Mashaly, G.; El-Kazzaz, S.; Zeid, M. Urine YKL-40 versus Urine NGAL as Potential Markers for Diagnosis of Urinary Tract Infection in Febrile Pediatric Patients. Open J. Immunol. 2020, 10, 10–20. [Google Scholar] [CrossRef]
- Henckel, E.; James, A.; Konradsen, J.R.; Nordlund, B.; Kjellberg, M.; Berggren-Broström, E.; Hedlin, G.; Degerman, S.; Bohlin, K. A Novel Association between YKL-40, a Marker of Structural Lung Disease, and Short Telomere Length in 10-Year-Old Children with Bronchopulmonary Dysplasia. Children 2021, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Dąbkowska, K.; Wojdas, M.; Kuźnik-Trocha, K.; Wisowski, G.; Gruenpeter, A.; Komosińska-Vassev, K.; Olczyk, K.; Winsz-Szczotka, K. GAAGs, COMP, and YKL-40 as Potential Markers of Cartilage Turnover in Blood of Children with Juvenile Idiopathic Arthritis Treated with Etanercept-Relationship with ADAMTS4, ADAMTS5, and PDGF-BB. J. Clin. Med. 2022, 11, 5069. [Google Scholar] [CrossRef] [PubMed]
- Bernard, I.; Ransy, D.G.; Brophy, J.; Kakkar, F.; Bitnun, A.; Samson, L.; Read, S.; Soudeyns, H.; Hawkes, M.T.; Epic Study Group. Chitinase-3-like Protein 1 Is Associated with Poor Virologic Control and Immune Activation in Children Living with HIV. Viruses 2022, 14, 2602. [Google Scholar] [CrossRef]
- Permain, J.; Appleton, L.; Ho, S.S.C.; Coffey, M.; Ooi, C.Y.; Keenan, J.I.; Day, A.S. Children with Cystic Fibrosis Have Elevated Levels of Fecal Chitinase-3-like-1. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Alarcón, A.A.; Gómez-Gómez, Y.; Organista-Nava, J.; Jiménez-López, M.A.; Rivera-Ramírez, A.B.; Ibarra-Sierra, E.; Saavedra-Herrera, M.V.; Illades-Aguiar, B.; Leyva-Vázquez, M.A. Plasma levels of YKL-40 as a prognostic factor in childhood acute lymphoblastic leukemia. Mol. Clin. Oncol. 2021, 15, 168. [Google Scholar] [CrossRef]
- El-Asrar, M.A.; Elbarbary, N.S.; Ismail, E.A.; Elshenity, A.M. Serum YKL-40 in young patients with β-thalassemia major: Relation to hepatitis C virus infection, liver stiffness by transient elastography and cardiovascular complications. Blood Cells Mol. Dis. 2016, 56, 1–8. [Google Scholar] [CrossRef]
- Yang, X.; Sheng, G. YKL-40 levels are associated with disease severity and prognosis of viral pneumonia, but not available in bacterial pneumonia in children. BMC Pediatr. 2018, 18, 381. [Google Scholar] [CrossRef] [PubMed]
- Volck, B.; Price, P.A.; Johansen, J.S.; Sørensen, O.; Benfield, T.L.; Nielsen, H.J.; Calafat, J.; Borregaard, N. YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc. Assoc. Am. Physicians 1998, 110, 351–360. [Google Scholar]

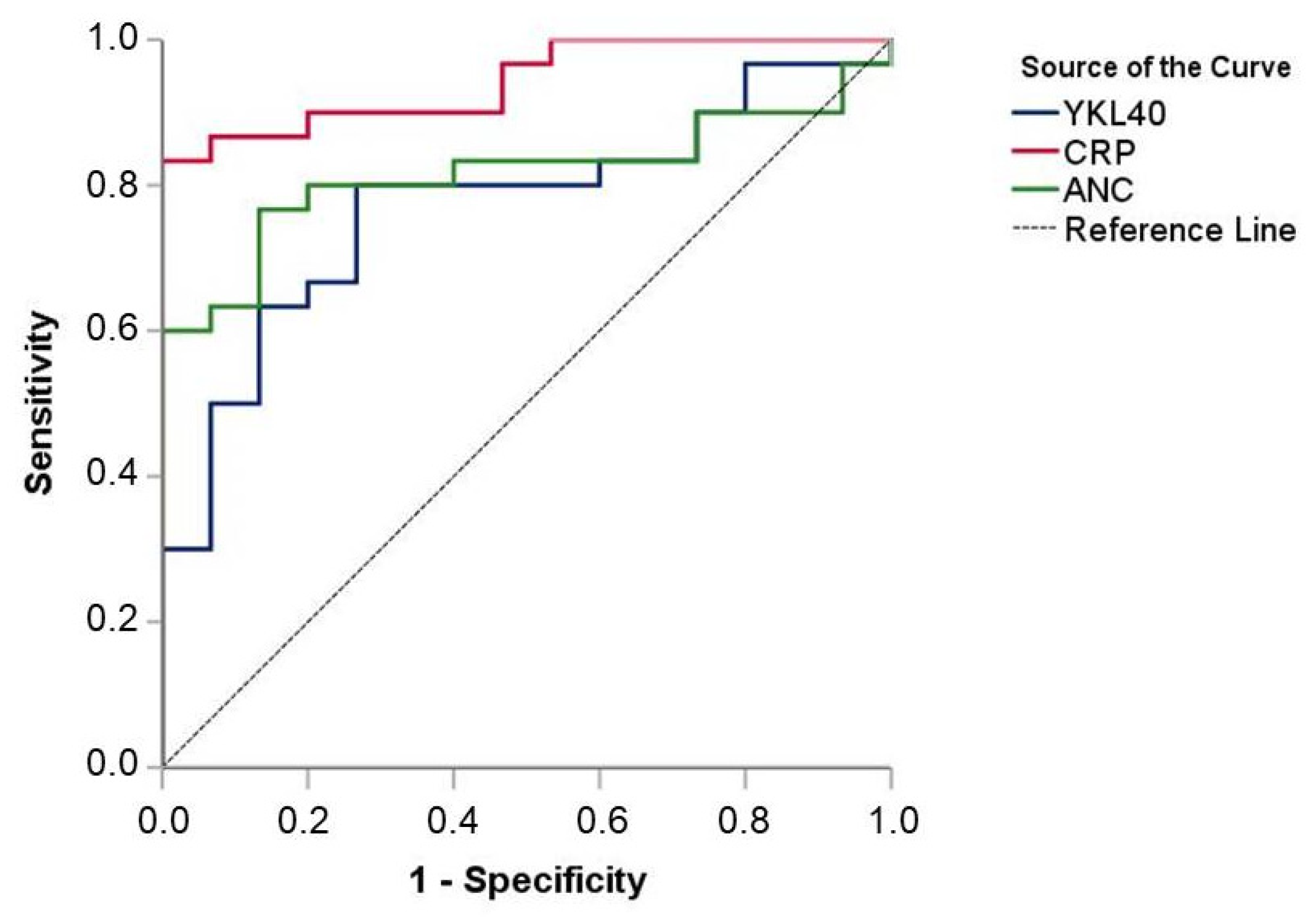
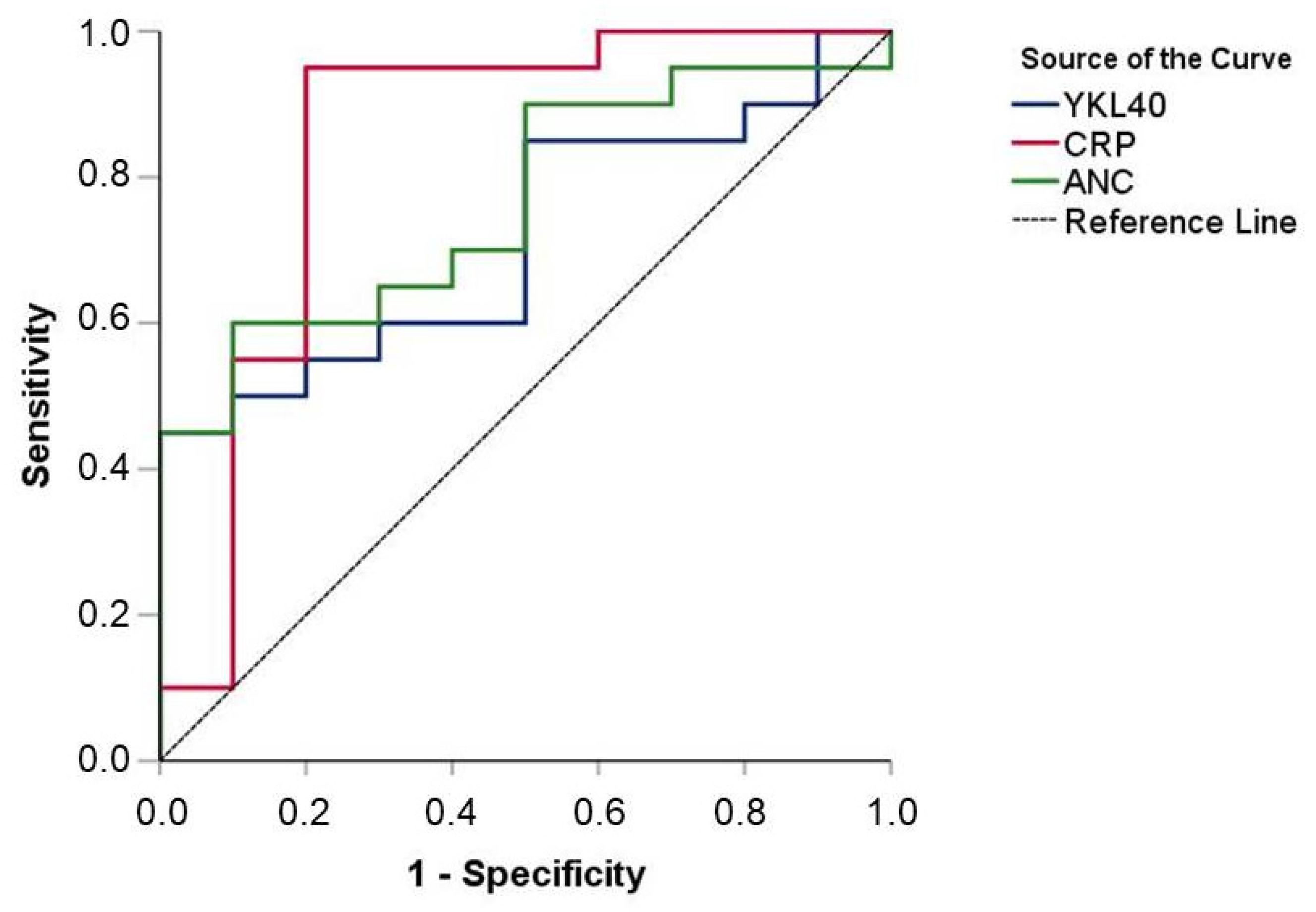
| Variable | Overall (n = 45) | Septic (n = 30) | Controls (n = 15) | p-Value |
|---|---|---|---|---|
| Gender | 0.660 | |||
| Female | 16 (35.6%) | 10 (33.3%) | 6 (40%) | |
| Male | 29 (64.4%) | 20 (66.7%) | 9 (60%) | |
| Age (days) | 18.0 (6.7) | 17.9 (6.6) | 18.1 (7.2) | 0.549 |
| Gestational age (weeks) | 38.4 (1.2) | 38.5 (1.2) | 38.4 (1.3) | 0.568 |
| Birth weight (grams) | 3,128 (363) | 3,155 (303) | 3,075 (467) | 0.752 |
| SIRS criteria | N/A | |||
| Core temperature of >38.5 °C or <36 °C | 30 (100%) | |||
| Tachycardia or bradycardia | 15 (50%) | |||
| Increased respiratory rate | 21 (70%) | |||
| Abnormal leukocyte count | 13 (43.3%) | |||
| Positive culture (blood, urine, or CSF) | N/A | |||
| No | 10 (33.3%) | |||
| Yes | 20 (66.7%) | |||
| Positive blood culture | N/A | |||
| No | 23 (76.7%) | |||
| Yes | 7 (23.3%) |
| Overall (n = 45) | Septic (n = 30) | Controls (n = 15) | p-Value * | |
|---|---|---|---|---|
| Upon admission (Day 1) | ||||
| WBC (/mm3) | ||||
| Median (25th–75th) | 11,030 (8660–15,940) | 13,165 (8570–19,260) | 10,250 (8660–11,030) | 0.017 |
| ANC (/mm3) | ||||
| Median (25th–75th) | 3242 (1964–7970) | 7089 (3192–11,388) | 2010 (1426–2854) | <0.001 |
| PLT (×103/mm3) | ||||
| Median (25th–75th) | 466 (369–609) | 459 (361–555) | 572 (418–702) | 0.059 |
| CRP (mg/L) | ||||
| Median (25th–75th) | 3.4 (1–52.3) | 30.3 (3.4–75) | 0.6 (0.2–1.1) | <0.001 |
| YKL-40 (pg/mL) | ||||
| Median (25th–75th) | 32.9 (21.3–40.4) | 35.5 (27.9–54.2) | 21.5 (18.9–31.2) | 0.003 |
| Upon Admission | Total Septic Group (n = 30) | Culture-Positive Group (n = 20) | Culture-Negative Group (n = 10) | p-Value |
|---|---|---|---|---|
| WBC (/mm3) | ||||
| Median (25th–75th) | 13,165 (8570–19,260) | 15,270 (10,690–20,225) | 10,255 (6500–13,960) | 0.053 |
| ANC (/mm3) | ||||
| Median (25th–75th) | 7089 (3192–11,388) | 8674 (3,521–12,014) | 3619 (1823–7199) | 0.010 |
| PLT (×103/mm3) | ||||
| Median (25th–75th) | 459 (361–555) | 462 (350–580) | 397 (363–510) | 0.395 |
| CRP (mg/L) | ||||
| Median (25th–75th) | 30.3 (3.4–75) | 54.7 (24.2–81.1) | 2.2 (1–3.8) | 0.002 |
| YKL-40 (pg/mL) | ||||
| Median (25th–75th) | 35.5 (27.9–54.2) | 38.6 (31–63.1) | 31.5 (21.3–35.9) | 0.061 |
| CRP | p-Value | YKL-40 | p-Value | |
|---|---|---|---|---|
| Overall (n = 45) | ||||
| YKL-40 | 0.59 | <0.001 | - | - |
| WBC | 0.51 | <0.001 | 0.39 | 0.009 |
| ANC | 0.61 | <0.001 | 0.43 | 0.003 |
| PLT | −0.08 | 0.599 | −0.02 | 0.877 |
| Septic group (n = 30) | ||||
| YKL-40 | 0.52 | 0.003 | - | - |
| WBC | 0.43 | 0.016 | 0.37 | 0.044 |
| ANC | 0.48 | 0.006 | 0.40 | 0.030 |
| PLT | 0.28 | 0.133 | 0.13 | 0.477 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steletou, E.; Metallinou, D.; Margeli, A.; Giannouchos, T.; Michos, A.; Kanaka-Gantenbein, C.; Papassotiriou, I.; Siahanidou, T. Serum YKL-40 as a Potential Biomarker for Sepsis in Term Neonates—A Pilot Study. Children 2023, 10, 772. https://doi.org/10.3390/children10050772
Steletou E, Metallinou D, Margeli A, Giannouchos T, Michos A, Kanaka-Gantenbein C, Papassotiriou I, Siahanidou T. Serum YKL-40 as a Potential Biomarker for Sepsis in Term Neonates—A Pilot Study. Children. 2023; 10(5):772. https://doi.org/10.3390/children10050772
Chicago/Turabian StyleSteletou, Evangelia, Dimitra Metallinou, Alexandra Margeli, Theodoros Giannouchos, Athanasios Michos, Christina Kanaka-Gantenbein, Ioannis Papassotiriou, and Tania Siahanidou. 2023. "Serum YKL-40 as a Potential Biomarker for Sepsis in Term Neonates—A Pilot Study" Children 10, no. 5: 772. https://doi.org/10.3390/children10050772
APA StyleSteletou, E., Metallinou, D., Margeli, A., Giannouchos, T., Michos, A., Kanaka-Gantenbein, C., Papassotiriou, I., & Siahanidou, T. (2023). Serum YKL-40 as a Potential Biomarker for Sepsis in Term Neonates—A Pilot Study. Children, 10(5), 772. https://doi.org/10.3390/children10050772








