Timing of Ketogenic Dietary Therapy (KDT) Introduction and Its Impact on Cognitive Profiles in Children with Glut1-DS—A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuropsychological Assessment
2.3. Statistical Analyses
3. Results
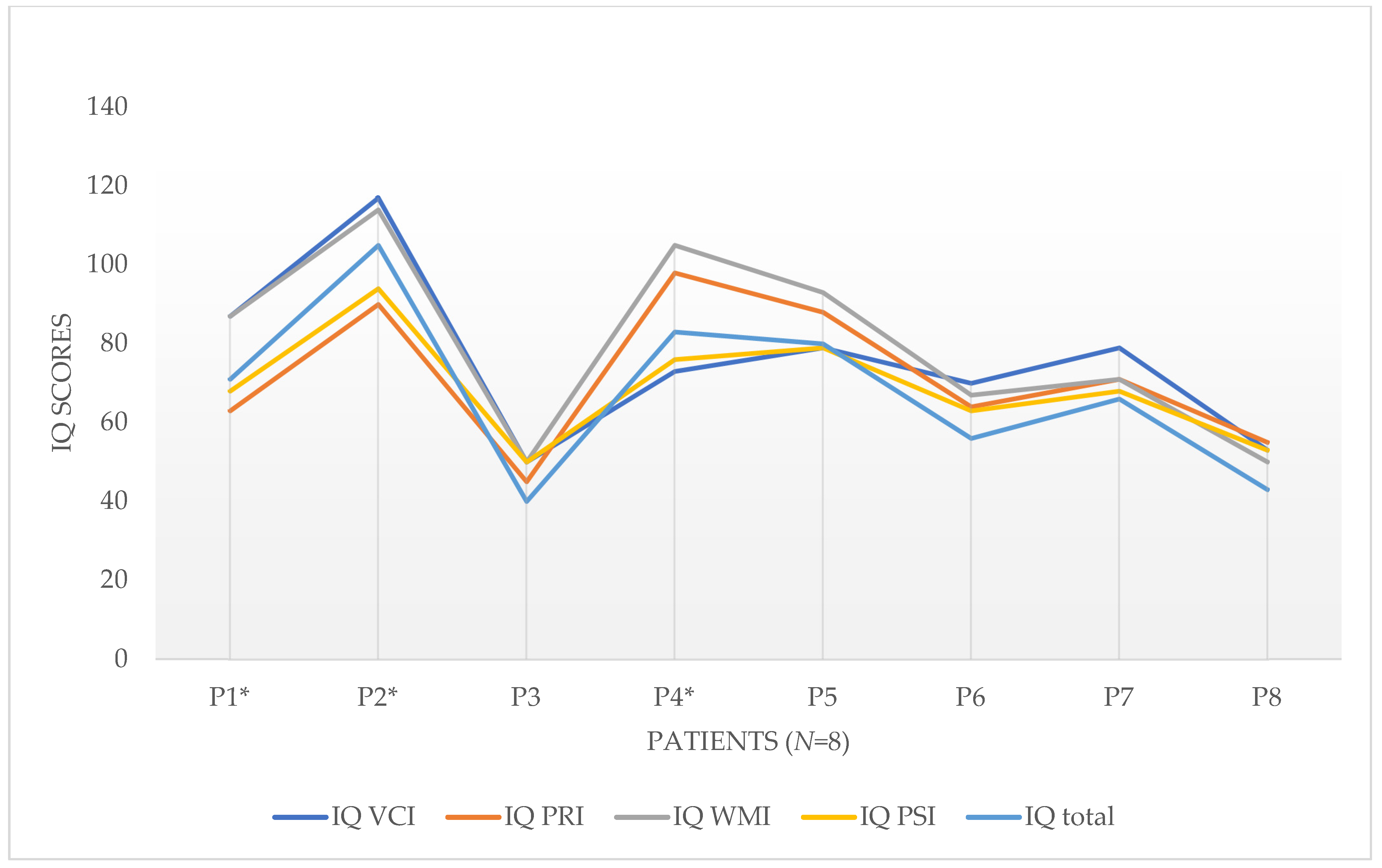
3.1. Cognitive Performance Profiles in Glut1DS
3.2. Correlations to KDT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klepper, J.; Akman, C.; Armeno, M.; Auvin, S.; Cervenka, M.; Cross, H.J.; De Giorgis, V.; Della Marina, A.; Engelstad, K.; Heussinger, N.; et al. Glut1 Deficiency Syndrome (Glut1DS): State of the art in 2020 and recommendations of the international Glut1DS study group. Epilepsia Open 2020, 5, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, K. Klinisches Spektrum des Glucosetransporter-Typ-1-Defizienz-Syndroms. Z. Epileptol. 2014, 27, 186–190. [Google Scholar] [CrossRef]
- Hully, M.; Vuillaumier-Barrot, S.; Le Bizec, C.; Boddaert, N.; Kaminska, A.; Lascelles, K.; de Lonlay, P.; Cances, C.; Portes, V.D.; Roubertie, A.; et al. From splitting GLUT1 deficiency syndromes to overlapping phenotypes. Eur. J. Med. Genet. 2015, 58, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Gras, D.; Roze, E.; Caillet, S.; Méneret, A.; Doummar, D.; de Villemeur, T.B.; Vidailhet, M.; Mochel, F. GLUT1 deficiency syndrome: An update. Rev. Neurol. 2014, 170, 91–99. [Google Scholar] [CrossRef]
- Klepper, J. Der Glukosetransporter (GLUT1)-Defekt: Definition einer neuen Erkrankung. Neuropädiatrie Klinik Praxis 2004, 3, 82–86. [Google Scholar]
- Wang, D.; Pascual, J.M.; de Vivo, D. Glucose Transporter Type 1 Deficiency Syndrome; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.A., Stephens, K., Eds.; GeneReviews®; University of Washington: Seattle, WA, USA, 2018. [Google Scholar]
- Alter, A.S.; Engelstad, K.; Hinton, V.J.; Montes, J.; Pearson, T.S.; Akman, C.I.; De Vivo, D.C. Long-Term Clinical Course of Glut1 Deficiency Syndrome. J. Child Neurol. 2014, 30, 160–169. [Google Scholar] [CrossRef]
- Leen, W.G.; Klepper, J.; Verbeek, M.M.; Leferink, M.; Hofste, T.; van Engelen, B.G.; Wevers, R.A.; Arthur, T.; Bahi-Buisson, N.; Ballhausen, D.; et al. Glucose transporter-1 deficiency syndrome: The expanding clinical and genetic spectrum of a treatable disorder. Brain 2010, 133, 655–670. [Google Scholar] [CrossRef]
- Pearson, T.S.; Akman, C.; Hinton, V.J.; Engelstad, K.; De Vivo, D.C. Phenotypic Spectrum of Glucose Transporter Type 1 Deficiency Syndrome (Glut1 DS). Curr. Neurol. Neurosci. Rep. 2013, 13, 342. [Google Scholar] [CrossRef]
- Pearson, T.S.; Pons, R.; Engelstad, K.; Kane, S.A.; de Vivo, D.C. Paroxysmal eye-head movements in Glut1 deficiency syn-drome. Neurology 2017, 88, 1666–1673. [Google Scholar] [CrossRef]
- De Giorgis, V.; Teutonico, F.; Cereda, C.; Balottin, U.; Bianchi, M.; Giordano, L.; Olivotto, S.; Ragona, F.; Tagliabue, A.; Zorzi, G.; et al. Sporadic and familial glut1ds Italian patients: A wide clinical variability. Seizure 2014, 24, 28–32. [Google Scholar] [CrossRef]
- De Giorgis, V.; Masnada, S.; Varesio, C.; Chiappedi, M.A.; Zanaboni, M.; Pasca, L.; Filippini, M.; Macasaet, J.A.; Valente, M.; Ferraris, C.; et al. Overall cognitive profiles in patients with GLUT 1 Deficiency Syndrome. Brain Behav. 2019, 9, e01224. [Google Scholar] [CrossRef]
- Ito, Y.; Oguni, H.; Ito, S.; Oguni, M.; Osawa, M. A modified Atkins diet is promising as a treatment for glucose transporter type 1 deficiency syndrome. Dev. Med. Child Neurol. 2011, 53, 658–663. [Google Scholar] [CrossRef]
- Ramm-Pettersen, A.; Stabell, K.E.; Nakken, K.O.; Selmer, K.K. Does ketogenic diet improve cognitive function in patients with GLUT1-DS? A 6- to 17-month follow-up study. Epilepsy Behav. 2014, 39, 111–115. [Google Scholar] [CrossRef]
- Varesio, C.; Pasca, L.; Parravicini, S.; Zanaboni, M.P.; Ballante, E.; Masnada, S.; Ferraris, C.; Bertoli, S.; Tagliabue, A.; Veggiotti, P.; et al. Quality of Life in Chronic Ketogenic Diet Treatment: The GLUT1DS Population Perspective. Nutrients 2019, 11, 1650. [Google Scholar] [CrossRef]
- Ito, Y.; Takahashi, S.; Kagitani-Shimono, K.; Natsume, J.; Yanagihara, K.; Fujii, T.; Oguni, H. Nationwide survey of glucose transporter-1 deficiency syndrome (GLUT-1DS) in Japan. Brain Dev. 2014, 37, 780–789. [Google Scholar] [CrossRef]
- Kolic, I.; Nisevic, J.R.; Cicvaric, I.V.; Ahel, I.B.; Tomulic, K.L.; Segulja, S.; Dekanic, K.B.; Serifi, S.; Ovuka, A.; Prpic, I. GLUT1 Deficiency Syndrome—Early Treatment Maintains Cognitive Development? (Literature Review and Case Report). Genes 2021, 12, 1379. [Google Scholar] [CrossRef]
- Pascual, J.M.; Wang, D.; Hinton, V.; Engelstad, K.; Saxena, C.M.; Van Heertum, R.L.; De Vivo, D.C. Brain Glucose Supply and the Syndrome of Infantile Neuroglycopenia. Arch. Neurol. 2007, 64, 507–513. [Google Scholar] [CrossRef]
- Ragona, F.; Matricardi, S.; Castellotti, B.; Patrini, M.; Freri, E.; Binelli, S.; Granata, T. Refractory Absence Epilepsy and Glut1 Deficiency Syndrome: A New Case Report and Literature Review. Neuropediatrics 2014, 45, 328–332. [Google Scholar] [CrossRef]
- Zanaboni, M.; Pasca, L.; Villa, B.; Faggio, A.; Grumi, S.; Provenzi, L.; Varesio, C.; De Giorgis, V. Characterization of Speech and Language Phenotype in GLUT1DS. Children 2021, 8, 344. [Google Scholar] [CrossRef]
- Amalou, S.; Gras, D.; Ilea, A.; Greneche, M.-O.; Francois, L.; Bellavoine, V.; Delanoe, C.; Auvin, S. Use of modified Atkins diet in glucose transporter type 1 deficiency syndrome. Dev. Med. Child Neurol. 2016, 58, 1193–1199. [Google Scholar] [CrossRef]
- Pong, A.W.; Geary, B.R.; Engelstad, K.M.; Natarajan, A.; Yang, H.; De Vivo, D.C. Glucose transporter type I deficiency syndrome: Epilepsy phenotypes and outcomes. Epilepsia 2012, 53, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Gramer, G.; Wolf, N.I.; Vater, D.; Bast, T.; Santer, R.; Kamsteeg, E.-J.; Wevers, R.A.; Ebinger, F. Glucose Transporter-1 (GLUT1) Deficiency Syndrome: Diagnosis and Treatment in Late Childhood. Neuropediatrics 2012, 43, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Leen, W.G.; Taher, M.; Verbeek, M.; Kamsteeg, E.J.; Van De Warrenburg, B.P.; Willemsen, M.A. GLUT1 deficiency syndrome into adulthood: A follow-up study. J. Neurol. 2014, 261, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Veggiotti, P.; Teutonico, F.; Alfei, E.; Nardocci, N.; Zorzi, G.; Tagliabue, A.; De Giorgis, V.; Balottin, U. Glucose transporter type 1 deficiency: Ketogenic diet in three patients with atypical phenotype. Brain Dev. 2010, 32, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. WAIS. Wechsler Adult Intelligence Scale; Standardizzazione Italiana a Cura di Laicardi C. e Orsini A.; Organizzazioni Speciali: Florence, Italy, 1997. [Google Scholar]
- Wechsler, D. Scala D’intelligenza Wechsler per Bambini; Organizzazioni Speciali: Florence, Italy, 2012. [Google Scholar]
- Wood, A.M.; Geddes, G.C.; Marashly, A. Language regression, hemichorea and focal subclinical seizures in a 6-year-old girl with GLUT-1 deficiency. Epilepsy Behav. Rep. 2019, 14, 100340. [Google Scholar] [CrossRef] [PubMed]
- Petermann, F.; Petermann, U. Wechsler Intelligence Scale for Children (WISC-IV); Pearson: Munich, Germany, 2011. [Google Scholar]
- Daseking, M.; Petermann, F.; Petermann, U. HAWIK-IV: Grundlagen und Auswertungsstrategien. In Fallbuch HAWIK-IV; Petermann, F., Daseking, M.H., Eds.; Hogrefe: Göttingen, Germany, 2009; pp. 13–36. [Google Scholar]
- Renner, G.; Schroeder, A. Testinformation zur Wechsler Intelligence Scale—Fifth Edition (WISC-V) (Dia-Inform Verfahrensinformationen 003-01); Pädagogische Hochschule Ludwigsburg: Ludwigsburg, Germany, 2018. [Google Scholar]
- Petermann, F.; Wechsler, D. Hamburg-Wechsler-Intelligenztest für Kinder-IV: HAWIK-IV; Huber: Berne, Germany, 2010. [Google Scholar]
- Ziegler, W.; Schölderle, T.; Staiger, A.; Vogel, M. BoDyS: Bogenhausener Dysarthrieskalen; Hogrefe: Göttingen, Germany, 2018. [Google Scholar]
- Haas, E.; Ziegler, W.; Schölderle, T. Dysarthriediagnostik mit Kindern—Das Testmaterial der BoDyS-KiD. Sprache Stimme·Gehör 2020, 44, 189–193. [Google Scholar] [CrossRef]
| ID Patient | Sex a | Age at Diagnosis | Mutation | CSF b/ Blood | Epilepsy | Movement Disorder | Other Symptoms f | KDT | KDT Efficacy | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ratio | Y/N c | Type d | Y/N | Type e | Start Age | Type g | Epilepsy | Cognition | |||||
| 1 | F | 5 years | Mut Q304X in Ex7 (nonsense, heterozygous) | / | Y | GS | Y | D, Hy, S, A, PED | ED, SEM | 5.2 | C | Y | N |
| 2 | F | 1 year | c.679+2T>G (heterozygous) | 0.36 | Y | ABS | Y | D, A | OMD | 1.0 | C | Y | / |
| 3 | F | 8.5 year | c1199C>T (point mutation) | 0.39 | Y | ABS, GS, FS | Y | D, Hy, A, PED | H | 14.3 | C | N | Y |
| 4 | F | 3 years | R153L (missense, heterozygous) | 0.39 | N | / | Y | D, A, PED | / | 3.1 | C, MAD, other | / | / |
| 5 | F | 3 years | R153L (missense, heterozygous) | 0.37 | N | / | Y | D, A, PED | / | 3.1 | C, MAD, other | / | / |
| 6 | F | 2.5 years | c.26-27insT, Arg11Ser78 (frameshift, heterozygous) | 0.33 | Y | FS | Y | D, Hy, A, PED | SEM | 2.7 | C | Y | Y |
| 7 | F | 3.8 years | c.485T>G(p.(Leu162Arg), Ex 4 (heterozygous) | 0.39 | Y | ABS, FS | Y | D, A | ED, M | 3.8 | MAD | Y | Y |
| 8 | M | 6.6 years | c.138_141dupGACA, (duplicated, heterozygous) | 0.37 | Y | ABS, FS | Y | D, S, A | / | 6.6 | C | Y | N |
| ID Patient | Sex a | Test | Test Date | Age at the Time of Testing | Dimensions of Intelligence: IQ-Score b | Total IQ- Score | |||
|---|---|---|---|---|---|---|---|---|---|
| VC | PR | WM | PS | ||||||
| 1 | F | WISC-IV | 29.04.16 | 13.1 | 87 | 63 | 87 | 68 | 71 |
| 2 | F | WISC-IV | 20.04.18 | 8.4 | 117 | 90 | 114 | 94 | 105 |
| 3 | F | WISC-IV | 30.09.16 | 15.5 | 50 | 45 | 50 | 50 | 40 |
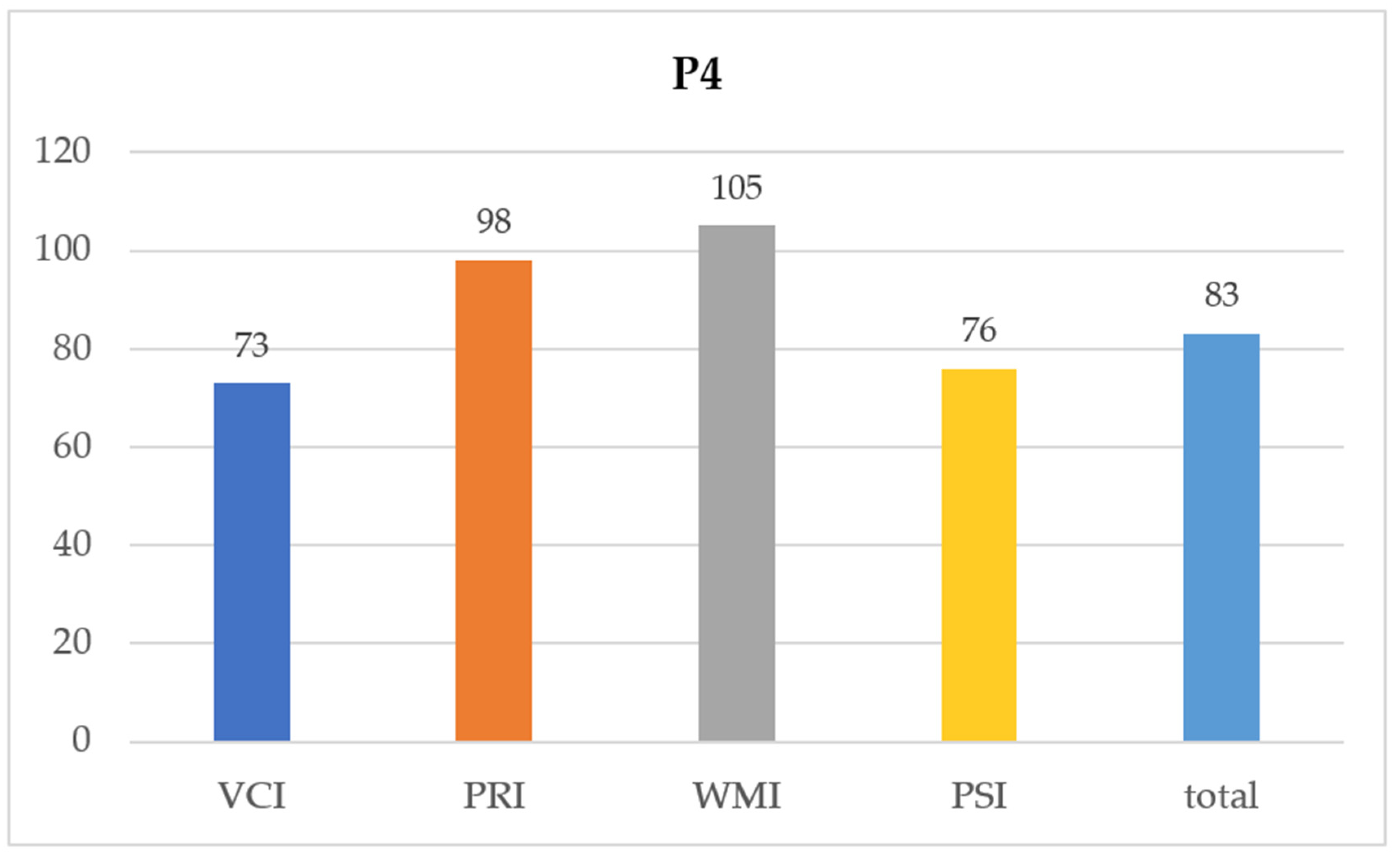
| 4 | F | WISC-IV | 18.03.16 | 14.3 | 73 | 98 | 105 | 76 | 83 |
| 5 | F | WISC-IV | 18.03.16 | 14.3 | 79 | 88 | 93 | 79 | 80 |
| 6 | F | WISC-IV | 30.08.21 | 9.1 | 70 | / | 67 | 63 | 56 |
| 7 | F | WISC-IV | 16.01.20 | 7.2 | 79 | 71 | 71 | 68 | 66 |
| 8 | M | WISC-IV | 25.08.17 | 10.5 | 53 | 55 | 50 | 53 | 43 |
| Timing of KD Introduction (Age in Years) | Duration of Treatment with KD (in Years) | IQ Total | Verbal Comprehension Index (VCI) | Perceptual Reasoning Index (PRI) | Working Memory Index (WMI) | Processing Speed Index (PSI) | |||
|---|---|---|---|---|---|---|---|---|---|
| Spearman’s Rho | Timing of KD introduction (age in years) | Correlation Coefficient | 1.000 | −0.663 * | −0.611 | −0.440 | −0.719 * | −0.602 | −0.749 * |
| Sig. (1-tailed) | 0.037 | 0.054 | 0.138 | 0.022 | 0.057 | 0.016 | |||
| Duration of treatment with KD (in years) | Correlation Coefficient | 1.000 | 0.347 | 0.078 | 0.383 | 0.361 | 0.430 | ||
| Sig. (1-tailed) | 0.200 | 0.427 | 0.174 | 0.190 | 0.144 | ||||
| Timing of KD Introduction (Age in Years) | Duration of Treatment with KD (in Years) | Coding | Symbol Search | |||
|---|---|---|---|---|---|---|
| Spearman’s Rho | Timing of KD introduction (age in years) | Correlation Coefficient | 1.000 | −0.663 * | −0.639 * | −0.855 ** |
| Sig. (1-tailed) | 0.037 | 0.044 | 0.003 | |||
| Duration of treatment with KD (in years) | Correlation Coefficient | 1.000 | 0.253 | 0.624 * | ||
| Sig. (1-tailed) | 0.273 | 0.049 | ||||
| Timing of KD Introduction (Age in Years) | Duration of Treatment with KD (in Years) | Block Design | Picture Concepts | Matrix Reasoning | |||
|---|---|---|---|---|---|---|---|
| Spearman’s Rho | Timing of KD introduction (age in years) | Correlation Coefficient | 1.000 | −0.663 * | −0.604 | −0.712 * | −0.739 * |
| Sig. (1-tailed) | 0.037 | 0.056 | 0.024 | 0.018 | |||
| Duration of treatment with KD (in years) | Correlation Coefficient | 1.000 | 0.252 | 0.491 | 0.533 | ||
| Sig. (1-tailed) | 0.274 | 0.108 | 0.087 | ||||
| Timing of KD Introduction (Age in Years) | Duration of Treatment with KD (in Years) | Digit Span | Letter-Number Sequencing | |||
|---|---|---|---|---|---|---|
| Spearman’s Rho | Timing of KD introduction (age in years) | Correlation Coefficient | 1.000 | −0.663 * | −0.673 * | −0.457 |
| Sig. (1-tailed) | 0.037 | 0.034 | 0.128 | |||
| Duration of treatment with KD (in years) | Correlation Coefficient | 1.000 | 0.515 | 0.235 | ||
| Sig. (1-tailed) | 0.096 | 0.288 | ||||
| Timing of KD Introduction (Age in Years) | Duration of Treatment with KD (in Years) | Similarities | Vocabulary | Comprehension | |||
|---|---|---|---|---|---|---|---|
| Spearman’s Rho | Timing of KD introduction (age in years) | Correlation Coefficient | 1.000 | −0.663 * | −0.565 | −0.424 | −0.467 |
| Sig. (1-tailed) | 0.037 | 0.072 | 0.147 | 0.122 | |||
| Duration of treatment with KD (in years) | Correlation Coefficient | 1.000 | 0.319 | −0.024 | 0.224 | ||
| Sig. (1-tailed) | 0.221 | 0.477 | 0.297 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barthold, M.; Jurkutat, A.; Goetz, R.; Schubring, L.; Spiegler, J.; Fries, A.-S.; Kiesel, L.; Klepper, J. Timing of Ketogenic Dietary Therapy (KDT) Introduction and Its Impact on Cognitive Profiles in Children with Glut1-DS—A Preliminary Study. Children 2023, 10, 681. https://doi.org/10.3390/children10040681
Barthold M, Jurkutat A, Goetz R, Schubring L, Spiegler J, Fries A-S, Kiesel L, Klepper J. Timing of Ketogenic Dietary Therapy (KDT) Introduction and Its Impact on Cognitive Profiles in Children with Glut1-DS—A Preliminary Study. Children. 2023; 10(4):681. https://doi.org/10.3390/children10040681
Chicago/Turabian StyleBarthold, Martina, Anne Jurkutat, Regina Goetz, Lucia Schubring, Juliane Spiegler, Ann-Sophie Fries, Lucia Kiesel, and Joerg Klepper. 2023. "Timing of Ketogenic Dietary Therapy (KDT) Introduction and Its Impact on Cognitive Profiles in Children with Glut1-DS—A Preliminary Study" Children 10, no. 4: 681. https://doi.org/10.3390/children10040681
APA StyleBarthold, M., Jurkutat, A., Goetz, R., Schubring, L., Spiegler, J., Fries, A.-S., Kiesel, L., & Klepper, J. (2023). Timing of Ketogenic Dietary Therapy (KDT) Introduction and Its Impact on Cognitive Profiles in Children with Glut1-DS—A Preliminary Study. Children, 10(4), 681. https://doi.org/10.3390/children10040681






