Neuromodulation in Pediatric Migraine using Repetitive Neuromuscular Magnetic Stimulation: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Study Enrollment
2.2. Subjects
2.3. Prospective Study Design and rNMS Intervention
2.4. Outcome Measures
2.5. Data Management
2.6. Statistics
3. Results
3.1. Screening
3.2. Subject Characteristics
3.3. Treatment Characteristics
3.4. Adherence
3.5. Safety
3.6. Satisfaction
3.7. Headache Characteristics
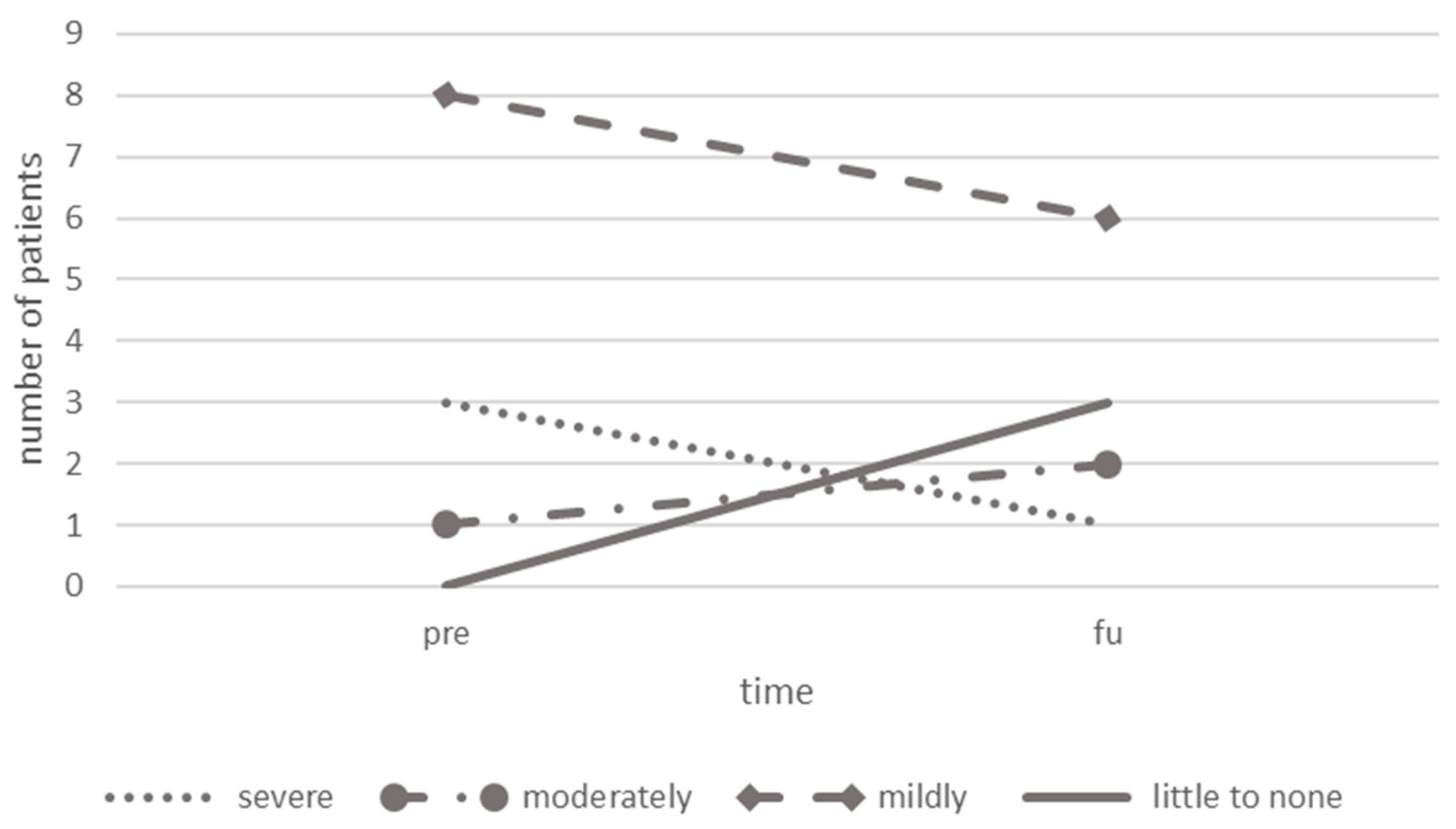
3.8. Headache-Related Disability
3.9. Muscular Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Albers, L.; Straube, A.; Landgraf, M.N.; Filippopulos, F.; Heinen, F.; von Kries, R. Migraine and tension type headache in adolescents at grammar school in Germany—Burden of disease and health care utilization. J. Headache Pain 2015, 16, 52. [Google Scholar] [CrossRef]
- Albers, L.; von Kries, R.; Heinen, F.; Straube, A. Headache in School Children: Is the Prevalence Increasing? Curr. Pain Headache Rep. 2015, 19, 4. [Google Scholar] [CrossRef]
- Abu-Arafeh, I.; Razak, S.; Sivaraman, B.; Graham, C. Prevalence of headache and migraine in children and adolescents: A systematic review of population-based studies. Dev. Med. Child Neurol. 2010, 52, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- E Youssef, P.; Mack, K.J. Episodic and chronic migraine in children. Dev. Med. Child Neurol. 2020, 62, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, M.; Matsumori, Y.; Kawahara, J.; Yamagishi, C.; Koh, A.; Kawamura, S.; Kashiwagi, K.; Kito, T.; Oguri, M.; Mizuno, S.; et al. School-based online survey on chronic headache, migraine, and medication-overuse headache prevalence among children and adolescents in Japanese one city—Itoigawa Benizuwaigani study. Clin. Neurol. Neurosurg. 2023, 226, 107610. [Google Scholar] [CrossRef]
- Bonfert, M.V.; Börner, C.; Gerstl, L.; Hannibal, I.; Mathonia, N.; Huß, K.; Rahmsdorf, B.; Kainz, C.; Klose, B.; Koenig, H.; et al. Migraine in childhood and adolescence-neurostimulation as a future innovative approach in terms of a multimodal treatment regimen. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2020, 63, 872–880. [Google Scholar] [CrossRef]
- Orr, S.L.; Kabbouche, M.A.; O’brien, H.L.; Kacperski, J.; Powers, S.W.; Hershey, A.D. Paediatric migraine: Evidence-based management and future directions. Nat. Rev. Neurol. 2018, 14, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, M.N.; Heinen, F.; Gerstl, L.; Kainz, C.; Ruscheweyh, R.; Straube, A.; Scheidt, J.; von Mutius, S.; Obermeier, V.; von Kries, R. Comparison of a pediatric practice-based therapy and an interdisciplinary ambulatory treatment in social pediatric centers for migraine in children: A nation-wide randomized-controlled trial in Germany: “Moma—Modules on migraine activity”. BMC Pediatr. 2021, 21, 294. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). National Institute for Health and Care Excellence: Clinical Guidelines; National Institute for Health and Care Excellence (NICE): London, UK, 2021.
- Oskoui, M.; Pringsheim, T.; Billinghurst, L.; Potrebic, S.; Gersz, E.M.; Gloss, D.; Holler-Managan, Y.; Leininger, E.; Licking, N.; Mack, K.; et al. Practice guideline update summary: Pharmacologic treatment for pediatric migraine prevention: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2019, 93, 500–509. [Google Scholar] [CrossRef]
- Oskoui, M.; Pringsheim, T.; Holler-Managan, Y.; Potrebic, S.; Billinghurst, L.; Gloss, D.; Hershey, A.D.; Licking, N.; Sowell, M.; Victorio, M.C.; et al. Practice guideline update summary: Acute treatment of migraine in children and adolescents: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2019, 93, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Straube, A.; Schroeder, A.S.; Reilich, P.; Ebinger, F.; Heinen, F.; Bonfert, M. Primary Headache in Children and Adolescents: Update on Pharmacotherapy of Migraine and Tension-Type Headache. Neuropediatrics 2013, 44, 3–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diener, H.-C.; Charles, A.; Goadsby, P.J.; Holle, D. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015, 14, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Charles, A. The pathophysiology of migraine: Implications for clinical management. Lancet Neurol. 2018, 17, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.; Ashwal, S.; Hershey, A.; Hirtz, D.; Yonker, M.; Silberstein, S. Practice Parameter: Pharmacological treatment of migraine headache in children and adolescents: Report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology 2004, 63, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, T.; Goadsby, P.J. The trigeminocervical complex and migraine: Current concepts and synthesis. Curr. Pain Headache Rep. 2003, 7, 371–376. [Google Scholar] [CrossRef]
- Busch, V.; Frese, A.; Bartsch, T. The trigemino-cervical complex. Integration of peripheral and central pain mechanisms in primary headache syndromes. Schmerz 2004, 18, 404–410. [Google Scholar] [CrossRef]
- Ashina, M.; Hansen, J.M.; Do, T.P.; Melo-Carrillo, A.; Burstein, R.; A Moskowitz, M. Migraine and the trigeminovascular system—40 years and counting. Lancet Neurol. 2019, 18, 795–804. [Google Scholar] [CrossRef]
- Landgraf, M.; Ertl-Wagner, B.; Koerte, I.; Thienel, J.; Langhagen, T.; Straube, A.; von Kries, R.; Reilich, P.; Pomschar, A.; Heinen, F. Alterations in the trapezius muscle in young patients with migraine—A pilot case series with MRI. Eur. J. Paediatr. Neurol. 2015, 19, 372–376. [Google Scholar] [CrossRef]
- Landgraf, M.N.; von Kries, R.; Heinen, F.; Langhagen, T.; Straube, A.; Albers, L. Self-reported neck and shoulder pain in adolescents is associated with episodic and chronic migraine. Cephalalgia 2016, 36, 807–811. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Dommerholt, J. International Consensus on Diagnostic Criteria and Clinical Considerations of Myofascial Trigger Points: A Delphi Study. Pain Med. 2018, 19, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Fernández-De-Las-Peñas, C.; Madeleine, P.; Caminero, A.; Cuadrado, M.; Arendt-Nielsen, L.; Pareja, J. Generalized Neck-Shoulder Hyperalgesia in Chronic Tension-Type Headache and Unilateral Migraine Assessed by Pressure Pain Sensitivity Topographical Maps of the Trapezius Muscle. Cephalalgia 2010, 30, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Fernández-De-Las-Peñas, C. Myofascial Head Pain. Curr. Pain Headache Rep. 2015, 19, 28. [Google Scholar] [CrossRef]
- Blaschek, A.; Decke, S.; Albers, L.; Schroeder, A.S.; Lehmann, S.; Straube, A.; Landgraf, M.N.; Heinen, F.; von Kries, R. Self-reported neck pain is associated with migraine but not with tension-type headache in adolescents. Cephalalgia 2014, 34, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Blaschek, A.; Milde-Busch, A.; Straube, A.; Schankin, C.; Langhagen, T.; Jahn, K.; Schröder, S.A.; Reiter, K.; von Kries, R.; Heinen, F. Self-reported muscle pain in adolescents with migraine and tension-type headache. Cephalalgia 2012, 32, 241–249. [Google Scholar] [CrossRef]
- Luedtke, K.; Starke, W.; May, A. Musculoskeletal dysfunction in migraine patients. Cephalalgia 2018, 38, 865–875. [Google Scholar] [CrossRef]
- Simons, D.G.; Travell, J.G.; Simons, L.S. Travell & Simons’ Myofascial Pain and Dysfunction: Upper Half of Body; Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- Ferracini, G.N.; Florencio, L.L.; Dach, F.; Grossi, D.B.; Palacios-Ceña, M.; Ordás-Bandera, C.; Chaves, T.C.; Speciali, J.G.; Fernández-De-Las-Peñas, C. Musculoskeletal disorders of the upper cervical spine in women with episodic or chronic migraine. Eur. J. Phys. Rehabil. Med. 2017, 53, 342–350. [Google Scholar] [CrossRef]
- Ashina, S.; Bendtsen, L.; Lyngberg, A.C.; Lipton, R.B.; Hajiyeva, N.; Jensen, R. Prevalence of neck pain in migraine and tension-type headache: A population study. Cephalalgia 2015, 35, 211–219. [Google Scholar] [CrossRef]
- Do, T.P.; Heldarskard, G.F.; Kolding, L.T.; Hvedstrup, J.; Schytz, H.W. Myofascial trigger points in migraine and tension-type headache. J. Headache Pain 2018, 19, 84. [Google Scholar] [CrossRef]
- Giamberardino, M.A.; Tafuri, E.; Savini, A.; Fabrizio, A.; Affaitati, G.; Lerza, R.; Di Ianni, L.; Lapenna, D.; Mezzetti, A. Contribution of Myofascial Trigger Points to Migraine Symptoms. J. Pain 2007, 8, 869–878. [Google Scholar] [CrossRef]
- Renner, T.; Sollmann, N.; Heinen, F.; Albers, L.; Trepte-Freisleder, F.; Klose, B.; König, H.; Krieg, S.M.; Bonfert, M.V.; Landgraf, M.N. Alleviation of migraine symptoms by application of repetitive peripheral magnetic stimulation to myofascial trigger points of neck and shoulder muscles—A randomized trial. Sci. Rep. 2020, 10, 5954. [Google Scholar] [CrossRef] [PubMed]
- Renner, T.; Sollmann, N.; Trepte-Freisleder, F.; Albers, L.; Mathonia, N.M.; Bonfert, M.V.; König, H.; Klose, B.; Krieg, S.M.; Heinen, F.; et al. Repetitive Peripheral Magnetic Stimulation (rPMS) in Subjects With Migraine—Setup Presentation and Effects on Skeletal Musculature. Front. Neurol. 2019, 10, 738. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Trepte-Freisleder, F.; Albers, L.; Jung, N.H.; Mall, V.; Meyer, B.; Heinen, F.; Krieg, S.M.; Landgraf, M.N. Magnetic stimulation of the upper trapezius muscles in patients with migraine—A pilot study. Eur. J. Paediatr. Neurol. 2016, 20, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Staisch, J.; Börner, C.; Lang, M.; Hauser, A.; Hannibal, I.; Huß, K.; Klose, B.; Lechner, M.F.; Sollmann, N.; Heinen, F.; et al. Repetitive neuromuscular magnetic stimulation in children with headache. Eur. J. Paediatr. Neurol. 2022, 39, 40–48. [Google Scholar] [CrossRef]
- Börner, C.; Staisch, J.; Lang, M.; Hauser, A.; Hannibal, I.; Huß, K.; Klose, B.; Lechner, M.F.; Sollmann, N.; Heinen, F.; et al. Repetitive Neuromuscular Magnetic Stimulation for Pediatric Headache Disorders: Muscular Effects and Factors Affecting Level of Response. Brain Sci. 2022, 12, 932. [Google Scholar] [CrossRef]
- Smania, N.; Corato, E.; Fiaschi, A.; Pietropoli, P.; Aglioti, S.M.; Tinazzi, M. Repetitive magnetic stimulation A novel therapeutic approach for myofascial pain syndrome. J. Neurol. 2005, 252, 307–314. [Google Scholar] [CrossRef]
- Pujol, J.; Pascual-Leone, A.; Dolz, C.; Delgado, E.; Dolz, J.L.; Aldomà, J. The effect of repetitive magnetic stimulation on localized musculoskeletal pain. NeuroReport 1998, 9, 1745–1748. [Google Scholar] [CrossRef]
- Bartsch, T.; Goadsby, P.J. Central mechanisms of peripheral nerve stimulation in headache disorders. Prog. Neurol. Surg. 2011, 24, 16–26. [Google Scholar] [CrossRef]
- Beaulieu, L.; Schneider, C. Effects of repetitive peripheral magnetic stimulation on normal or impaired motor control. A review. Neurophysiol. Clin. 2013, 43, 251–260. [Google Scholar] [CrossRef]
- Beaulieu, L.-D.; Schneider, C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: A literature review on parameters of application and afferents recruitment. Neurophysiol. Clin. 2015, 45, 223–237. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef] [PubMed]
- Fernández-De-Las-Peñas, C.; Simons, D.G.; Cuadrado, M.L.; Pareja, J.A. The role of myofascial trigger points in musculoskeletal pain syndromes of the head and neck. Curr. Pain Headache Rep. 2007, 11, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.; Tassorelli, C.; Rossi, P.; Allena, M.; Osipova, V.; Steiner, T.; Sandrini, G.; Olesen, J.; Nappi, G.; Barrientos, N.; et al. A basic diagnostic headache diary (BDHD) is well accepted and useful in the diagnosis of headache. A multicentre European and Latin American study. Cephalalgia 2011, 31, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Hershey, A.D.; Powers, S.W.; Vockell, A.-L.B.; LeCates, S.; Kabbouche, M.; Maynard, M.K. PedMIDAS: Development of a questionnaire to assess disability of migraines in children. Neurology 2001, 57, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Ravens-Sieberer, U.; Bullinger, M. Assessing health-related quality of life in chronically ill children with the German KINDL: First psychometric and content analytical results. Qual. Life Res. 1998, 7, 399–407. [Google Scholar] [CrossRef]
- Lacourt, T.E.; Houtveen, J.H.; van Doornen, L.J. Experimental pressure-pain assessments: Test-retest reliability, convergence and dimensionality. Scand. J. Pain 2012, 3, 31–37. [Google Scholar] [CrossRef]
- Tassorelli, C.; Diener, H.-C.; Dodick, D.W.; Silberstein, S.D.; Lipton, R.B.; Ashina, M.; Becker, W.J.; Ferrari, M.D.; Goadsby, P.J.; Pozo-Rosich, P.; et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia 2018, 38, 815–832. [Google Scholar] [CrossRef]
- Börner, C.; Renner, T.; Trepte-Freisleder, F.; Urban, G.; Schandelmaier, P.; Lang, M.; Lechner, M.F.; Koenig, H.; Klose, B.; Albers, L.; et al. Response Predictors of Repetitive Neuromuscular Magnetic Stimulation in the Preventive Treatment of Episodic Migraine. Front. Neurol. 2022, 13, 919623. [Google Scholar] [CrossRef]
- Ravens-Sieberer, U.; Ellert, U.; Erhart, M. Health-related quality of life of children and adolescents in Germany. Norm data from the German Health Interview and Examination Survey (KiGGS). Bundesgesundheitsblatt—Gesundheitsforschung—Gesundheitsschutz 2007, 50, 810–818. [Google Scholar] [CrossRef]
- Ravens-Sieberer, U.; Erhart, M.; Wille, N.; Bullinger, M.; The BELLA Study Group. Health-related quality of life in children and adolescents in Germany: Results of the BELLA study. Eur. Child Adolesc. Psychiatry 2008, 17, 148–156. [Google Scholar] [CrossRef]
- Bono, F.; Salvino, D.; Mazza, M.R.; Curcio, M.; Trimboli, M.; Vescio, B.; Quattrone, A. The influence of ictal cutaneous allodynia on the response to occipital transcutaneous electrical stimulation in chronic migraine and chronic tension-type headache: A randomized, sham-controlled study. Cephalalgia 2015, 35, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Schoenen, J.; Vandersmissen, B.; Jeangette, S.; Herroelen, L.; Vandenheede, M.; Gérard, P.; Magis, D. Migraine prevention with a supraorbital transcutaneous stimulator: A randomized controlled trial. Neurology 2013, 80, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Chou, D.E.; Yugrakh, M.S.; Winegarner, D.; Rowe, V.; Kuruvilla, D.; Schoenen, J. Acute migraine therapy with external trigeminal neurostimulation (ACME): A randomized controlled trial. Cephalalgia 2019, 39, 3–14. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, D.L.; Li, M.; Liu, C.; Liu, Q.; Zhang, Y.; Tan, G. Combination of flunarizine and transcutaneous supraorbital neurostimulation improves migraine prophylaxis. Acta Neurol. Scand. 2019, 139, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Hokenek, N.M.; Erdogan, M.O.; Hokenek, U.D.; Algin, A.; Tekyol, D.; Seyhan, A.U. Treatment of migraine attacks by transcutaneous electrical nerve stimulation in emergency department: A randomize controlled trial. Am. J. Emerg. Med. 2021, 39, 80–85. [Google Scholar] [CrossRef]
- Straube, A.; Ellrich, J.; Eren, O.; Blum, B.; Ruscheweyh, R. Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): A randomized, monocentric clinical trial. J. Headache Pain 2015, 16, 543. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Calhoun, A.H.; Lipton, R.B.; Grosberg, B.M.; Cady, R.K.; Dorlas, S.; Simmons, K.A.; Mullin, C.; Liebler, E.J.; Goadsby, P.J.; et al. Chronic migraine headache prevention with noninvasive vagus nerve stimulation. Neurology 2016, 87, 529–538. [Google Scholar] [CrossRef]
- Yarnitsky, D.; Dodick, D.W.; Grosberg, B.M.; Burstein, R.; Ironi, A.; Harris, D.; Lin, T.; Silberstein, S.D. Remote Electrical Neuromodulation (REN) Relieves Acute Migraine: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Headache J. Head Face Pain 2019, 59, 1240–1252. [Google Scholar] [CrossRef]
- Kersch, A.; Perera, P.; Mercado, M.; Gorrie, A.; Sainsbury, D.; McGrath, T.; Aouad, P.; Sarraf, S.; Jaaniste, T.; Champion, D. Somatosensory Testing in Pediatric Patients with Chronic Pain: An Exploration of Clinical Utility. Children 2020, 7, 275. [Google Scholar] [CrossRef]
- Florencio, L.L.; Giantomassi, M.C.M.; Carvalho, G.F.; Gonçalves, M.C.; Dach, F.; Fernández-De-Las-Peñas, C.; Bevilaqua-Grossi, D. Generalized Pressure Pain Hypersensitivity in the Cervical Muscles in Women with Migraine. Pain Med. 2015, 16, 1629–1634. [Google Scholar] [CrossRef]
- Blankenburg, M.; Boekens, H.; Hechler, T.; Maier, C.; Krumova, E.; Scherens, A.; Magerl, W.; Aksu, F.; Zernikow, B. Reference values for quantitative sensory testing in children and adolescents: Developmental and gender differences of somatosensory perception. Pain 2010, 149, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Castien, R.F.; van der Wouden, J.C.; De Hertogh, W. Pressure pain thresholds over the cranio-cervical region in headache: A systematic review and meta-analysis. J. Headache Pain 2018, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Mathonia, N.; Weidlich, D.; Bonfert, M.; Schroeder, S.A.; Badura, K.A.; Renner, T.; Trepte-Freisleder, F.; Ganter, C.; Krieg, S.M.; et al. Quantitative magnetic resonance imaging of the upper trapezius muscles—Assessment of myofascial trigger points in patients with migraine. J. Headache Pain 2019, 20, 8. [Google Scholar] [CrossRef]
- Sollmann, N.; Schandelmaier, P.; Weidlich, D.; Börner, C.; Urban, G.; Lang, M.; Zimmer, C.; Karampinos, D.C.; Landgraf, M.N.; Heinen, F.; et al. Patients with episodic migraine show increased T2 values of the trapezius muscles—An investigation by quantitative high-resolution magnetic resonance imaging. Cephalalgia 2021, 41, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K. Does inflammation have a role in migraine? Nat. Rev. Neurol. 2019, 15, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L. Role of CGRP in Migraine. Handb. Exp. Pharmacol. 2019, 255, 121–130. [Google Scholar] [CrossRef]
- Börner, C.; Urban, G.; Beaulieu, L.-D.; Sollmann, N.; Krieg, S.M.; Straube, A.; Renner, T.; Schandelmaier, P.; Lang, M.; Lechner, M.; et al. The bottom-up approach: Non-invasive peripheral neurostimulation methods to treat migraine: A scoping review from the child neurologist’s perspective. Eur. J. Paediatr. Neurol. 2021, 32, 16–28. [Google Scholar] [CrossRef]
- Lipton, R.B.; Dodick, D.W.; Silberstein, S.D.; Saper, J.R.; Aurora, S.K.; Pearlman, S.H.; E Fischell, R.; Ruppel, P.L.; Goadsby, P.J. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: A randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010, 9, 373–380. [Google Scholar] [CrossRef]
- Stilling, J.M.; Monchi, O.; Amoozegar, F.; Debert, C.T. Transcranial Magnetic and Direct Current Stimulation (TMS/tDCS) for the Treatment of Headache: A Systematic Review. Headache J. Head Face Pain 2019, 59, 339–357. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Z.; Wang, R.; Ao, R.; Han, X.; Tang, W.; Yu, S. Migraine Prevention Using Different Frequencies of Transcutaneous Occipital Nerve Stimulation: A Randomized Controlled Trial. J. Pain 2017, 18, 1006–1015. [Google Scholar] [CrossRef]
- Magis, D.; Sava, S.; d’Elia, T.S.; Baschi, R.; Schoenen, J. Safety and patients’ satisfaction of transcutaneous Supraorbital NeuroStimulation (tSNS) with the Cefaly device in headache treatment: A survey of 2313 headache sufferers in the general population. J. Headache Pain 2013, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.; Grosberg, B.; Mauskop, A.; Cady, R.; Simmons, K. Effect of noninvasive vagus nerve stimulation on acute migraine: An open-label pilot study. Cephalalgia 2014, 34, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Grazzi, L.; Egeo, G.; Calhoun, A.H.; McClure, C.K.; Liebler, E.; Barbanti, P. Non-invasive Vagus Nerve Stimulation (nVNS) as mini-prophylaxis for menstrual/menstrually related migraine: An open-label study. J. Headache Pain 2016, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.R.; Benjamin, D.K.; Cohen-Wolkowiez, M. Evidence-based guidelines for pediatric clinical trials: Focus on StaR Child Health. Expert Rev. Clin. Pharmacol. 2012, 5, 525–531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaptchuk, T.J.; Goldman, P.; A Stone, D.; Stason, W.B. Do medical devices have enhanced placebo effects? J. Clin. Epidemiology 2000, 53, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.J.; Stovner, L.J.; Vos, T.; Jensen, R.; Katsarava, Z. Migraine is first cause of disability in under 50s: Will health politicians now take notice? J. Headache Pain 2018, 19, 17. [Google Scholar] [CrossRef]
- Miller, S.; Matharu, M.S. Migraine is underdiagnosed and undertreated. Practitioner 2014, 258, 19–24. [Google Scholar]



| Characteristics | n (%) | Median (Range) | |
|---|---|---|---|
| Age | - | 12 (6–17) | |
| Sex | Female | 12 (92.3%) | - |
| Handedness | Right | 10 (76.9%) | - |
| Headache Diagnosis | |||
| Migraine with aura | 1 (7.7%) | - | |
| Migraine without aura | 5 (38.5%) | - | |
| Migraine with aura + TTH | 2 (15.4%) | - | |
| Migraine without aura + TTH | 5 (38.5%) | - | |
| Age at headache onset (years) | - | 9 (2–15) | |
| Time since headache onset (years) | - | 3 (2–13) | |
| Family history for migraine | Yes | 3 (23.1%) | - |
| No | 9 (69.2%) | - | |
| Not known | 1 (7.7%) | - | |
| Neck pain at baseline | Yes | 7 (53.8%) | - |
| No | 6 (46.6%) | - | |
| mTrP localization at baseline | Unilateral | 5 (38.5%) | - |
| Bilateral | 8 (61.5%) | - | |
| Left | 10 (45.5%) | - | |
| Right | 12 (54.5%) | - | |
| mTrP entity at baseline | Latent | 15 (68.2%) | - |
| Active | 7 (31.8%) | - | |
| Physiotherapy | During baseline | 6 (46.2%) | - |
| During intervention | 3 (23.1%) | - | |
| AE (n = 91) | n (%) | Serious/Severe | Unexpected | Related |
|---|---|---|---|---|
| No side effects | 64 (82.1%) | |||
| Side effects | 16 in 14 sessions (17.9%) | |||
| During stimulation | ||||
| Trembling (arm/hand) | 5 (6.4%) | X | ||
| Heaviness (at stimulation site) | 2 (2.6%) | X | ||
| Tingling (at stimulation site) | 1 (1.3%) | X | ||
| Arm pain | 1 (1.3%) | X | ||
| Tension in shoulder-neck region (hand) | 1 (1.3%) | X | ||
| In-between stimulations | ||||
| Headache | 5 (6.4%) | X | ||
| Sore muscles | 1 (1.3%) | X | ||
| Life events | ||||
| Suicide of school colleague | 1 (7.7%) | X | X | |
| Health-related absence of caregiver a | 1 (7.7%) | |||
| Accident on ice | 1 (7.7%) | X |
| Headache Characteristics | Pre | FU | Test Values | 95% CI of Mean Difference | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | t/Z | p | ||
| Headache frequency | 9.43 (5.86) | 9.00 (4.50–13.17) | 6.90 (4.53) | 5.60 (3.00–10.67) | t = 1.848 | 0.089 | −0.45–5.52 |
| Headache intensity | 5.50 (0.97) | 5.21 (4.75–6.73) | 6.27 (1.47) | 6.53 (4.24–7.09) | t = −1.68 | 0.142 | −1.86–0.31 |
| Headache duration | 6.27 (4.82) | 5.03 (3.56–7.35) | 6.50 (4.70) | 4.45 (2.59–9.41) | Z = −0.89 | 0.929 | - |
| Medication frequency | 4.42 (2.58) | 4.33 (2.67–5.33) | 2.73 (2.10) | 2.00 (0.75–4.66) | t = 1.94 | 0.081 | −0.25–3.65 |
| PedMIDAS | 35.00 (23.84) | 24.00 (21.00–51.00) | 20.67 (16.83) | 16.00 (7.75–30.75) | Z = −1.92 | 0.055 | - |
| KINDL Child | 65.23 (19.02) | 69.50 (46.13–82.75) | 67.08 (18.04) | 74.00 (58.38–79.25) | Z = −0.420 | 0.675 | - |
| KINDL Caregiver | 67.27 (11.99) | 68.75 (58.38–77.63) | 69.44 (9.64) | 70.75 (61.50–78.75) | t = −1.038 | 0.320 | −6.74–2.39 |
| Test Values | Mean_Pre (SD) | Mean_Post (SD) | Mean_FU (SD) | Post Hoc Test | |||
|---|---|---|---|---|---|---|---|
| F | p | η2 | p | ||||
| Left UTM | 6.46 | 0.016 * | 0.564 | 1.99 (0.77) | 3.02 (1.61) | 2.84 (1.13) | |
| Pre-post | 0.097 | ||||||
| Pre-FU | 0.015 * | ||||||
| Post-FU | 1.000 | ||||||
| Right UTM | 4.67 | 0.037 * | 0.483 | 2.04 (0.67) | 3.00 (1.55) | 2.70 (1.00) | |
| Pre-post | 0.126 | ||||||
| Pre-FU | 0.026 * | ||||||
| Post-FU | 1.000 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Börner-Schröder, C.; Lang, M.; Urban, G.; Zaidenstadt, E.; Staisch, J.; Hauser, A.; Hannibal, I.; Huß, K.; Klose, B.; Lechner, M.F.; et al. Neuromodulation in Pediatric Migraine using Repetitive Neuromuscular Magnetic Stimulation: A Feasibility Study. Children 2023, 10, 1764. https://doi.org/10.3390/children10111764
Börner-Schröder C, Lang M, Urban G, Zaidenstadt E, Staisch J, Hauser A, Hannibal I, Huß K, Klose B, Lechner MF, et al. Neuromodulation in Pediatric Migraine using Repetitive Neuromuscular Magnetic Stimulation: A Feasibility Study. Children. 2023; 10(11):1764. https://doi.org/10.3390/children10111764
Chicago/Turabian StyleBörner-Schröder, Corinna, Magdalena Lang, Giada Urban, Erik Zaidenstadt, Jacob Staisch, Ari Hauser, Iris Hannibal, Kristina Huß, Birgit Klose, Matthias F. Lechner, and et al. 2023. "Neuromodulation in Pediatric Migraine using Repetitive Neuromuscular Magnetic Stimulation: A Feasibility Study" Children 10, no. 11: 1764. https://doi.org/10.3390/children10111764
APA StyleBörner-Schröder, C., Lang, M., Urban, G., Zaidenstadt, E., Staisch, J., Hauser, A., Hannibal, I., Huß, K., Klose, B., Lechner, M. F., Sollmann, N., Landgraf, M. N., Heinen, F., & Bonfert, M. V. (2023). Neuromodulation in Pediatric Migraine using Repetitive Neuromuscular Magnetic Stimulation: A Feasibility Study. Children, 10(11), 1764. https://doi.org/10.3390/children10111764







