Abstract
Background: Acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) is the most frequent subtype of acute encephalopathy syndrome among Japanese children. Exanthem subitum is the most common causative infectious disease of AESD. We herein retrospectively analyzed serum and cerebrospinal fluid (CSF) concentrations of matrix metalloproteinase-9 (MMP-9), tissue inhibitor matrix metalloproteinase-1 (TIMP-1), and seven cytokines in patients with AESD or prolonged febrile seizure (FS) to assess the pathophysiology of AESD and detect biomarkers for diagnosing AESD in the early phase. Methods: Serum and CSF samples were obtained from 17 patients with AESD (1st seizure phase group, n = 7; 2nd seizure phase group, n = 10) and 8 with FS. The concentrations of MMP-9, TIMP-1, and seven cytokines were measured by enzyme-linked immunosorbent assays or cytometric bead arrays. Results: Serum concentrations of TIMP-1 were significantly higher in the 1st seizure phase group than in the 2nd seizure phase group. No significant differences were observed in serum concentrations of MMP-9 or the MMP-9/TIMP-1 ratio. Conclusions: The MMP-9-independent increase in circulating TIMP-1 concentrations observed in the present study may be associated with the pathophysiology of AESD in the 1st seizure phase.
1. Introduction
Acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) is a type of acute encephalopathy characterized by biphasic convulsions and impaired consciousness that is induced by infection [1]. AESD is the most common subtype of acute encephalopathy syndrome in Japanese children [1]. The median age of onset is 1 year and the annual number of cases is 100–200 [1]. Although the pathophysiology of AESD may be excitotoxic disorders associated with late-onset neuronal cell death, this has yet to be confirmed [2,3]. Human herpesvirus-6 (HHV-6) is the most common causative agent of AESD, followed by the influenza virus and HHV-7 [1]. There are currently no established biomarkers for diagnosing AESD or predicting the severity of neuronal damage in the early acute phase, which has been described as “the 1st seizure phase”.
Matrix metalloproteinase-9 (MMP-9), a member of the endopeptidase family [4], has been reported to disrupt the blood–brain barrier (BBB) by degrading all components of the extracellular matrix, including laminin, collagen, and fibronectin [5]. Tissue inhibitor matrix metalloproteinase-1 (TIMP-1) is a glycoprotein that regulates the degradation of the extracellular matrix by inhibiting the enzymatic activity of MMP-9 [6]. An increase in circulating MMP-9 degrades the BBB, whereas an increase in serum TIMP-1 prevents this degradation. Therefore, an increased MMP-9/TIMP-1 ratio has been suggested to impair the BBB, which ultimately leads to encephalopathy [7]. On the other hand, an increase in serum concentrations of TIMP-1, but not MMP-9 of the MMP-9/TIMP-1 ratio has been reported in patients with central nervous disorders, such as cerebral infarction, cranial hemorrhage, traumatic brain injury, hemolytic-uremic syndrome, and HHV-6 encephalitis [2,8,9,10,11,12]. Therefore, the role of TIMP-1 needs to be considered based on these two aspects, i.e., the MMP-9/TIMP-1 balance and MMP-9-independent increases in TIMP-1. However, there is currently no evidence to show elevated concentrations of TIMP-1 in patients with AESD.
In the present study, we retrospectively investigated serum and cerebrospinal fluid (CSF) concentrations of MMP-9 and TIMP-1 in patients with AESD or prolonged febrile seizure (FS). We also analyzed seven types of cytokines, i.e., interleukin (IL)-1β, -2, -4, -10, -17A, and -21 and macrophage inflammatory protein-1 alpha (MIP-1α), which may be involved in TIMP-1-induced inflammation. CD4+ T cell- and monocyte/macrophage-related cytokines were targeted in the present study based on previous findings [13,14,15,16,17,18,19,20,21]. This is the first study to report MMP-9-independent increases in serum concentrations of TIMP-1 in patients with AESD in the 1st seizure phase over those in the 2nd seizure phase, which may be partly responsible for the pathophysiology of AESD.
2. Materials and Methods
2.1. Subjects and Sample Collection
Seventeen patients with AESD (11 males and 6 females) and 8 with prolonged FS (4 males and 4 females) treated at Nagano Children’s Hospital between 4 April 2014 and 9 July 2020 were retrospectively enrolled. Patients were diagnosed with AESD by pediatric neurologists based on standard clinical criteria: (1) convulsions, mostly status epilepticus, 24 h or less after the onset of fever; (2) the transient amelioration of impaired awareness; (3) the reappearance of convulsions, mostly clustered focal seizures, followed by disturbed consciousness on the fourth to sixth day after onset; (4) causative pathogens of antecedent infection, mainly influenza virus or HHV-6/7; (5) a wide range of neurological prognoses; (6) normal magnetic resonance imaging (MRI) results within two days of the onset; and (7) the bright tree appearance on cerebral MRI: high-intensity lesions in the subcortical white matter, called U-fibers, on diffusion-weighted images on the third to the ninth day of illness [1]. Serum and CSF samples (serum, n = 21; CSF, n = 23) were collected in pairs from 19 patients on admission. A CSF sample was only obtained from 4 patients and a serum sample solely from 2. All 25 patients were divided into three groups. Patients in “the 1st seizure phase group” (serum, n =7; CSF, n = 6) and “the 2nd seizure phase group” (serum n = 8, CSF n = 9) presented to our hospital in the 1st and 2nd seizure phases of AESD, respectively. “The prolonged FS group” (serum, n = 6; CSF, n = 8) included patients who developed prolonged FS. FS was defined as seizures provoked by fever ≥38.0 °C according to a previous study, and a prolonged seizure was defined as a seizure lasting more than 30 min or recurrent seizures persisting for a total of more than 30 min without fully recovering consciousness [22]. Among patients in the prolonged FS group, those with other neurological disorders, such as encephalitis, encephalopathy, meningitis, brain abscess, brain tumor, and epilepsy, were excluded based on the clinical course, a blood examination, CSF test, electroencephalogram, and brain MRI. All patients in the prolonged FS group regained consciousness within 24 h without neurological sequelae. All samples were collected on admission and stored at −80 °C for later analyses.
2.2. MMP-9 and TIMP-1 Assays
The serum and CSF concentrations of MMP-9 and TIMP-1 were measured using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA). The detectable range for each ELISA kit was 0.313 to 20 ng/mL and 0.156 to 10 ng/mL, respectively. The ELISA kit for MMP-9 used in the present study measures the 92 kDa pro- and 82 kDa active forms of MMP-9. Measurements were performed according to the manufacturer’s instructions. Samples were assayed in triplicate and average values were adopted. All assays were completed in one day.
2.3. Cytokine Assay
Serum and CSF concentrations of IL-1β, -2, -4, -10, -17A, and -21 and MIP-1α were measured using the BD™ Cytometric Bead Array® (Becton, Dickinson, and Company [BD], East Rutherford, NJ, USA) and BD FACSCanto™ II Flow Cytometer (BD) equipped with FCAP Array™ v3.0 Software (BD, East Rutherford, NJ, USA) according to the manufacturer’s instructions. The detectable ranges for IL-1β, -2, -4, -10, and -17A and MIP-1α were 10 to 2500 pg/mL, while that for IL-21 was 40 to 10,000 pg/mL. Measurements were performed according to the manufacturer’s instructions. Samples were analyzed once. All assays were completed in one day.
2.4. Statistical Analysis
Values are presented as the median (range). Statistical comparisons of sex and the presence of a prolonged seizure of more than 40 min were performed using the chi-square test, and other clinical and laboratory findings and cytokine profiles were assessed by the Steel–Dwass method. To evaluate the significance of differences in TIMP-1 and MMP-9 concentrations among the three independent groups, the Tukey–Kramer method was used when data were parametric and the Steel–Dwass method when data were non-parametric. The relationship between the two parameters was analyzed by Spearman’s correlation test. All statistical analyses were conducted using Microsoft® Excel® for windows version 2016, Statcel 4 software (OMS Publishing Inc., Saitama, Japan). Cytokine levels below the lower detectable range were defined as 1.0 pg/mL for statistical analyses. The level of significance was defined as a p value < 0.05.
2.5. Ethics
The present study was approved by the Ethics Committee of the Nagano Children’s Hospital (approval number: 31-11; approval date: 9 September 2019).
3. Results
3.1. Clinical Characteristics
The clinical data and laboratory findings of the 1st and 2nd seizure phase groups and prolonged FS group are shown in Table 1. No significant differences were observed in age, sex, or the number of patients with a prolonged seizure of more than 40 min. Laboratory data, such as blood values of aspartate aminotransferase, creatinine, and sugar and CSF levels of the cell count, total protein, and lactate, did not significantly differ among the three groups. On the other hand, a significant difference was observed in neuron-specific enolase (NSE) in the CSF and C-reactive protein (CRP). The CSF concentrations of NSE in the 1st and 2nd seizure phase groups and prolonged FS group were 12.6 (range, 6.0–61.0), 81.1 (range, 17.1–366.0), and 7.8 (range, 6.3–13.2) ng/mL, respectively. Significant differences were observed between the 1st and 2nd seizure phase groups (p < 0.05) and between the 2nd seizure phase group and prolonged FS group (p < 0.01) (Table 1 and Supplemental Figure S1A). In addition, CRP levels were significantly higher in the 1st seizure phase group (0.90 mg/dL; range, 0.38–5.11) than in the 2nd seizure phase (0.30 mg/dL; range, 0.08–1.66). The localization of the bright tree appearance on brain MRI was as follows: three patients each from the 1st and 2nd seizure phase groups had a bilateral widespread lesion, one from the 1st seizure phase group and four from the 2nd seizure phase group had bilateral frontal lobe lesions, three from the 1st seizure phase group and one from the 2nd seizure phase group had a unilaterally widespread lesion, and two from 2nd seizure phase group with a unilaterally frontal lobe lesion. All patients in the 1st and 2nd seizure phase groups received 72 h of targeted temperature management and were administered of dextromethorphan and vitamins. Six patients from the 1st seizure phase group and all patients from the 2nd seizure phase group were administered edaravone. Methylprednisolone pulse therapy was initiated for four patients from the 1st seizure phase group and one from the 2nd seizure phase group, and intravenous immunoglobulin was administered to one each from the 1st and 2nd seizure groups. Five patients each in the 1st and 2nd seizure phage groups had residual neurologic sequelae, including intellectual disability, developmental disability, and epilepsy. Ten (58.8%) out of the seventeen patients with AESD in the 1st and 2nd seizure groups had neurological sequelae.

Table 1.
Clinical and laboratory findings of patients with acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) or prolonged febrile seizure (FS).
3.2. Serum Concentrations of MMP-9 and TIMP-1
Serum TIMP-1 concentrations were significantly higher in the 1st seizure phase group (289.0 ng/mL; range, 146.6–390.9) than in the 2nd seizure phase (140.9 ng/mL; range, 108.3–212.7) (p < 0.05) (Figure 1B). Significant differences were not observed in serum MMP-9 concentrations or MMP-9/TIMP-1 ratios among the three groups (Figure 1A,C).
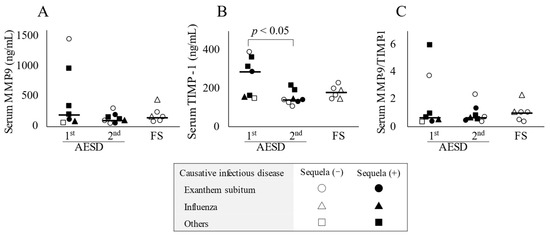
Figure 1.
Serum concentrations of biomarkers in patients with acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) in the 1st or 2nd seizure phase or with prolonged febrile seizure (FS). Serum concentrations of matrix metalloproteinase-9 (MMP-9) (A) and tissue inhibitor matrix metalloproteinase-1 (TIMP-1) (B) and the MMP-9/TIMP-1 ratio in serum (C) in all patients with AESD or prolonged FS. The horizontal bar represents the median value. Statistical comparisons by the Tukey–Kramer or Steel–Dwass test. Circles, triangles, and squares represent patients with AESD or FS due to exanthem subitum, influenza, and other infectious diseases, respectively. Filled marks donate patients with sequela (e), and open marks without sequela.
3.3. CSF Concentrations of MMP-9 and TIMP-1
As shown in Figure 2A, CSF concentrations of MMP-9 were below the lower detection limit, while TIMP-1 concentrations and MMP-9/TIMP-1 ratios in CSF were not significantly different among the three groups (Figure 2B,C).
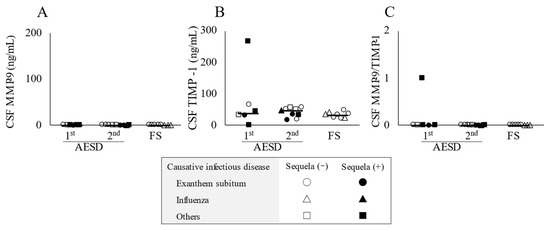
Figure 2.
Cerebrospinal fluid (CSF) concentrations of the biomarkers in patients with acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) in the 1st or 2nd seizure phase or with prolonged febrile seizure (FS). CSF levels of matrix metalloproteinase-9 (MMP-9) (A) and tissue inhibitor matrix metalloproteinase-1 (TIMP-1) (B) and the MMP-9/TIMP-1 ratio in CSF (C) in all patients with AESD or prolonged FS. Statistical comparisons by the Tukey–Kramer or Steel–Dwass test. Circles, triangles, and squares represent patients with AESD or FS due to exanthem subitum, influenza, and other infectious diseases, respectively. Filled marks donate patients with sequela (e), and open marks without sequela.
3.4. Relationship between Serum Concentrations of MMP-9 and TIMP-1
A positive correlation was observed between serum concentrations of MMP-9 and TIMP-1 (p < 0.05, Rs = 0.474) (Figure 3A), whereas no interaction was detected by a group-based analysis (Figure 3B–D).
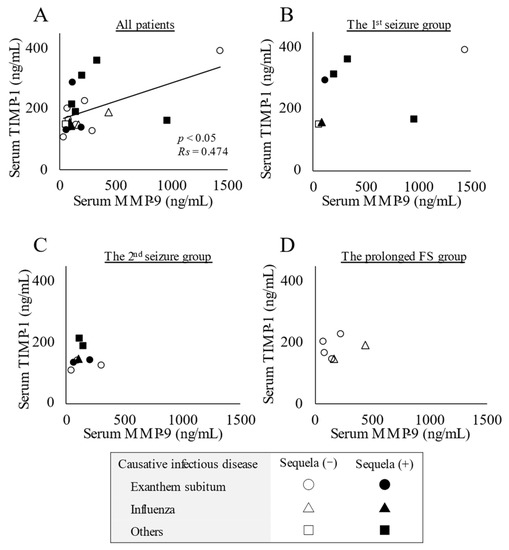
Figure 3.
Relationship between serum concentrations of matrix metalloproteinase-9 (MMP-9) and tissue inhibitor matrix metalloproteinase-1 (TIMP-1). (A) Positive correlation between serum concentrations of MMP-9 and TIMP-1 in all patients with acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) or prolonged febrile seizure (FS). Statistical comparisons by Spearman’s correlation test. (B) The 1st seizure phase group. (C) The 2nd seizure phase group. (D) The prolonged FS group. Circles, triangles, and squares represent patients with AESD or FS due to exanthem subitum, influenza, and other infectious diseases, respectively. Filled marks donate patients with sequela (e), and open marks without sequela.
3.5. Relationship between Serum TIMP-1 and CSF NSE Concentrations
Serum concentrations of TIMP-1 did not correlate with CSF concentrations of NSE (Supplemental Figure S1B).
3.6. Relationship between Serum Concentrations of TIMP-1 and Serum or CSF Concentrations of Seven Types of Cytokines
Serum concentrations of TIMP-1 did not correlate with serum or CSF concentrations of seven types of cytokines (IL-1β, -2, -4, -10, -17A, and -21 and MIP-1α).
3.7. Cytokine Profiles in Serum and CSF
As shown in Table 2, serum and CSF concentrations of seven types of cytokines (IL-1β, -2, -4, -10, -17A, and -21 and MIP-1α) did not significantly differ in patients with AESD or prolonged FS among the three groups.

Table 2.
Serum and cerebrospinal fluid (CSF) cytokine profiles in patients with acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) or prolonged febrile seizure (FS).
4. Discussion
We retrospectively analyzed clinical characteristics and serum and CSF concentrations of MMP-9, TIMP-1, and seven types of cytokines in children with AESD. Serum concentrations of TIMP-1 were significantly higher in the 1st seizure phase group than in the 2nd seizure phase group. Serum concentrations of MMP-9 and MMP-9/TIMP-1 ratios did not significantly differ among the three groups. CSF concentrations of NSE were significantly higher in the 2nd seizure phase group than in the prolonged FS group, which may reflect subacute neuronal damage caused by AESD [23]. CSF concentrations of NSE did not correlate with serum concentrations of TIMP-1.
The role of TIMP-1, the main factor examined in the present study, needs to be discussed based on two aspects, i.e., the MMP-9/TIMP-1 balance and MMP-9-independent increases in TIMP-1. MMPs are a family of zinc- and calcium-dependent endopeptidases composed of 24 known subtypes, ranging from MMP-1 to -28 [24]. MMPs are classified into the following five categories according to the domain composition and substrate preference: collagenases, gelatinases, stromelysins, matrilysins, and membrane types. MMP-9 is a gelatinase that digests components of the extracellular matrix, including collagen, laminin, and fibronectin [24]. Therefore, increases in serum concentrations of MMP-9 may be destructive to the BBB because the basement membrane of the BBB is composed of these matrixes [7]. On the other hand, TIMPs are a family of endogenous inhibitors of MMPs comprised of four subgroups: TIMP-1 to -4 [6,25]. The enzymatic activity of MMP-9 is inhibited by one-to-one non-covalent binding with TIMP-1; therefore, TIMP-1 may protect the BBB from destruction by MMP-9. Previous studies reported elevated concentrations of MMP-9 and/or a high MMP-9/TIMP-1 ratio in the serum of patients with central nervous diseases, such as intracerebral hemorrhage, traumatic brain injury, multiple sclerosis, and febrile convulsive disorders, including FS and acute encephalitis or encephalopathy [7,9,11,26,27]. Ichiyama et al. demonstrated that serum concentrations of MMP-9 and the MMP-9/TIMP-1 ratio increased in influenza-associated encephalopathy with a poor prognosis [7]. In children with AESD, Suenaga et al. showed that serum concentrations of MMP-9 were significantly higher in patients with AESD than in those with simple FS or epilepsy and controls; serum concentrations of TIMP-1 in AESD were significantly lower than those in patients with FS or epilepsy and controls [22]. However, these findings need to be carefully interpreted because this study did not describe the clinical phase of AESD, i.e., the 1st or 2nd seizure phase.
In contrast to these findings, no significant increases were observed in serum concentrations of MMP-9 or the MMP-9/TIMP-1 ratio in the present study, suggesting that the increase in circulating TIMP-1 was independent of MMP-9. Leonard et al. reported an MMP-9-independent increase in TIMP-1 concentrations in patients with cerebral infarction [10], and Shiraishi et al. detected elevated TIMP-1 concentrations in patients with encephalopathy due to hemolytic uremic syndrome [8]. However, the source or cause of the MMP-9-independent increase in TIMP-1 in these studies and the present study remains unclear. We have two hypotheses: the innate immune response following viral infection and TIMP-1-secreting cells. Innate immunity is mainly controlled by dendritic cells, natural killer cells [20], and monocytes/macrophages [15,19,21], and these cells have been proposed as candidate cells for the secretion of TIMP-1 in vitro [13]. In vivo, patients with sepsis were found to have high TIMP-1 and low MMP-9 concentrations in serum, which predicted a monocyte-derived increase in TIMP-1 concentrations in peripheral blood [16,17]. Therefore, these immune cells or related cytokines may be involved in the MMP-9-independent increase in TIMP-1 and the development of AESD. In addition, HHV-6 and -7, which are causative viruses of exanthem subitum, infect several immunocytes, including monocytes/macrophages and CD4+ T cells, the latter of which have been identified as a candidate secreting TIMP-1 in vitro [15,18,19,21]. In children with AESD due to exanthem subitum, Kawamura et al. analyzed cytokine and chemokine profiles in serum and CSF: significant increases in serum concentrations of IL-10 and a significant decrease in CSF concentrations of IL-1β were observed [28]. Additionally, a few studies or case reports showed the increases of serum concentrations of IL-6 and -10 and tumor necrosis factor-α in patients with AESD, although the cause of increases of these cytokines had not been demonstrated [27,29,30,31]. Based on these findings, we herein focused on seven types of cytokines related to CD4+ T cells and monocytes/macrophages (IL-1β, -2, -4, -10, -17A, and -21 and MIP-1α). However, no significant differences were observed in any of the seven cytokines among the three groups, and, thus, further analyses are warranted with a larger study design.
Furthermore, an imbalance in the MMP-9/TIMP-1 system may have affected the development of AESD in children in the present study. A positive correlation was observed between serum concentrations of MMP-9 and TIMP-1 (Figure 3A), although there was no significant increase in circulating concentrations of MMP-9 or the MMP-9/TIMP-1 ratio (Figure 1). Therefore, damage to the BBB based on an imbalance in MMP-9/TIMP-1 may partially contribute to the development of AESD, which is consistent with previous findings [22].
5. Conclusions
An MMP-9-independent increase in serum concentrations of TIMP-1 may be associated with the pathophysiology of AESD in the 1st seizure phase. The source or cause of the increase in serum concentrations of TIMP-1 remains unclear and, thus, further studies with a large number of patients are warranted.
Supplementary Materials
The following supporting information may be downloaded at https://www.mdpi.com/article/10.3390/children10010078/s1, Figure S1: A: Cerebrospinal fluid (CSF) concentrations of neuron-specific enolase (NSE) in patients with acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) in the 1st or 2nd seizure phase or with prolonged febrile seizure (FS).; B: Correlation between the concentrations of NSE in CSF and tissue inhibitor matrix metalloproteinase-1 (TIMP-1) in serum.
Author Contributions
M.M. and A.K. designed the study; M.M., A.K., J.K. and M.N. performed experiments and collected and analyzed data; M.M. and A.K. wrote the manuscript; Y.I. and N.K. gave technical support and conceptual advice. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was supported in part by a Grant for Clinical and Medical Research from Nagano Children’s Hospital (2019-003) and JSPS KAKENHI Grant Number 19H0388301 (YI).
Institutional Review Board Statement
The present study was approved by the Ethics Committee of the Nagano Children’s Hospital (approval number: 31-11; approval date: 9 September 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to express our gratitude to the patients and their families for their participation in this study. We are also grateful to Minoru Tozuka and the clinical team, including Maki Shirai, Shihoko Takeuchi, Ken Imai, and Yuka Misawa, for their valuable advice.
Conflicts of Interest
The authors declare that they have no potential conflict of interest to disclose.
References
- Hoshino, A.; Saitoh, M.; Oka, A.; Okumura, A.; Kubota, M.; Saito, Y.; Takanashi, J.; Hirose, S.; Yamagata, T.; Yamanouchi, H.; et al. Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev. 2012, 34, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Sugata, K.; Ihira, M.; Mihara, T.; Mutoh, T.; Asano, Y.; Yoshikawa, T. Different characteristics of human herpesvirus 6 encephalitis between primary infection and viral reactivation. J. Clin. Virol. 2011, 51, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, M.; Kashiwagi, M.; Tanabe, T.; Nakahara, H.; Ohta, K.; Tamai, H. Overlapping MERS and mild AESD caused by HHV-6 infection. Brain Dev. 2015, 37, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Asahi, M.; Wang, X.; Mori, T.; Sumii, T.; Jung, J.C.; Moskowitz, M.A.; Fini, M.E.; Lo, E.H. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci. 2001, 21, 7724–7732. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Gomis-Rüth, F.X.; Maskos, K.; Betz, M.; Bergner, A.; Huber, R.; Suzuki, K.; Yoshida, N.; Nagase, H.; Brew, K.; Bourenkov, G.P.; et al. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature 1997, 389, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, T.; Morishima, T.; Kajimoto, M.; Matsushige, T.; Matsubara, T.; Furukawa, S. Matrix metalloproteinase-9 and tissue inhibitors of metalloproteinases 1 in influenza-associated encephalopathy. Pediatr. Infect. Dis. J. 2007, 26, 542–544. [Google Scholar] [CrossRef]
- Shiraishi, M.; Ichiyama, T.; Matsushige, T.; Iwaki, T.; Iyoda, K.; Fukuda, K.; Makata, H.; Matsubara, T.; Furukawa, S. Soluble tumor necrosis factor receptor 1 and tissue inhibitor of metalloproteinase-1 in hemolytic uremic syndrome with encephalopathy. J. Neuroimmunol. 2008, 196, 147–152. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Ramos, L.; Argueso, M.; Cáceres, J.J.; Solé-Violán, J.; Jiménez, A.; Borreguero-León, J.M.; González-Rivero, A.F.; Orbe, J.; et al. Persistently high circulating tissue inhibitor of matrix metalloproteinase-1 levels in non-survivor brain trauma injury patients. J. Crit. Care 2019, 51, 117–121. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Ramos, L.; Argueso, M.; Cáceres, J.J.; Solé-Violán, J.; Jiménez, A.; Borreguero-León, J.M.; González-Rivero, A.F.; Orbe, J.; et al. High serum levels of tissue inhibitor of matrix metalloproteinase-1 during the first week of a malignant middle cerebral artery infarction in non-surviving patients. BMC Neurol. 2019, 19, 167. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Ramos, L.; Argueso, M.; Cáceres, J.J.; Solé-Violán, J.; Jiménez, A. High Serum Tissue Inhibitor of Matrix Metalloproteinase-1 Levels and Mortality in Patients with Spontaneous Intracerebral Hemorrhage. World Neurosurg. 2020, 134, e476–e480. [Google Scholar] [CrossRef] [PubMed]
- Jordakieva, G.; Budge-Wolfram, R.M.; Budinsky, A.C.; Nikfardjam, M.; Delle-Karth, G.; Girard, A.; Godnic-Cvar, J.; Crevenna, R.; Heinz, G. Plasma MMP-9 and TIMP-1 levels on ICU admission are associated with 30-day survival. Wien Klin Wochenschr 2021, 133, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; McCluskey, K.; Fujii, K.; Wahl, L.M. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J. Immunol. 1998, 161, 3071–3076. [Google Scholar] [PubMed]
- Wurster, A.L.; Rodgers, V.L.; Satoskar, A.R.; Whitters, M.J.; Young, D.A.; Collins, M.; Grusby, M.J. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J. Exp. Med. 2002, 196, 969–977. [Google Scholar] [CrossRef]
- Takahashi, K.; Sonoda, S.; Higashi, K.; Kondo, T.; Takahashi, H.; Takahashi, M.; Yamanishi, K. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J. Virol. 1989, 63, 3161–3163. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Martín, M.M.; Labarta, L.; Díaz, C.; Solé-Violán, J.; Blanquer, J.; Orbe, J.; Rodríguez, J.A.; Jiménez, A.; Borreguero-León, J.M.; et al. Matrix metalloproteinase-9, -10, and tissue inhibitor of matrix metalloproteinases-1 blood levels as biomarkers of severity and mortality in sepsis. Crit. Care 2009, 13, R158. [Google Scholar] [CrossRef]
- Lacraz, S.; Nicod, L.P.; Chicheportiche, R.; Welgus, H.G.; Dayer, J.M. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J. Clin. Invest. 1995, 96, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Johnatty, R.N.; Taub, D.D.; Reeder, S.P.; Turcovski-Corrales, S.M.; Cottam, D.W.; Stephenson, T.J.; Rees, R.C. Cytokine and chemokine regulation of proMMP-9 and TIMP-1 production by human peripheral blood lymphocytes. J. Immunol. 1997, 158, 2327–2333. [Google Scholar] [PubMed]
- Frenkel, N.; Schirmer, E.C.; Wyatt, L.S.; Katsafanas, G.; Roffman, E.; Danovich, R.M.; June, C.H. Isolation of a new herpesvirus from human CD4+ T cells. Proc. Natl. Acad. Sci. USA 1990, 87, 748–752. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Agut, H.; Bonnafous, P.; Gautheret-Dejean, A. Update on infections with human herpesviruses 6A, 6B, and 7. Med. Et Mal. Infect. 2017, 47, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, N.; Ichiyama, T.; Kubota, M.; Isumi, H.; Tohyama, J.; Furukawa, S. Roles of matrix metalloproteinase-9 and tissue inhibitors of metalloproteinases 1 in acute encephalopathy following prolonged febrile seizures. J. Neurol. Sci. 2008, 266, 126–130. [Google Scholar] [CrossRef]
- Schmechel, D.; Marangos, P.J.; Brightman, M. Neurone-specific enolase is a molecular marker for peripheral and central neuroendocrine cells. Nature 1978, 276, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Lee, M.A.; Palace, J.; Stabler, G.; Ford, J.; Gearing, A.; Miller, K. Serum gelatinase B, TIMP-1 and TIMP-2 levels in multiple sclerosis. A longitudinal clinical and MRI study. Brain 1999, 122 Pt 2, 191–197. [Google Scholar] [CrossRef]
- Kawamura, Y.; Nakai, H.; Sugata, K.; Asano, Y.; Yoshikawa, T. Serum biomarker kinetics with three different courses of HHV-6B encephalitis. Brain Dev. 2013, 35, 590–595. [Google Scholar] [CrossRef]
- Kawamura, Y.; Yamazaki, Y.; Ohashi, M.; Ihira, M.; Yoshikawa, T. Cytokine and chemokine responses in the blood and cerebrospinal fluid of patients with human herpesvirus 6B-associated acute encephalopathy with biphasic seizures and late reduced diffusion. J. Med. Virol. 2014, 86, 512–518. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Nagase, H.; Ito, Y.; Matsunoshita, N.; Mizutani, M.; Matsushige, T.; Ishida, Y.; Toyoshima, D.; Kasai, M.; Kurosawa, H.; et al. Acute focal bacterial nephritis characterized by acute encephalopathy with biphasic seizures and late reduced diffusion. J Infect Chemother 2018, 24, 932–935. [Google Scholar] [CrossRef]
- Shiba, T.; Hamahata, K.; Yoshida, A. Acute encephalopathy with biphasic seizures and late reduced diffusion in Kawasaki disease. Pediatr. Int. 2017, 59, 1276–1278. [Google Scholar] [CrossRef]
- Ichiyama, T.; Suenaga, N.; Kajimoto, M.; Tohyama, J.; Isumi, H.; Kubota, M.; Mori, M.; Furukawa, S. Serum and CSF levels of cytokines in acute encephalopathy following prolonged febrile seizures. Brain Dev. 2008, 30, 47–52. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).