Preserving Fertility in Children and Adolescents with Cancer
Abstract
:1. Introduction
2. Fertility Preservation in Females
2.1. Risk for Infertility
2.2. Options for Fertility Cryopreservation
2.2.1. Oocyte and Embryo Cryopreservation
2.2.2. Use of Gonadotropin Releasing Hormone Analogues
2.2.3. Ovarian Tissue Cryopreservation
2.2.4. Protection of Ovarian Function
2.2.5. Decision Making
2.3. Considerations Post-Therapy
2.4. Risk for Infertility in Males
2.5. Options for Fertility Preservation
2.5.1. Sperm Banking
2.5.2. Testicular Tissue Cryopreservation
2.5.3. Protection of the Testes during Treatment
2.5.4. Decision Making
2.6. Considerations Post Therapy
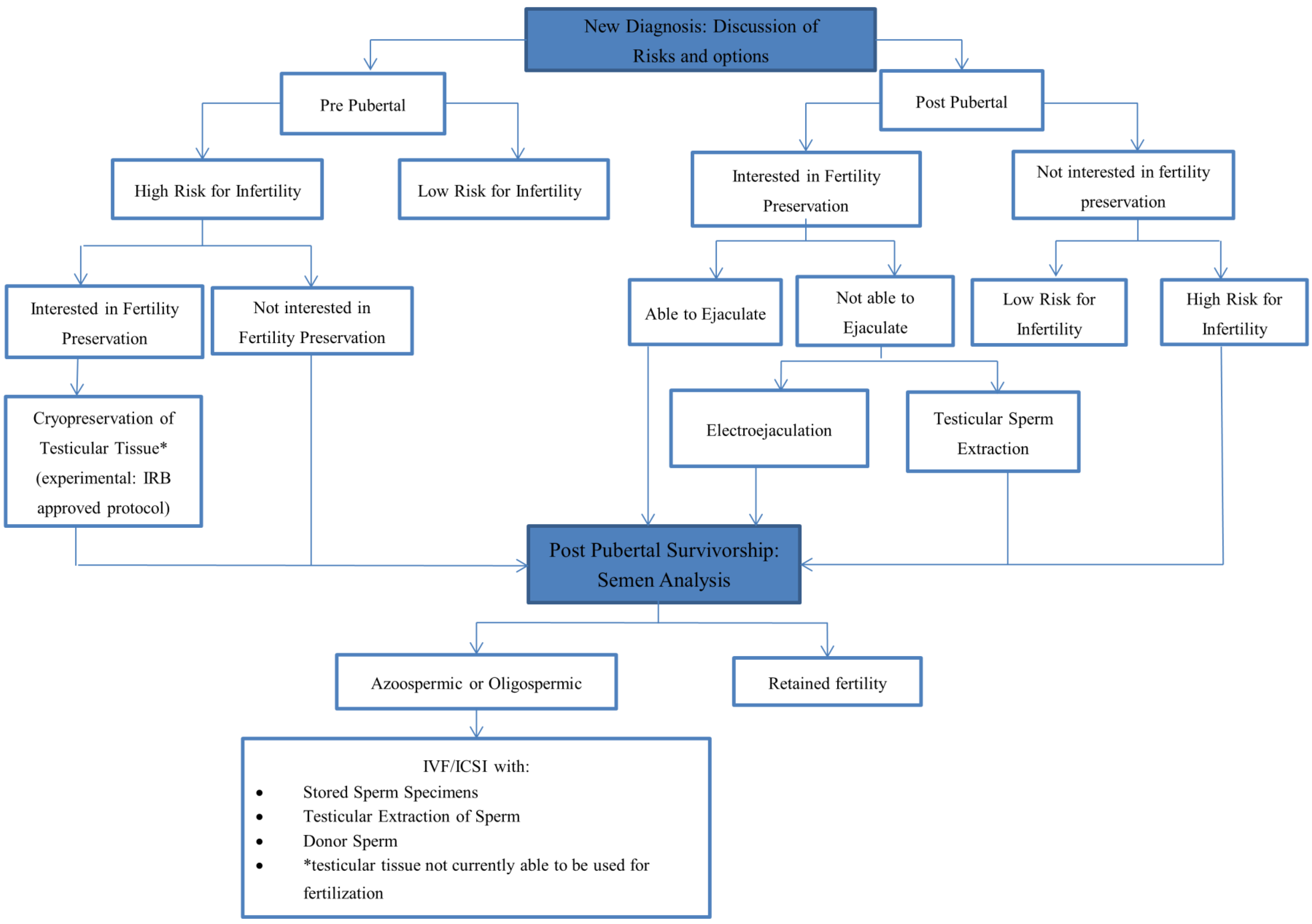
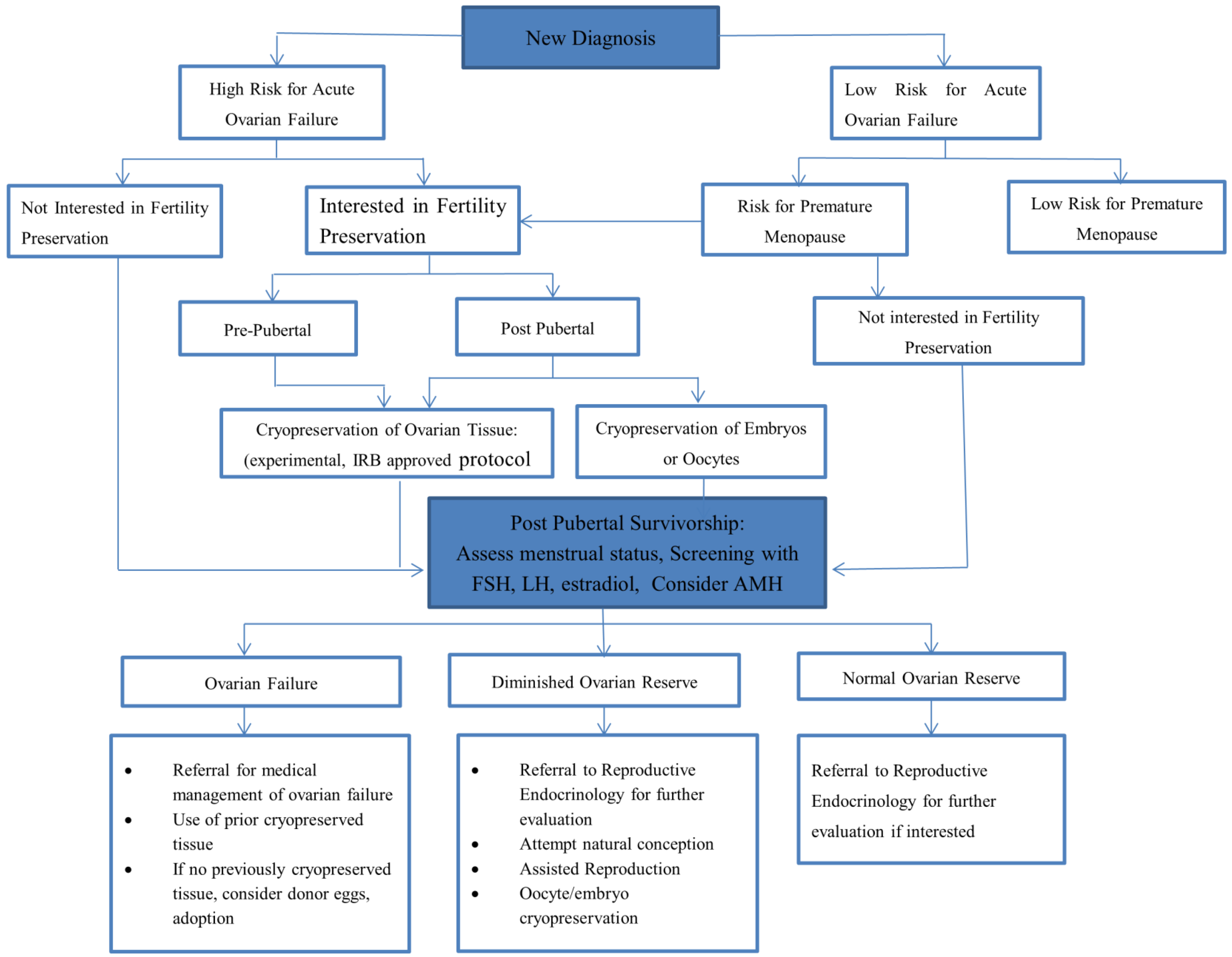
3. Conclusions
Conflicts of Interest
References
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Green, D.M.; Kawashima, T.; Stovall, M.; Leisenring, W.; Sklar, C.A.; Mertens, A.C.; Donaldson, S.S.; Byrne, J.; Robison, L.L. Fertility of male survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2010, 28, 332–339. [Google Scholar] [CrossRef]
- Green, D.M.; Sklar, C.A.; Boice, J.D., Jr.; Mulvihill, J.J.; Whitton, J.A.; Stovall, M.; Yasui, Y. Ovarian failure and reproductive outcomes after childhood cancer treatment: Results from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009, 27, 2374–2381. [Google Scholar] [CrossRef]
- Jeruss, J.S.; Woodruff, T.K. Preservation of fertility in patients with cancer. N. Engl. J. Med. 2009, 360, 902–911. [Google Scholar] [CrossRef]
- Schover, L.R. Psychosocial aspects of infertility and decisions about reproduction in young cancer survivors: A review. Med. Pediatr. Oncol. 1999, 33, 53–59. [Google Scholar] [CrossRef]
- Langeveld, N.E.; Grootenhuis, M.A.; Voute, P.A.; de Haan, R.J.; van den Bos, C. Quality of life, self-esteem and worries in young adult survivors of childhood cancer. Psychooncology 2004, 13, 867–881. [Google Scholar] [CrossRef]
- Levine, J.; Canada, A.; Stern, C.J. Fertility preservation in adolescents and young adults with cancer. J. Clin. Oncol. 2010, 28, 4831–4841. [Google Scholar] [CrossRef]
- Practice Committee of American Society for Reproductive, M. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil. Steril. 2013, 100, 1214–1223. [Google Scholar]
- National Institute for Health and Care Excellence. Fertility: Assessment and treatment for people with fertility problems: Clinical guideline 156. Available online: http://www.nice.org.uk/guidance/cg156/resources/guidance-fertility-pdf (accessed on 19 July 2014).
- Loren, A.W.; Mangu, P.B.; Beck, L.N.; Brennan, L.; Magdalinski, A.J.; Partridge, A.H.; Quinn, G.; Wallace, W.H.; Oktay, K.; American Society of Clinical, O. Fertility preservation for patients with cancer: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 2500–2510. [Google Scholar] [CrossRef]
- Coccia, P.F.; Pappo, A.S.; Altman, J.; Bhatia, S.; Borinstein, S.C.; Flynn, J.; Frazier, A.L.; George, S.; Goldsby, R.; Hayashi, R.; et al. Adolescent and young adult oncology, version 2.2014. J. Natl. Compr. Canc. Netw. 2014, 12, 21–32. [Google Scholar]
- AYA cancer fertility preservation guidance working group Cancer Council Australia. Fertility preservation for AYAs diagnosed with cancer: Guidance for health professionals. Available online: http://wiki.cancer.org.au/australia/COSA:AYA_cancer_fertility_preservation (accessed on 19 July 2014).
- National Child Cancer Network New Zealand. Fertility preservation for people with cancer. Available online https://www.starship.org.nz/for-health-professionals/national-guidelines-paediatric-oncology-and-haematology (accessed on 21 August 2014).
- International Society for Fertility Preservation; Kim, S.S.; Donnez, J.; Barri, P.; Pellicer, A.; Patrizio, P.; Rosenwaks, Z.; Nagy, P.; Falcone, T.; Andersen, C.; et al. Recommendations for fertility preservation in patients with lymphoma, leukemia, and breast cancer. J. Assist. Reprod. Genet. 2012, 29, 465–468. [Google Scholar]
- Eshre Task Force on Ethics. Taskforce 7: Ethical considerations for the cryopreservation of gametes and reproductive tissues for self use. Hum. Reprod. 2004, 19, 460–462. [Google Scholar]
- Yeomanson, D.J.; Morgan, S.; Pacey, A.A. Discussing fertility preservation at the time of cancer diagnosis: Dissatisfaction of young females. Pediatr. Blood Cancer 2013, 60, 1996–2000. [Google Scholar]
- Goodwin, T.; Elizabeth Oosterhuis, B.; Kiernan, M.; Hudson, M.M.; Dahl, G.V. Attitudes and practices of pediatric oncology providers regarding fertility issues. Pediatr. Blood Cancer 2007, 48, 80–85. [Google Scholar] [CrossRef]
- Quinn, G.P.; Vadaparampil, S.T.; King, L.; Miree, C.A.; Wilson, C.; Raj, O.; Watson, J.; Lopez, A.; Albrecht, T.L. Impact of physicians' personal discomfort and patient prognosis on discussion of fertility preservation with young cancer patients. Patient Educ. Couns. 2009, 77, 338–343. [Google Scholar] [CrossRef]
- Peddie, V.L.; Porter, M.A.; Barbour, R.; Culligan, D.; MacDonald, G.; King, D.; Horn, J.; Bhattacharya, S. Factors affecting decision making about fertility preservation after cancer diagnosis: A qualitative study. BJOG 2012, 119, 1049–1057. [Google Scholar] [CrossRef]
- Kohler, T.S.; Kondapalli, L.A.; Shah, A.; Chan, S.; Woodruff, T.K.; Brannigan, R.E. Results from the survey for preservation of adolescent reproduction (SPARE) study: Gender disparity in delivery of fertility preservation message to adolescents with cancer. J. Assist. Reprod. Genet. 2011, 28, 269–277. [Google Scholar] [CrossRef]
- Reinecke, J.D.; Kelvin, J.F.; Arvey, S.R.; Quinn, G.P.; Levine, J.; Beck, L.N.; Miller, A. Implementing a systematic approach to meeting patients' cancer and fertility needs: A review of the fertile hope centers of excellence program. J. Oncol. Pract. 2012, 8, 303–308. [Google Scholar] [CrossRef]
- Johnston, R.J.; Wallace, W.H. Normal ovarian function and assessment of ovarian reserve in the survivor of childhood cancer. Pediatr. Blood Cancer 2009, 53, 296–302. [Google Scholar] [CrossRef]
- Charpentier, A.M.; Chong, A.L.; Gingras-Hill, G.; Ahmed, S.; Cigsar, C.; Gupta, A.A.; Greenblatt, E.; Hodgson, D.C. Anti-Mullerian hormone screening to assess ovarian reserve among female survivors of childhood cancer. J. Cancer Surviv. 2014. [Google Scholar] [CrossRef]
- Meirow, D. Ovarian injury and modern options to preserve fertility in female cancer patients treated with high dose radio-chemotherapy for hemato-oncological neoplasias and other cancers. Leuk. Lymphoma 1999, 33, 65–76. [Google Scholar]
- Wallace, W.H.; Shalet, S.M.; Hendry, J.H.; Morris-Jones, P.H.; Gattamaneni, H.R. Ovarian failure following abdominal irradiation in childhood: The radiosensitivity of the human oocyte. Br. J. Radiol. 1989, 62, 995–998. [Google Scholar] [CrossRef]
- Metzger, M.L.; Meacham, L.R.; Patterson, B.; Casillas, J.S.; Constine, L.S.; Hijiya, N.; Kenney, L.B.; Leonard, M.; Lockart, B.A.; Likes, W.; et al. Female reproductive health after childhood, adolescent, and young adult cancers: Guidelines for the assessment and management of female reproductive complications. J. Clin. Oncol. 2013, 31, 1239–1247. [Google Scholar] [CrossRef]
- Dillon, K.E.; Sammel, M.D.; Prewitt, M.; Ginsberg, J.P.; Walker, D.; Mersereau, J.E.; Gosiengfiao, Y.; Gracia, C.R. Pretreatment antimullerian hormone levels determine rate of posttherapy ovarian reserve recovery: Acute changes in ovarian reserve during and after chemotherapy. Fertil. Steril. 2013, 99, 477–483. [Google Scholar] [CrossRef]
- Chemaitilly, W.; Mertens, A.C.; Mitby, P.; Whitton, J.; Stovall, M.; Yasui, Y.; Robison, L.L.; Sklar, C.A. Acute ovarian failure in the childhood cancer survivor study. J. Clin. Endocrinol. Metab. 2006, 91, 1723–1728. [Google Scholar] [CrossRef]
- Sklar, C.A.; Mertens, A.C.; Mitby, P.; Whitton, J.; Stovall, M.; Kasper, C.; Mulder, J.; Green, D.; Nicholson, H.S.; Yasui, Y.; et al. Premature menopause in survivors of childhood cancer: A report from the childhood cancer survivor study. J. Natl. Cancer Inst. 2006, 98, 890–896. [Google Scholar] [CrossRef]
- Signorello, L.B.; Mulvihill, J.J.; Green, D.M.; Munro, H.M.; Stovall, M.; Weathers, R.E.; Mertens, A.C.; Whitton, J.A.; Robison, L.L.; Boice, J.D., Jr. Stillbirth and neonatal death in relation to radiation exposure before conception: A retrospective cohort study. Lancet 2010, 376, 624–630. [Google Scholar] [CrossRef]
- Littley, M.D.; Shalet, S.M.; Beardwell, C.G.; Ahmed, S.R.; Applegate, G.; Sutton, M.L. Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q. J. Med. 1989, 70, 145–160. [Google Scholar]
- Green, D.M.; Nolan, V.G.; Kawashima, T.; Stovall, M.; Donaldson, S.S.; Srivastava, D.; Leisenring, W.; Robison, L.L.; Sklar, C.A. Decreased fertility among female childhood cancer survivors who received 22–27 Gy hypothalamic/pituitary irradiation: A report from the Childhood Cancer Survivor Study. Fertil. Steril. 2011, 95, 1922–1927. [Google Scholar] [CrossRef]
- Practice Committees of American Society for Reproductive, M.; Society for Assisted Reproductive, T. Mature oocyte cryopreservation: A guideline. Fertil. Steril. 2013, 99, 37–43. [Google Scholar]
- Society for Assisted Reproduction. Available online: http://www.sart.org/find_frm.html (accessed on 20 August 2014).
- Human Fertilisation and Embryology Authority. Available online: http://guide.hfea.gov.uk/guide/ (accessed on 20 August 2014).
- Noyes, N.; Porcu, E.; Borini, A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod. Biomed. Online 2009, 18, 769–776. [Google Scholar] [CrossRef]
- Bedoschi, G.M.; de Albuquerque, F.O.; Ferriani, R.A.; Navarro, P.A. Ovarian stimulation during the luteal phase for fertility preservation of cancer patients: Case reports and review of the literature. J. Assist. Reprod. Genet. 2010, 27, 491–494. [Google Scholar] [CrossRef]
- Livestrong Foundation. Available online: http://www.livestrong.org/we-can-help/fertility-services/fertility-preservation-options-women/ (accessed on 28 May 2014).
- Livestrong Foundation. Available online: http://www.livestrong.org/we-can-help/fertility/#tab2 (accessed on 20 August 2014).
- Partridge, A.H. Ovarian suppression for prevention of premature menopause and infertility: Empty promise or effective therapy? J. Clin. Oncol. 2012, 30, 479–481. [Google Scholar] [CrossRef]
- Beck-Fruchter, R.; Weiss, A.; Shalev, E. GnRH agonist therapy as ovarian protectants in female patients undergoing chemotherapy: A review of the clinical data. Hum. Reprod. Update. 2008, 14, 553–561. [Google Scholar]
- Badawy, A.; Elnashar, A.; El-Ashry, M.; Shahat, M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: Prospective randomized study. Fertil. Steril. 2009, 91, 694–697. [Google Scholar] [CrossRef]
- Del Mastro, L.; Boni, L.; Michelotti, A.; Gamucci, T.; Olmeo, N.; Gori, S.; Giordano, M.; Garrone, O.; Pronzato, P.; Bighin, C.; et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: A randomized trial. JAMA 2011, 306, 269–276. [Google Scholar]
- Sverrisdottir, A.; Nystedt, M.; Johansson, H.; Fornander, T. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: Results from a randomized trial. Breast Cancer Res. Treat. 2009, 117, 561–567. [Google Scholar] [CrossRef]
- Gerber, B.; von Minckwitz, G.; Stehle, H.; Reimer, T.; Felberbaum, R.; Maass, N.; Fischer, D.; Sommer, H.L.; Conrad, B.; Ortmann, O.; et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: The GBG 37 ZORO study. J. Clin. Oncol. 2011, 29, 2334–2341. [Google Scholar] [CrossRef]
- Munster, P.N.; Moore, A.P.; Ismail-Khan, R.; Cox, C.E.; Lacevic, M.; Gross-King, M.; Xu, P.; Carter, W.B.; Minton, S.E. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J. Clin. Oncol. 2012, 30, 533–538. [Google Scholar] [CrossRef]
- Behringer, K.; Wildt, L.; Mueller, H.; Mattle, V.; Ganitis, P.; van den Hoonaard, B.; Ott, H.W.; Hofer, S.; Pluetschow, A.; Diehl, V.; et al. No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced-stage Hodgkin lymphoma. Final results of a phase II trial from the German Hodgkin Study Group. Ann. Oncol. 2010, 21, 2052–2060. [Google Scholar]
- Moore, H.C.F.; Unger, J.M.; Phillips, K.; Boyle, F.M.; Hitre, E.; Porter, D.J.; Francis, P.A.; Minasian, L.M.; Gelber, R.D.; Goldstein, L.J.; et al. Phase III trial (Prevention of Early Menopause Study [POEMS]-SWOG S0230) of LHRH analog during chemotherapy (CT) to reduce ovarian failure in early-stage, hormone receptor-negative breast cancer: An international Intergroup trial of SWOG, IBCSG, ECOG, and CALGB (Alliance). J. Clin. Oncol. 2014, 32. Abstract number: LBA505. [Google Scholar]
- Gracia, C.R.; Chang, J.; Kondapalli, L.; Prewitt, M.; Carlson, C.A.; Mattei, P.; Jeffers, S.; Ginsberg, J.P. Ovarian tissue cryopreservation for fertility preservation in cancer patients: Successful establishment and feasibility of a multidisciplinary collaboration. J. Assist. Reprod. Genet. 2012, 29, 495–502. [Google Scholar] [CrossRef]
- Kim, S.S. Fertility preservation in female cancer patients: Current developments and future directions. Fertil. Steril. 2006, 85, 1–11. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Fertility preservation in women. Nat. Rev. Endocrinol. 2013, 9, 735–749. [Google Scholar] [CrossRef]
- Babayev, S.N.; Arslan, E.; Kogan, S.; Moy, F.; Oktay, K. Evaluation of ovarian and testicular tissue cryopreservation in children undergoing gonadotoxic therapies. J. Assist. Reprod. Genet. 2013, 30, 3–9. [Google Scholar] [CrossRef]
- Smitz, J.; Dolmans, M.M.; Donnez, J.; Fortune, J.E.; Hovatta, O.; Jewgenow, K.; Picton, H.M.; Plancha, C.; Shea, L.D.; Stouffer, R.L.; et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: Implications for fertility preservation. Hum. Reprod. Update 2010, 16, 395–414. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Marinescu, C.; Saussoy, P.; Van Langendonckt, A.; Amorim, C.; Donnez, J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood 2010, 116, 2908–2914. [Google Scholar] [CrossRef]
- Terenziani, M.; Piva, L.; Meazza, C.; Gandola, L.; Cefalo, G.; Merola, M. Oophoropexy: A relevant role in preservation of ovarian function after pelvic irradiation. Fertil. Steril. 2009, 91, 935.e15–935.e16. [Google Scholar] [CrossRef]
- Anderson, R.A.; Weddell, A.; Spoudeas, H.A.; Douglas, C.; Shalet, S.M.; Levitt, G.; Wallace, W.H. Do doctors discuss fertility issues before they treat young patients with cancer? Hum. Reprod. 2008, 23, 2246–2251. [Google Scholar]
- Quinn, G.P.; Murphy, D.; Knapp, C.; Stearsman, D.K.; Bradley-Klug, K.L.; Sawczyn, K.; Clayman, M.L. Who decides? Decision making and fertility preservation in teens with cancer: A review of the literature. J. Adolesc. Health 2011, 49, 337–346. [Google Scholar] [CrossRef]
- Anderson, R.A.; Wallace, W.H. Antimullerian hormone, the assessment of the ovarian reserve, and the reproductive outcome of the young patient with cancer. Fertil. Steril. 2013, 99, 1469–1475. [Google Scholar] [CrossRef]
- Peigne, M.; Decanter, C. Serum AMH level as a marker of acute and long-term effects of chemotherapy on the ovarian follicular content: A systematic review. Reprod. Biol. Endocrinol. 2014, 12, 26. [Google Scholar] [CrossRef]
- Bath, L.E.; Wallace, W.H.; Shaw, M.P.; Fitzpatrick, C.; Anderson, R.A. Depletion of ovarian reserve in young women after treatment for cancer in childhood: Detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum. Reprod. 2003, 18, 2368–2374. [Google Scholar] [CrossRef]
- Dillon, K.E.; Sammel, M.D.; Ginsberg, J.P.; Lechtenberg, L.; Prewitt, M.; Gracia, C.R. Pregnancy after cancer: Results from a prospective cohort study of cancer survivors. Pediatr. Blood Cancer 2013, 60, 2001–2006. [Google Scholar]
- Barton, S.E.; Najita, J.S.; Ginsburg, E.S.; Leisenring, W.M.; Stovall, M.; Weathers, R.E.; Sklar, C.A.; Robison, L.L.; Diller, L. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: A report from the childhood cancer survivor study cohort. Lancet Oncol. 2013, 14, 873–881. [Google Scholar] [CrossRef]
- Meistrich, M.L. Male gonadal toxicity. Pediatr. Blood Cancer 2009, 53, 261–266. [Google Scholar] [CrossRef]
- Shetty, G.; Meistrich, M.L. Hormonal approaches to preservation and restoration of male fertility after cancer treatment. J. Natl. Cancer Inst. Monogr. 2005, 34, 36–39. [Google Scholar] [CrossRef]
- Wyrobek, A.J.; Schmid, T.E.; Marchetti, F. Relative susceptibilities of male germ cells to genetic defects induced by cancer chemotherapies. J. Natl. Cancer Inst. Monogr. 2005, 34, 31–35. [Google Scholar] [CrossRef]
- Kenney, L.B.; Laufer, M.R.; Grant, F.D.; Grier, H.; Diller, L. High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer 2001, 91, 613–621. [Google Scholar] [CrossRef]
- Trottmann, M.; Becker, A.J.; Stadler, T.; Straub, J.; Soljanik, I.; Schlenker, B.; Stief, C.G. Semen quality in men with malignant diseases before and after therapy and the role of cryopreservation. Eur. Urol. 2007, 52, 355–367. [Google Scholar] [CrossRef]
- Jahnukainen, K.; Stukenborg, J.B. Clinical review: Present and future prospects of male fertility preservation for children and adolescents. J. Clin. Endocrinol. Metab. 2012, 97, 4341–4351. [Google Scholar] [CrossRef]
- Kenney, L.B.; Cohen, L.E.; Shnorhavorian, M.; Metzger, M.L.; Lockart, B.; Hijiya, N.; Duffey-Lind, E.; Constine, L.; Green, D.; Meacham, L. Male reproductive health after childhood, adolescent, and young adult cancers: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 3408–3416. [Google Scholar] [CrossRef]
- Vigersky, R.A.; Chapman, R.M.; Berenberg, J.; Glass, A.R. Testicular dysfunction in untreated hodgkin's disease. Am. J. Med. 1982, 73, 482–486. [Google Scholar] [CrossRef]
- Petersen, P.M.; Skakkebaek, N.E.; Vistisen, K.; Rorth, M.; Giwercman, A. Semen quality and reproductive hormones before orchiectomy in men with testicular cancer. J. Clin. Oncol. 1999, 17, 941–947. [Google Scholar]
- Howell, S.J.; Shalet, S.M. Spermatogenesis after cancer treatment: Damage and recovery. J. Natl. Cancer Inst. Monogr. 2005, 34, 12–17. [Google Scholar] [CrossRef]
- Shin, D.; Lo, K.C.; Lipshultz, L.I. Treatment options for the infertile male with cancer. J. Natl. Cancer Inst. Monogr. 2005, 34, 48–50. [Google Scholar] [CrossRef]
- Feldschuh, J.; Brassel, J.; Durso, N.; Levine, A. Successful sperm storage for 28 years. Fertil. Steril. 2005, 84, 1017. [Google Scholar]
- Ginsberg, J.P. New advances in fertility preservation for pediatric cancer patients. Curr. Opin. Pediatr. 2011, 23, 9–13. [Google Scholar] [CrossRef]
- Pacey, A.A. Fertility issues in survivors from adolescent cancers. Cancer Treat. Rev. 2007, 33, 646–655. [Google Scholar] [CrossRef]
- Crawshaw, M.A.; Glaser, A.W.; Pacey, A.A. The use of pornographic materials by adolescent male cancer patients when banking sperm in the UK: Legal and ethical dilemmas. Hum. Fertil. Camb. 2007, 10, 159–163. [Google Scholar] [CrossRef]
- Berookhim, B.M.; Mulhall, J.P. Outcomes of operative sperm retrieval strategies for fertility preservation among males scheduled to undergo cancer treatment. Fertil. Steril. 2014, 101, 805–811. [Google Scholar] [CrossRef]
- Hagenas, I.; Jorgensen, N.; Rechnitzer, C.; Sommer, P.; Holm, M.; Schmiegelow, K.; Daugaard, G.; Jacobsen, N.; Juul, A. Clinical and biochemical correlates of successful semen collection for cryopreservation from 12–18-year-old patients: A single-center study of 86 adolescents. Hum. Reprod. 2010, 25, 2031–2038. [Google Scholar] [CrossRef]
- Chong, A.L.; Gupta, A.; Punnett, A.; Nathan, P.C. A cross Canada survey of sperm banking practices in pediatric oncology centers. Pediatr. Blood Cancer 2010, 55, 1356–1361. [Google Scholar] [CrossRef]
- Ginsberg, J.P.; Ogle, S.K.; Tuchman, L.K.; Carlson, C.A.; Reilly, M.M.; Hobbie, W.L.; Rourke, M.; Zhao, H.; Meadows, A.T. Sperm banking for adolescent and young adult cancer patients: Sperm quality, patient, and parent perspectives. Pediatr. Blood Cancer 2008, 50, 594–598. [Google Scholar] [CrossRef]
- Shnorhavorian, M.; Kroon, L.; Jeffries, H.; Johnson, R. Creating a standardized process to offer the standard of care: Continuous process improvement methodology is associated with increased rates of sperm cryopreservation among adolescent and young adult males with cancer. J. Pediatr. Hematol. Oncol. 2012, 34, e315–e319. [Google Scholar] [CrossRef]
- Ginsberg, J.P.; Carlson, C.A.; Lin, K.; Hobbie, W.L.; Wigo, E.; Wu, X.; Brinster, R.L.; Kolon, T.F. An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: A report of acceptability and safety. Hum. Reprod. 2010, 25, 37–41. [Google Scholar] [CrossRef]
- Orwig, K.E.; Schlatt, S. Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J. Natl. Cancer Inst. Monogr. 2005, 34, 51–56. [Google Scholar] [CrossRef]
- Wyns, C.; Curaba, M.; Vanabelle, B.; Van Langendonckt, A.; Donnez, J. Options for fertility preservation in prepubertal boys. Hum. Reprod. Update 2010, 16, 312–328. [Google Scholar] [CrossRef]
- Jahnukainen, K.; Hou, M.; Petersen, C.; Setchell, B.; Soder, O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001, 61, 706–710. [Google Scholar]
- Ginsberg, J.P.; Li, Y.; Carlson, C.A.; Gracia, C.R.; Hobbie, W.L.; Miller, V.A.; Mulhall, J.; Shnorhavorian, M.; Brinster, R.L.; Kolon, T.F. Testicular tissue cryopreservation in prepubertal male children: An analysis of parental decision-making. Pediatr. Blood Cancer 2014, 61, 1673–1678. [Google Scholar] [CrossRef]
- Pacey, A.A.; Eiser, C. Banking sperm is only the first of many decisions for men: What healthcare professionals and men need to know. Hum. Fertil. Camb. 2011, 14, 208–217. [Google Scholar] [CrossRef]
- Pacey, A.; Merrick, H.; Arden-Close, E.; Morris, K.; Rowe, R.; Stark, D.; Eiser, C. Implications of sperm banking for health-related quality of life up to 1 year after cancer diagnosis. Br. J. Cancer 2013, 108, 1004–1011. [Google Scholar] [CrossRef]
- Yee, S.; Fuller-Thomson, E.; Dwyer, C.; Greenblatt, E.; Shapiro, H. “Just what the doctor ordered”: Factors associated with oncology patients’ decision to bank sperm. Can. Urol. Assoc. J. 2012, 6, E174–E178. [Google Scholar] [CrossRef]
- Wasilewski-Masker, K.; Seidel, K.D.; Leisenring, W.; Mertens, A.C.; Shnorhavorian, M.; Ritenour, C.W.; Stovall, M.; Green, D.M.; Sklar, C.A.; Armstrong, G.T.; et al. Male infertility in long-term survivors of pediatric cancer: A report from the childhood cancer survivor study. J. Cancer Surviv. 2014, 8, 437–447. [Google Scholar]
- Green, D.M.; Zhu, L.; Zhang, N.; Sklar, C.A.; Ke, R.W.; Kutteh, W.H.; Klosky, J.L.; Spunt, S.L.; Metzger, M.L.; Navid, F.; et al. Lack of specificity of plasma concentrations of inhibin B and follicle-stimulating hormone for identification of azoospermic survivors of childhood cancer: A report from the St Jude lifetime cohort study. J. Clin. Oncol. 2013, 31, 1324–1328. [Google Scholar] [CrossRef]
- Romerius, P.; Stahl, O.; Moell, C.; Relander, T.; Cavallin-Stahl, E.; Wiebe, T.; Giwercman, Y.L.; Giwercman, A. Hypogonadism risk in men treated for childhood cancer. J. Clin. Endocrinol. Metab. 2009, 94, 4180–4186. [Google Scholar] [CrossRef]
- Dohle, G.R. Male infertility in cancer patients: Review of the literature. Int. J. Urol. 2010, 17, 327–331. [Google Scholar] [CrossRef]
- Signorello, L.B.; Mulvihill, J.J.; Green, D.M.; Munro, H.M.; Stovall, M.; Weathers, R.E.; Mertens, A.C.; Whitton, J.A.; Robison, L.L.; Boice, J.D., Jr. Congenital anomalies in the children of cancer survivors: A report from the childhood cancer survivor study. J. Clin. Oncol. 2012, 30, 239–245. [Google Scholar] [CrossRef]
- Winther, J.F.; Boice, J.D., Jr.; Mulvihill, J.J.; Stovall, M.; Frederiksen, K.; Tawn, E.J.; Olsen, J.H. Chromosomal abnormalities among offspring of childhood-cancer survivors in Denmark: A population-based study. Am. J. Hum. Genet. 2004, 74, 1282–1285. [Google Scholar] [CrossRef]
- Eiser, C.; Arden-Close, E.; Morris, K.; Pacey, A.A. The legacy of sperm banking: How fertility monitoring and disposal of sperm are linked with views of cancer treatment. Hum. Reprod. 2011, 26, 2791–2798. [Google Scholar] [CrossRef]
- Merrick, H.; Wright, E.; Pacey, A.A.; Eiser, C. Finding out about sperm banking: What information is available online for men diagnosed with cancer? Hum. Fertil. Camb. 2012, 15, 121–128. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.H.; Mersereau, J.E. Building a successful fertility preservation program at a major cancer center. J. Gynecol. Oncol. 2014, 25, 148–154. [Google Scholar] [CrossRef]
- Blough, K.; Mansfield, C.; Kondapalli, L.A. Seamless integration of clinical care and research in an innovative fertility preservation program: The Colorado Oncofertility Program model. J. Cancer Surviv. 2014. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Levine, J.M. Preserving Fertility in Children and Adolescents with Cancer. Children 2014, 1, 166-185. https://doi.org/10.3390/children1020166
Levine JM. Preserving Fertility in Children and Adolescents with Cancer. Children. 2014; 1(2):166-185. https://doi.org/10.3390/children1020166
Chicago/Turabian StyleLevine, Jennifer M. 2014. "Preserving Fertility in Children and Adolescents with Cancer" Children 1, no. 2: 166-185. https://doi.org/10.3390/children1020166
APA StyleLevine, J. M. (2014). Preserving Fertility in Children and Adolescents with Cancer. Children, 1(2), 166-185. https://doi.org/10.3390/children1020166



