Nephroprotective Role of Chrysophanol in Hypoxia/Reoxygenation-Induced Renal Cell Damage via Apoptosis, ER Stress, and Ferroptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Culture and Hypoxia/Reoxygenation (H/R) Conditions
2.3. Cell Viability Assay
2.4. Western Blotting
2.5. Lipid ROS Detection
2.6. Nuclear Fraction Extraction
2.7. Immunofluorescence Staining
2.8. Statistical Analyses
3. Results
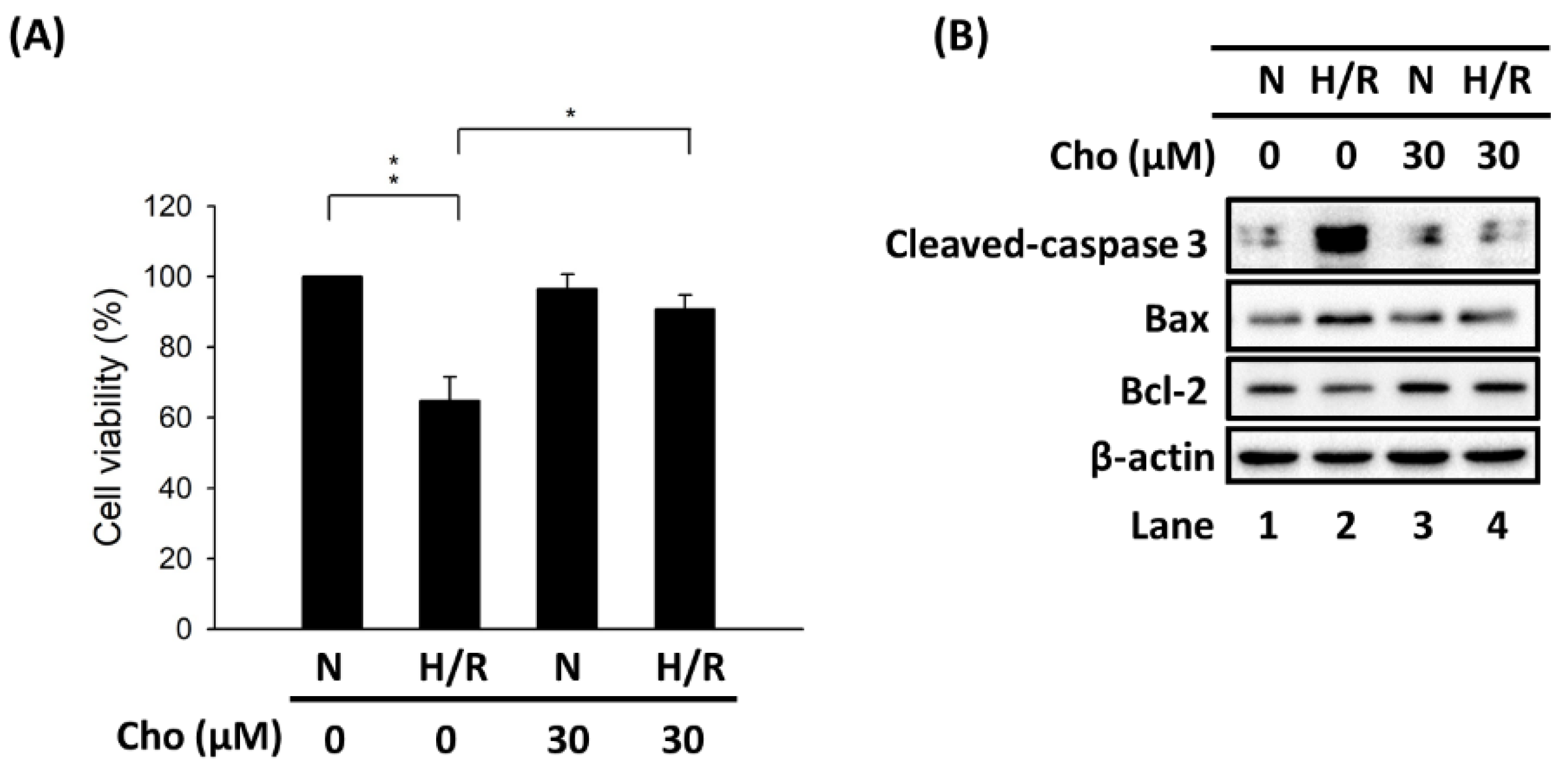
3.1. Chrysophanol Attenuated H/R-Induced Cell Death via Apoptosis
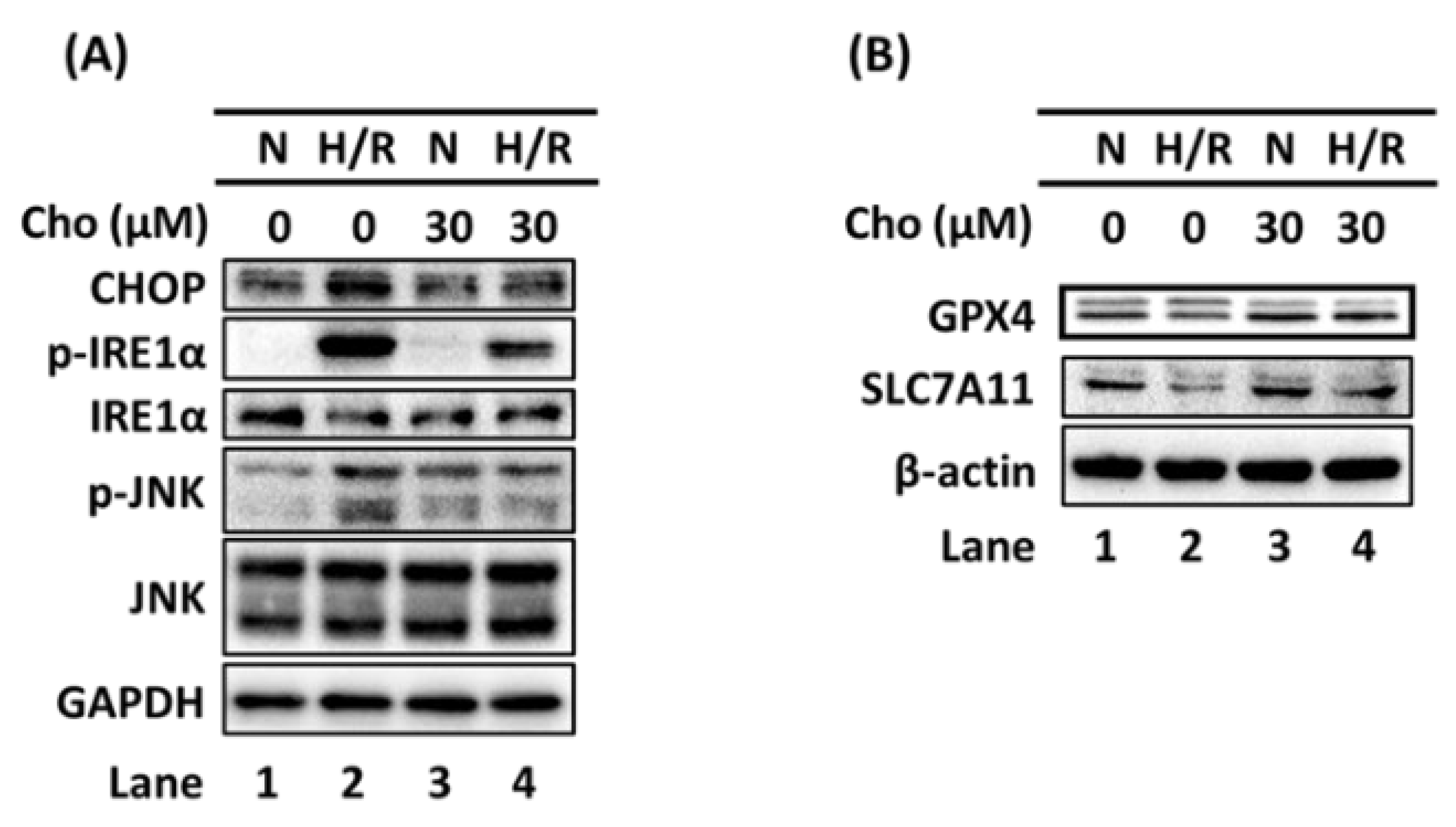
3.2. Chrysophanol Regulated ER Stress and Ferroptosis Pathway under H/R Conditions
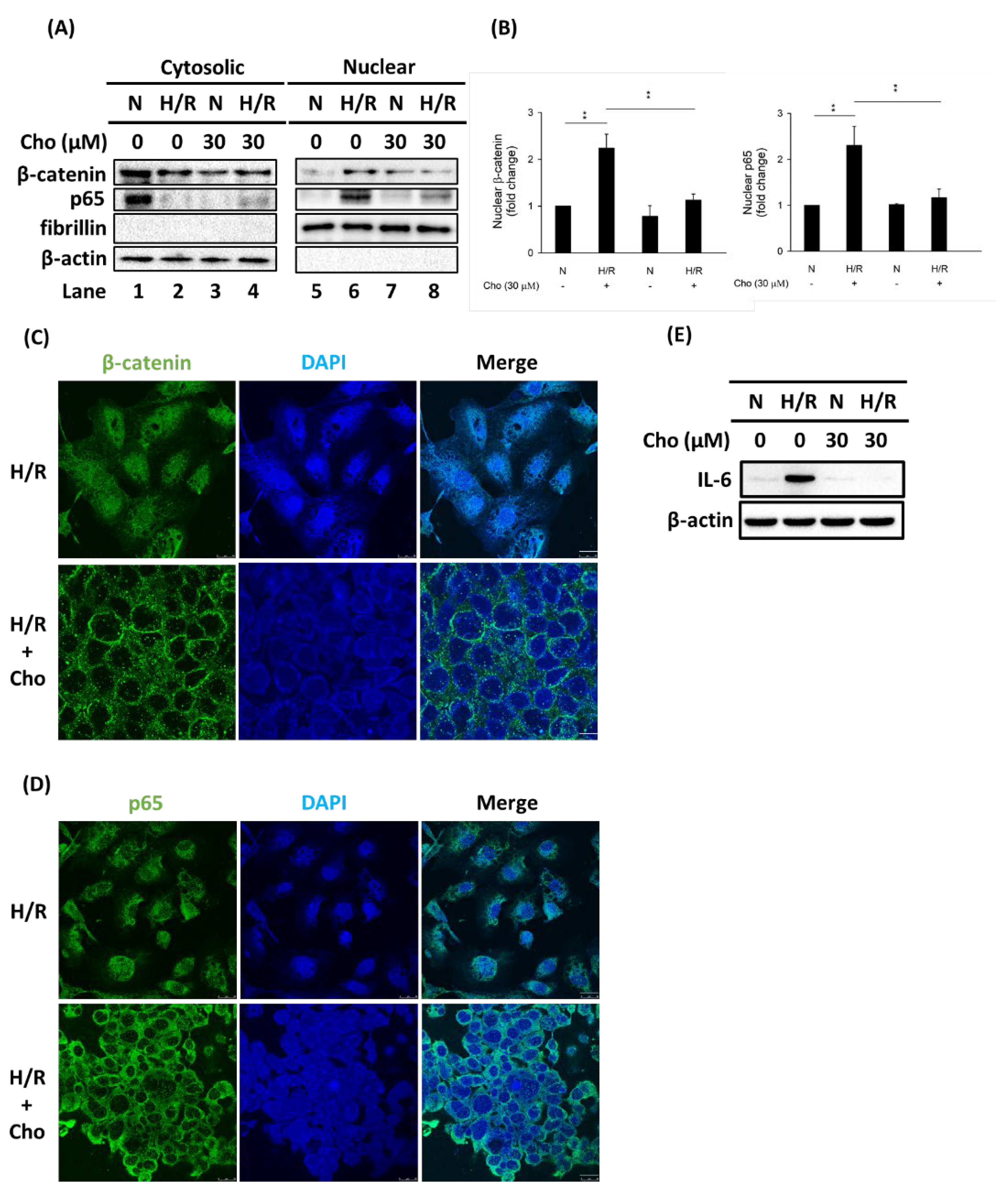
3.3. Chrysophanol Treatment Attenuated p-JNK Expression and NF-κB Nuclear Translocation under H/R Conditions
4. Discussion
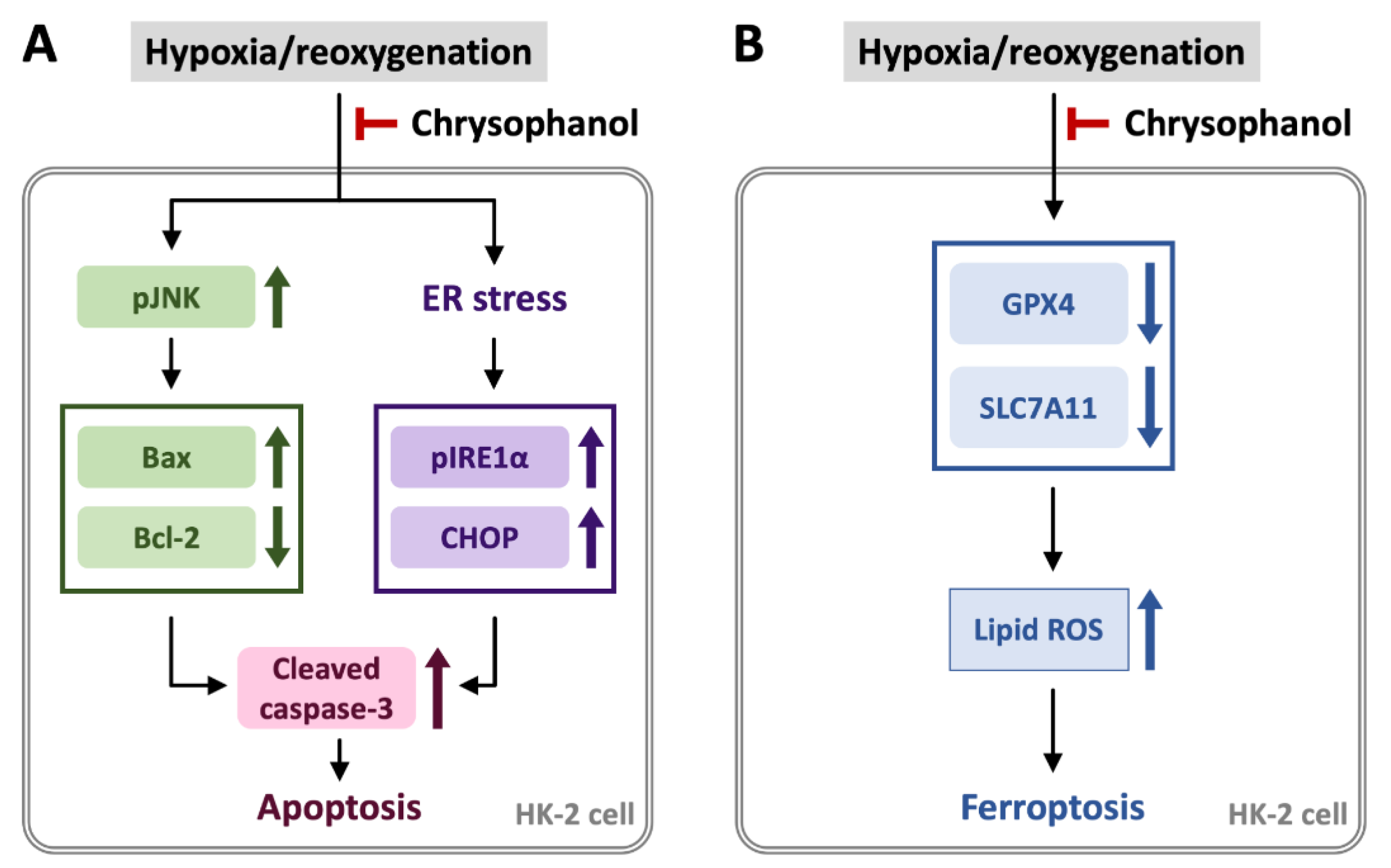
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Li, H.D.; Meng, X.M.; Huang, C.; Zhang, L.; Lv, X.W.; Li, J. Application of herbal traditional Chinese medicine in the treatment of acute kidney injury. Front. Pharmacol. 2019, 10, 376. [Google Scholar] [CrossRef]
- Yang, B.; Xie, Y.; Guo, M.; Rosner, M.H.; Yang, H.; Ronco, C. Nephrotoxicity and Chinese herbal medicine. Clin. J. Am. Soc. Nephrol. 2018, 13, 1605–1611. [Google Scholar] [CrossRef]
- Meng, X.M.; Li, H.D.; Wu, W.F.; Ming-Kuen Tang, P.; Ren, G.L.; Gao, L.; Li, X.F.; Yang, Y.; Xu, T.; Ma, T.T.; et al. Wogonin protects against cisplatin-induced acute kidney injury by targeting RIPK1-mediated necroptosis. Lab. Investig. 2018, 98, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Isnard Bagnis, C.; Deray, G.; Baumelou, A.; Le Quintrec, M.; Vanherweghem, J.L. Herbs and the kidney. Am. J. Kidney Dis. 2004, 44, 1–11. [Google Scholar] [CrossRef]
- Valerian Bunel, F.Q.; Duez, P.; Xu, Q.-H. Herbal medicines for acute kidney injury: Evidence, gapsand frontiers. World J. Tradit. Chin. Med. 2015, 1, 47–66. [Google Scholar] [CrossRef]
- Xie, L.; Tang, H.; Song, J.; Long, J.; Zhang, L.; Li, X. Chrysophanol: A review of its pharmacology, toxicity and pharmacokinetics. J. Pharm. Pharmacol. 2019, 71, 1475–1487. [Google Scholar] [CrossRef] [Green Version]
- Prateeksha; Yusuf, M.A.; Singh, B.N.; Sudheer, S.; Kharwar, R.N.; Siddiqui, S.; Abdel-Azeem, A.M.; Fernandes Fraceto, L.; Dashora, K.; Gupta, V.K. Chrysophanol: A Natural Anthraquinone with Multifaceted Biotherapeutic Potential. Biomolecules 2019, 9, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.; Yang, H.C.; You, L.; Wang, Y.Y.; He, W.J.; Hao, C.M. The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2013, 83, 404–413. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Tanaka, T.; Nangaku, M. Hypoxia as a key player in the AKI-to-CKD transition. Am. J. Physiol. Renal Physiol. 2014, 307, F1187–F1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Yang, Y.; Cao, H.; Huang, Y.; Wang, H.; Zhao, B. Resveratrol protects human renal proximal tubular cells from high glucose and hypoxia/ reoxygenation induced injury via inhibiting p38-MAPK and thioredoxin-interacting protein (TXNIP) pathways. Int. J. Clin. Exp. Med. 2019, 12, 2243–2254. [Google Scholar]
- Luo, L.; Lu, J.; Wei, L.; Long, D.; Guo, J.Y.; Shan, J.; Li, F.S.; Lu, P.Y.; Li, P.Y.; Feng, L. The role of HIF-1 in up-regulating MICA expression on human renal proximal tubular epithelial cells during hypoxia/reoxygenation. BMC Cell Biol. 2010, 11, 91. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Guo, Q. Apoptosis antagonizing transcription factor protects renal tubule cells against oxidative damage and apoptosis induced by ischemia-reperfusion. J. Am. Soc. Nephrol. 2006, 17, 3336–3346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; Shu, S.; Guo, C.; Tang, C.; Dong, Z. Endoplasmic reticulum stress in ischemic and nephrotoxic acute kidney injury. Ann. Med. 2018, 50, 381–390. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Huang, L.; Zheng, Z.; Vlassara, H.; Striker, G.; Zhang, X.; Guan, Y.; Zheng, F. Excessive oxidative stress contributes to increased acute ER stress kidney injury in aged mice. Oxid. Med. Cell Longev. 2019, 2019, 2746521. [Google Scholar] [CrossRef]
- Inagi, R. Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron Exp. Nephrol. 2009, 112, e1–e9. [Google Scholar] [CrossRef]
- Cybulsky, A.V. Endoplasmic reticulum stress in proteinuric kidney disease. Kidney Int. 2010, 77, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, H.; Yang, S.K.; Wu, X.; He, D.; Cao, K.; Zhang, W. Emerging role of ferroptosis in acute kidney injury. Oxid. Med. Cell Longev. 2019, 2019, 8010614. [Google Scholar] [CrossRef]
- Ma, D.; Li, C.; Jiang, P.; Jiang, Y.; Wang, J.; Zhang, D. Inhibition of ferroptosis attenuates acute kidney injury in rats with severe acute pancreatitis. Dig. Dis. Sci. 2020, 66, 483–492. [Google Scholar] [CrossRef]
- Borawski, B.; Malyszko, J. Iron, ferroptosis, and new insights for prevention in acute kidney injury. Adv. Med. Sci. 2020, 65, 361–370. [Google Scholar] [CrossRef]
- Shu, S.; Zhu, J.; Liu, Z.; Tang, C.; Cai, J.; Dong, Z. Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine 2018, 37, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malek, M.; Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Renal Inj. Prev. 2015, 4, 20–27. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, S.W.; Sviderskiy, V.O.; Terzi, E.M.; Papagiannakopoulos, T.; Moreira, A.L.; Adams, S.; Sabatini, D.M.; Birsoy, K.; Possemato, R. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 2017, 551, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sheng, M.; Xu, R.; Yu, J.; Cui, K.; Tong, J.; Shi, L.; Ren, H.; Du, H. Berberine protects human renal proximal tubular cells from hypoxia/reoxygenation injury via inhibiting endoplasmic reticulum and mitochondrial stress pathways. J. Transl. Med. 2013, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Jiang, D.; Xiao, J.; Fu, C.; Zhang, Z.; Ye, Z.; Zhang, X. Ischemic preconditioning attenuates ischemia/reperfusion-induced kidney injury by activating autophagy via the SGK1 signaling pathway. Cell Death Dis. 2018, 9, 338. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Chiu, V.; Hsieh, P.C.; Huang, C.Y.; Huang, S.J.; Tzeng, I.S.; Tsai, F.M.; Chen, M.L.; Liu, C.T.; Chen, Y.R. Chrysophanol attenuates hepatitis B virus X protein-induced hepatic stellate cell fibrosis by regulating endoplasmic reticulum stress and ferroptosis. J. Pharmacol. Sci. 2020, 144, 172–182. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Lin, C.H.; Hsu, T. VHL inactivation in precancerous kidney cells induces an inflammatory response via ER stress-activated IRE1alpha signaling. Cancer Res. 2017, 77, 3406–3416. [Google Scholar] [CrossRef] [Green Version]
- Havasi, A.; Borkan, S.C. Apoptosis and acute kidney injury. Kidney Int. 2011, 80, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Cai, C.; Lin, H.; Zhang, W.; Peng, Y.; Wu, K. Baicalein protects renal tubular epithelial cells againsthypoxia-reoxygenation injury. Ren. Fail. 2018, 40, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Deng, Y.; Chen, Y.; Chuang, P.Y.; Cijiang He, J. Therapeutic use of traditional Chinese herbal medications for chronic kidney diseases. Kidney Int. 2013, 84, 1108–1118. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.R.; Jeong, J.H.; Yu, K.S.; Lee, N.S.; Jeong, Y.G.; Kim, D.K.; Na, C.S.; Na, D.S.; Hwang, W.M.; Han, S.Y. Extract of Rhus verniciflua stokes protects against renal ischemia-reperfusion injury by enhancing Nrf2-mediated induction of antioxidant enzymes. Exp. Ther. Med. 2018, 15, 3827–3835. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zheng, Z.; Xie, Y.; Zhu, N.; Bao, J.; Yu, Q.; Zhou, Z.; Liu, J. Protective effect of taraxasterol on ischemia/reperfusion-induced acute kidney injury via inhibition of oxidative stress, inflammation, and apoptosis. Int. Immunopharmacol. 2020, 89, 107169. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Gao, H.; Zhou, N.; Wang, P.; Zhang, Y.; Zhang, A.; Jia, Z.; Huang, S. Trehalose attenuates renal ischemia-reperfusion injury by enhancing autophagy and inhibiting oxidative stress and inflammation. Am. J. Physiol. Renal Physiol. 2020, 318, F994–F1005. [Google Scholar] [CrossRef]
- Tian, R.; Wang, P.; Huang, L.; Li, C.; Lu, Z.; Lu, Z.; Wu, A.; Bao, K.; Mao, W.; Huang, Q.; et al. Sanqi oral solution ameliorates renal ischemia/reperfusion injury via reducing apoptosis and enhancing autophagy: Involvement of ERK/mTOR pathways. Front. Pharmacol. 2020, 11, 537147. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Guan, C.; Zhang, Y.; Yang, C.; Zhao, L.; Luan, H.; Zhou, B.; Che, L.; Wang, Y.; et al. Nicotiflorin attenuates cell apoptosis in renal ischemia-reperfusion injury through ATF3. Nephrology 2020, 26, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Bienholz, A.; Mae Pang, R.; Guberina, H.; Rauen, U.; Witzke, O.; Wilde, B.; Petrat, F.; Feldkamp, T.; Kribben, A. Resveratrol does not protect from ischemia-induced acute kidney injury in an in vivo rat model. Kidney Blood Press. Res. 2017, 42, 1090–1103. [Google Scholar] [CrossRef] [PubMed]
- Mahfoudh-Boussaid, A.; Zaouali, M.A.; Hadj-Ayed, K.; Miled, A.H.; Saidane-Mosbahi, D.; Rosello-Catafau, J.; Ben Abdennebi, H. Ischemic preconditioning reduces endoplasmic reticulum stress and upregulates hypoxia inducible factor-1alpha in ischemic kidney: The role of nitric oxide. J. Biomed. Sci. 2012, 19, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahfoudh-Boussaid, A.; Zaouali, M.A.; Hauet, T.; Hadj-Ayed, K.; Miled, A.H.; Ghoul-Mazgar, S.; Saidane-Mosbahi, D.; Rosello-Catafau, J.; Ben Abdennebi, H. Attenuation of endoplasmic reticulum stress and mitochondrial injury in kidney with ischemic postconditioning application and trimetazidine treatment. J. Biomed. Sci. 2012, 19, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.Y.; Wu, Y.B. Huaier extract attenuates acute kidney injury to chronic kidney disease transition by inhibiting endoplasmic reticulum stress and apoptosis via miR-1271 upregulation. Biomed. Res. Int. 2020, 2020, 9029868. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef]
- Li, D.; Liu, B.; Fan, Y.; Liu, M.; Han, B.; Meng, Y.; Xu, X.; Song, Z.; Liu, X.; Hao, Q.; et al. Nuciferine protects against folic acid-induced acute kidney injury by inhibiting ferroptosis. Br. J. Pharmacol. 2021, 178, 1182–1199. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, F.; Zhou, N.; Liu, S.; Wang, P.; Zhang, S.; Zhang, Y.; Zhang, A.; Jia, Z.; Huang, S. Dimethyl fumarate prevents ferroptosis to attenuate acute kidney injury by acting on NRF2. Clin. Transl. Med. 2021, 11, e382. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Thaiss, F.; Guo, L. NFkappaB and kidney injury. Front. Immunol. 2019, 10, 815. [Google Scholar] [CrossRef]
- Marko, L.; Vigolo, E.; Hinze, C.; Park, J.K.; Roel, G.; Balogh, A.; Choi, M.; Wubken, A.; Cording, J.; Blasig, I.E.; et al. Tubular epithelial NF-kappaB activity regulates ischemic AKI. J. Am. Soc. Nephrol. 2016, 27, 2658–2669. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.W.; Yiu, W.H.; Wu, H.J.; Li, R.X.; Liu, Y.; Chan, K.W.; Leung, J.C.; Chan, L.Y.; Lai, K.N.; Tang, S.C. Downregulation of renal tubular Wnt/beta-catenin signaling by Dickkopf-3 induces tubular cell death in proteinuric nephropathy. Cell Death Dis. 2016, 7, e2155. [Google Scholar] [CrossRef]
- Zhou, D.; Tan, R.J.; Fu, H.; Liu, Y. Wnt/beta-catenin signaling in kidney injury and repair: A double-edged sword. Lab. Investig. 2016, 96, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Pastor, C.; Blazquez-Serra, R.; Bosch, R.J.; Lucio Cazana, F.J.; Fernandez-Martinez, A.B. Apoptosis and cell proliferation in proximal tubular cells exposed to apoptotic bodies. Novel pathophysiological implications in cisplatin-induced renal injury. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2504–2515. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ma, Y.; Wang, L.; Zhang, Y.; Zhou, J. Multispectroscopic studies on the interaction of maltol, a food additive, with bovine serum albumin. Food Chem. 2012, 133, 264–270. [Google Scholar] [CrossRef]
- Mi, X.J.; Hou, J.G.; Wang, Z.; Han, Y.; Ren, S.; Hu, J.N.; Chen, C.; Li, W. The protective effects of maltol on cisplatin-induced nephrotoxicity through the AMPK-mediated PI3K/Akt and p53 signaling pathways. Sci. Rep. 2018, 8, 15922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridzuan, N.R.A.; Rashid, N.A.; Othman, F.; Budin, S.B.; Hussan, F.; Teoh, S.L. Protective role of natural products in cisplatin-induced nephrotoxicity. Mini Rev. Med. Chem. 2019, 19, 1134–1143. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Tseng, H.-F.; Hsieh, P.-C.; Chiu, V.; Lin, T.-Y.; Lan, C.-C.; Tzeng, I.-S.; Chao, H.-N.; Hsu, C.-C.; Kuo, C.-Y. Nephroprotective Role of Chrysophanol in Hypoxia/Reoxygenation-Induced Renal Cell Damage via Apoptosis, ER Stress, and Ferroptosis. Biomedicines 2021, 9, 1283. https://doi.org/10.3390/biomedicines9091283
Lin C-H, Tseng H-F, Hsieh P-C, Chiu V, Lin T-Y, Lan C-C, Tzeng I-S, Chao H-N, Hsu C-C, Kuo C-Y. Nephroprotective Role of Chrysophanol in Hypoxia/Reoxygenation-Induced Renal Cell Damage via Apoptosis, ER Stress, and Ferroptosis. Biomedicines. 2021; 9(9):1283. https://doi.org/10.3390/biomedicines9091283
Chicago/Turabian StyleLin, Chih-Hung, Han-Fang Tseng, Po-Chun Hsieh, Valeria Chiu, Ting-Yun Lin, Chou-Chin Lan, I-Shiang Tzeng, Huan-Nung Chao, Chia-Chen Hsu, and Chan-Yen Kuo. 2021. "Nephroprotective Role of Chrysophanol in Hypoxia/Reoxygenation-Induced Renal Cell Damage via Apoptosis, ER Stress, and Ferroptosis" Biomedicines 9, no. 9: 1283. https://doi.org/10.3390/biomedicines9091283
APA StyleLin, C.-H., Tseng, H.-F., Hsieh, P.-C., Chiu, V., Lin, T.-Y., Lan, C.-C., Tzeng, I.-S., Chao, H.-N., Hsu, C.-C., & Kuo, C.-Y. (2021). Nephroprotective Role of Chrysophanol in Hypoxia/Reoxygenation-Induced Renal Cell Damage via Apoptosis, ER Stress, and Ferroptosis. Biomedicines, 9(9), 1283. https://doi.org/10.3390/biomedicines9091283







