The Impact of Monoclonal Antibodies on Airway Smooth Muscle Contractility in Asthma: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Review Question
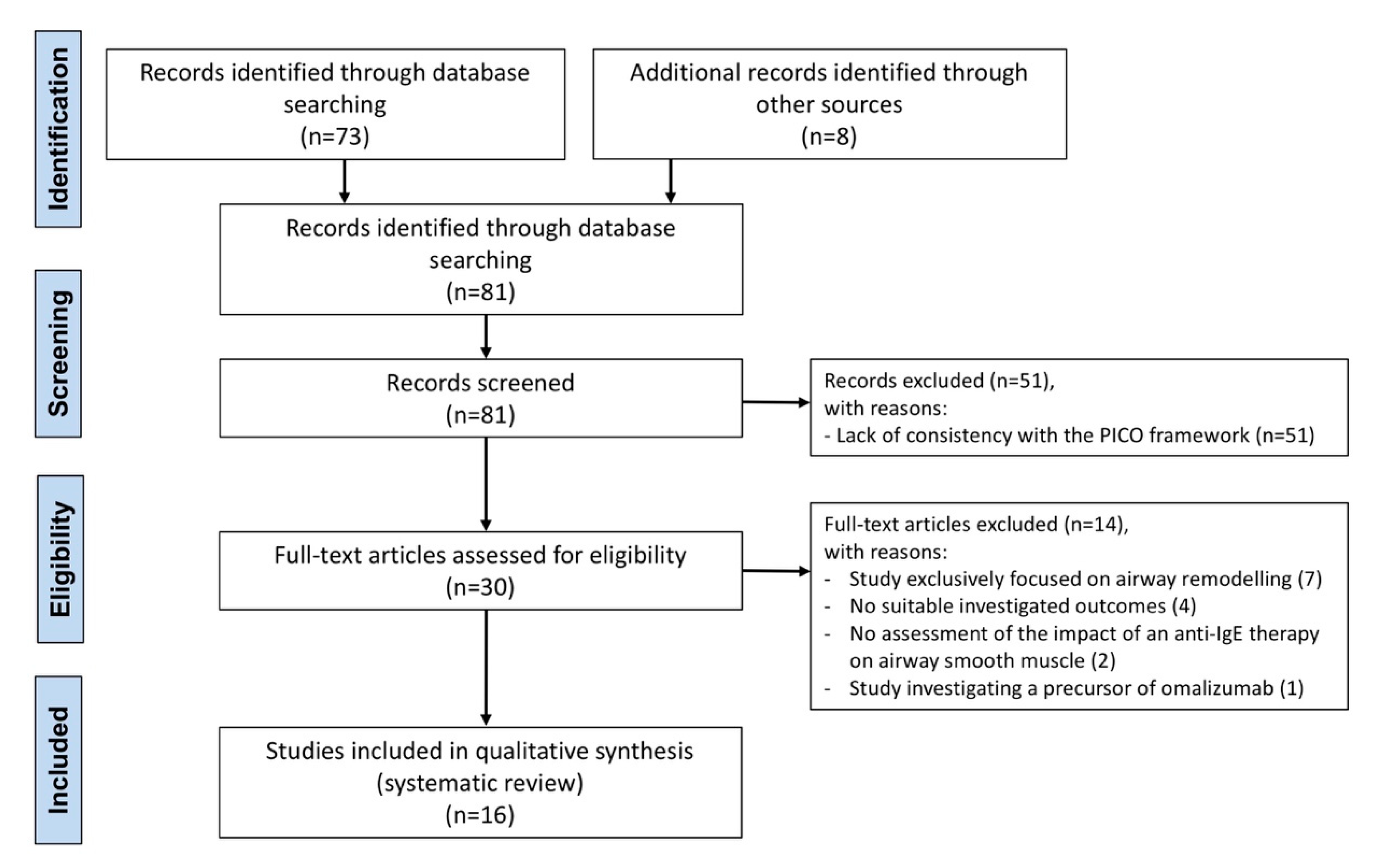
2.2. Search Strategy and Study Eligibility
2.3. Data Extraction
2.4. Endpoints
2.5. Strategy for Data Analysis
3. Results
3.1. Study Characteristics
3.2. Omalizumab
3.3. Mepolizumab
3.4. Benralizumab
3.5. Dupilumab
3.6. Tezepelumab
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pelaia, C.; Pelaia, G.; Crimi, C.; Longhini, F.; Lombardo, N.; Savino, R.; Sciacqua, A.; Vatrella, A. Biologics in severe asthma. Minerva Med. 2021. [Google Scholar] [CrossRef]
- Pelaia, C.; Crimi, C.; Benfante, A.; Caiaffa, M.F.; Calabrese, C.; Carpagnano, G.E.; Ciotta, D.; D’Amato, M.; Macchia, L.; Nolasco, S.; et al. Therapeutic Effects of Benralizumab Assessed in Patients with Severe Eosinophilic Asthma: Real-Life Evaluation Correlated with Allergic and Non-Allergic Phenotype Expression. J. Asthma Allergy 2021, 14, 163–173. [Google Scholar] [CrossRef]
- Kardas, G.; Kuna, P.; Panek, M. Biological Therapies of Severe Asthma and Their Possible Effects on Airway Remodeling. Front. Immunol. 2020, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Bossé, Y. Smooth muscle in abnormal airways. Curr. Opin. Physiol. 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Siddiqui, S.; Redhu, N.S.; Ojo, O.O.; Liu, B.; Irechukwu, N.; Billington, C.; Janssen, L.; Moir, L.M. Emerging airway smooth muscle targets to treat asthma. Pulm. Pharmacol. Ther. 2013, 26, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Rutting, S.; Xenaki, D.; Reddy, K.D.; Baraket, M.; Chapman, D.G.; King, G.G.; Oliver, B.G.; Tonga, K.O. Airway smooth muscle cells from severe asthma patients with fixed airflow obstruction are responsive to steroid and bronchodilator treatment in vitro. ERJ Open Res. 2021, 7, 00117–02021. [Google Scholar] [CrossRef]
- Ozier, A.; Allard, B.; Bara, I.; Girodet, P.-O.; Trian, T.; Marthan, R.; Berger, P. The Pivotal Role of Airway Smooth Muscle in Asthma Pathophysiology. J. Allergy 2011, 2011, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Quoc, Q.L.; Park, H.S. Biomarkers for severe Asthma: Lessons from longitudinal cohort studies. Allergy Asthma Immunol. Res. 2021, 13, 375–389. [Google Scholar] [CrossRef]
- Woodruff, P.G.; Modrek, B.; Choy, D.F.; Jia, G.; Abbas, A.R.; Ellwanger, A.; Arron, J.R.; Koth, L.L.; Fahy, J.V. T-helper Type 2–driven Inflammation Defines Major Subphenotypes of Asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 388. [Google Scholar] [CrossRef]
- Peters, M.C.; Mekonnen, Z.K.; Yuan, S.; Bhakta, N.R.; Woodruff, P.G.; Fahy, J.V. Measures of gene expression in sputum cells can identify T H2-high and TH2-low subtypes of asthma. J. Allergy Clin. Immunol. 2014, 133, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf (accessed on 5 July 2021).
- Licari, A.; Castagnoli, R.; Brambilla, I.; Marseglia, A.; Tosca, M.A.; Marseglia, G.L.; Ciprandi, G. New approaches for identifying and testing potential new anti-asthma agents. Expert Opin. Drug Discov. 2018, 13, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Henderson, I.; Caiazzo, E.; McSharry, C.; Guzik, T.J.; Maffia, P. Why do some asthma patients respond poorly to glucocorticoid therapy? Pharmacol. Res. 2020, 160, 105189. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.M. Severe asthma: Epidemiology, burden of illness, and heterogeneity. Allergy Asthma Proc. 2015, 36, 418–424. [Google Scholar] [CrossRef] [PubMed]
- McGregor, M.C.; Krings, J.G.; Nair, P.; Castro, M. Role of biologics in asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 433–445. [Google Scholar] [CrossRef]
- Watson, N.; Bodtke, K.; Coleman, R.A.; Dent, G.; Morton, B.E.; Rühlmann, E.; Magnussen, H.; Rabe, K.F. Role of IgE in hyperresponsiveness induced by passive sensitization of human airways. Am. J. Respir. Crit. Care Med. 1997, 155, 839–844. [Google Scholar] [CrossRef]
- Bryborn, M.; Adner, M.; Cardell, L.O. Interleukin-4 increases murine airway response to kinins, via up-regulation of bradykinin B1-receptors and altered signalling along mitogen-activated protein kinase pathways. Clin. Exp. Allergy 2004, 34, 1291–1298. [Google Scholar] [CrossRef]
- Manson, M.L.; Säfholm, J.; James, A.; Johnsson, A.K.; Bergman, P.; Al-Ameri, M.; Orre, A.C.; Kärrman-Mårdh, C.; Dahlén, S.E.; Adner, M. IL-13 and IL-4, but not IL-5 nor IL-17A, induce hyperresponsiveness in isolated human small airways. J. Allergy Clin. Immunol. 2020, 145, 808–817.e2. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, C.A.; Yang, R.; Greenfeder, S.; Egan, R.W.; Pauwels, R.A.; Hey, J.A. The IL-5 receptor on human bronchus selectively primes for hyperresponsiveness. J. Allergy Clin. Immunol. 2002, 109, 404–409. [Google Scholar] [CrossRef]
- Tliba, O.; Deshpande, D.; Chen, H.; Van Besien, C.; Kannan, M.; Panettieri, R.A.; Amrani, Y. IL-13 enhances agonist-evoked calcium signals and contractile responses in airway smooth muscle. Br. J. Pharmacol. 2003, 140, 1159–1162. [Google Scholar] [CrossRef] [Green Version]
- Chiba, Y.; Nakazawa, S.; Todoroki, M.; Shinozaki, K.; Sakai, H.; Misawa, M. Interleukin-13 augments bronchial smooth muscle contractility with an Up-regulation of RhoA protein. Am. J. Respir. Cell Mol. Biol. 2009, 40, 159–167. [Google Scholar] [CrossRef]
- Smelter, D.F.; Sathish, V.; Thompson, M.A.; Pabelick, C.M.; Vassallo, R.; Prakash, Y.S. Thymic Stromal Lymphopoietin in Cigarette Smoke-Exposed Human Airway Smooth Muscle. J. Immunol. 2010, 185, 3035–3040. [Google Scholar] [CrossRef]
- Zhou, B.; Comeau, M.R.; De Smedt, T.; Liggitt, H.D.; Dahl, M.E.; Lewis, D.B.; Gyarmati, D.; Aye, T.; Campbell, D.J.; Ziegler, S.F. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005, 6, 1047–1053. [Google Scholar] [CrossRef]
- Rottem, M. Omalizumab reduces corticosteroid use in patients with severe allergic asthma: Real-life experience in israel. J. Asthma 2012, 49, 78–82. [Google Scholar] [CrossRef]
- Calzetta, L.; Ritondo, B.L.; Matera, M.G.; Facciolo, F.; Rogliani, P. Targeting IL-5 pathway against airway hyperresponsiveness: A challenge between benralizumab and mepolizumab. Br. J. Pharmacol. 2020, 177, 4750–4765. [Google Scholar] [CrossRef]
- Walsh, G.M. Reslizumab, a humanized anti-IL-5 mAb for the treatment of eosinophil-mediated infammatory conditions. Curr. Opin. Mol. Ther. 2009, 11, 329–336. [Google Scholar] [PubMed]
- Moran, A.; Pavord, I.D. Anti-IL-4/IL-13 for the treatment of asthma: The story so far. Expert Opin. Biol. Ther. 2020, 20, 283–294. [Google Scholar] [CrossRef]
- FDA Grants Priority Review for Investigative Monoclonal Antibody Tezepelumab. Available online: https://www.astrazeneca.com/media-centre/press-releases/2021/tezepelumab-granted-fda-priority-review-for-asthma.html (accessed on 5 July 2021).
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inf. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samitas, K.; Delimpoura, V.; Zervas, E.; Gaga, M. Anti-IgE treatment, airway inflammation and remodelling in severe allergic asthma: Current knowledge and future perspectives. Eur. Respir. Rev. 2015, 24, 594–601. [Google Scholar] [CrossRef]
- Pedder, H.; Sarri, G.; Keeney, E.; Nunes, V.; Dias, S. Data extraction for complex meta-analysis (DECiMAL) guide. Syst. Rev. 2016, 5, 212. [Google Scholar] [CrossRef] [Green Version]
- Sverrild, A.; Hansen, S.; Hvidtfeldt, M.; Clausson, C.-M.; Cozzolino, O.; Cerps, S.; Uller, L.; Backer, V.; Erjefält, J.; Porsbjerg, C. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM). Eur. Respir. J. 2021, 2101296. [Google Scholar] [CrossRef]
- Haldar, P.; Brightling, C.E.; Hargadon, B.; Gupta, S.; Monteiro, W.; Sousa, A.; Marshall, R.P.; Bradding, P.; Green, R.H.; Wardlaw, A.J.; et al. Mepolizumab and Exacerbations of Refractory Eosinophilic Asthma. N. Engl. J. Med. 2009, 360, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Prieto, L.; Gutiérrez, V.; Colás, C.; Tabar, A.; Pérez-Francés, C.; Bruno, L.; Uixera, S. Effect of omalizumab on adenosine 5′-monophosphate responsiveness in subjects with allergic asthma. Int. Arch. Allergy Immunol. 2006, 139, 122–131. [Google Scholar] [CrossRef]
- Djukanović, R.; Wilson, S.J.; Kraft, M.; Jarjour, N.N.; Steel, M.; Chung, K.F.; Bao, W.; Fowler-Taylor, A.; Matthews, J.; Busse, W.W.; et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 583–893. [Google Scholar] [CrossRef]
- Flood-Page, P.T.; Menzies-Gow, A.N.; Kay, A.B.; Robinson, D.S. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am. J. Respir. Crit. Care Med. 2003, 167, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noga, O.; Hanf, G.; Kunkel, G. Immunological and Clinical Changes in Allergic Asthmatics following Treatment with Omalizumab. Int. Arch. Allergy Immunol. 2003, 131, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Leckie, M.J.; Ten Brinke, A.; Khan, J.; Diamant, Z.; O’xonnor, B.J.; Walls, C.M.; Mathur, A.K.; Cowley, H.C.; Chung, K.F.; Djukanovic, R.; et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000, 356, 2144–2148. [Google Scholar] [CrossRef]
- Boulet, L.P.; Chapman, K.R.; Côté, J.; Kalra, S.; Bhagat, R.; Swystun, V.A.; Laviolette, M.; Cleland, L.D.; Deschesnes, F.; Su, J.Q.; et al. Inhibitory effects of an anti-IgE antibody E25 on allergen-induced early asthmatic response. Am. J. Respir. Crit. Care Med. 1997, 155, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V.; Fleming, H.E.; Wong, H.H.; Liu, J.T.; Su, J.Q.; Reimann, J.; Fick, R.B.; Boushey, H.A. The effect of an anti-IgE monoclonal antibody on the early- and late- phase responses to allergen inhalation in asthmatic subjects. Am. J. Respir. Crit. Care Med. 1997, 155, 1828–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diver, S.; Khalfaoui, L.; Emson, C.; Wenzel, S.E.; Menzies-Gow, A.; Wechsler, M.E.; Johnston, J.; Molfino, N.; Parnes, J.R.; Megally, A.; et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021. [Google Scholar] [CrossRef]
- Kang, J.Y.; Kim, J.W.; Kim, J.S.; Kim, S.J.; Lee, S.H.; Kwon, S.S.; Kim, Y.K.; Moon, H.S.; Song, J.S.; Park, S.H.; et al. Inhibitory effects of anti-immunoglobulin E antibodies on airway remodeling in a murine model of chronic asthma. J. Asthma 2010, 47, 374–380. [Google Scholar] [CrossRef]
- Roth, M.; Tamm, M. The effects of omalizumab on IgE-induced cytokine synthesis by asthmatic airway smooth muscle cells. Ann. Allergy Asthma Immunol. 2010, 104, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.; Scotto-Gomez, E.; Molimard, M.; Marthan, R.; Le Gros, V.; Tunon-de-Lara, J.M. Omalizumab decreases nonspecific airway hyperresponsiveness in vitro. Allergy Eur. J. Allergy Clin. Immunol. 2007, 62, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, T.; Niimi, A.; Matsumoto, H.; Ito, I.; Oguma, T.; Otsuka, K.; Takeda, T.; Nakaji, H.; Inoue, H.; Iwata, T.; et al. Comprehensive efficacy of omalizumab for severe refractory asthma: A time-series observational study. Ann. Allergy Asthma Immunol. 2014, 113, 470–475.e2. [Google Scholar] [CrossRef]
- Solèr, M.; Matz, J.; Townley, R.; Buhl, R.; O’Brien, J.; Fox, H.; Thirlwell, J.; Gupta, N.; Della Cioppa, G. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur. Respir. J. 2001, 18, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buhl, R.; Solèr, M.; Matz, J.; Townley, R.; O’Brien, J.; Noga, O.; Champain, K.; Fox, H.; Thirlwell, J.; Della Cioppa, G. Omalizumab provides long-term control in patients with moderate-to-severe allergic asthma. Eur. Respir. J. 2002, 20, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzetta, L.; Matera, M.G.; Coppola, A.; Rogliani, P. Prospects for severe asthma treatment. Curr. Opin. Pharmacol. 2021, 56, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tumas, D.B.; Chan, B.; Werther, W.; Wrin, T.; Vennari, J.; Desjardin, N.; Shields, R.L.; Jardieu, P. Anti-IgE efficacy in murine asthma models is dependent on the method of allergen sensitization. J. Allergy Clin. Immunol. 2001, 107, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Laporte, J.C.; Moore, P.E.; Baraldo, S.; Jouvin, M.H.; Church, T.L.; Schwartzman, I.N.; Panettieri, J.; Kinet, J.P.; Shore, S.A. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am. J. Respir. Crit. Care Med. 2001, 164, 141–148. [Google Scholar] [CrossRef]
- Gounni, A.S.; Wellemans, V.; Yang, J.; Bellesort, F.; Kassiri, K.; Gangloff, S.; Guenounou, M.; Halayko, A.J.; Hamid, Q.; Lamkhioued, B. Human Airway Smooth Muscle Cells Express the High Affinity Receptor for IgE (FcεRI): A Critical Role of FcεRI in Human Airway Smooth Muscle Cell Function. J. Immunol. 2005, 175, 2613–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chachi, L.; Diver, S.; Kaul, H.; Rebelatto, M.C.; Boutrin, A.; Nisa, P.; Newbold, P.; Brightling, C. Computational modelling prediction and clinical validation of impact of benralizumab on airway smooth muscle mass in asthma. Eur. Respir. J. 2019, 54, 1900930. [Google Scholar] [CrossRef]
- Walker, C.; Kaegi, M.K.; Braun, P.; Blaser, K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J. Allergy Clin. Immunol. 1991, 88, 935–942. [Google Scholar] [CrossRef]
- Smith, S.G.; Chen, R.; Kjarsgaard, M.; Huang, C.; Oliveria, J.P.; O’Byrne, P.M.; Gauvreau, G.M.; Boulet, L.P.; Lemiere, C.; Martin, J.; et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J. Allergy Clin. Immunol. 2016, 137, 75–86.e8. [Google Scholar] [CrossRef] [Green Version]
- Roufosse, F. Targeting the Interleukin-5 Pathway for Treatment of Eosinophilic Conditions Other than Asthma. Front. Med. 2018, 5, 49. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.K.; Hu, S.; Cheung, P.F.Y.; Lam, C.W.K. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: Implications in allergic inflammation. Am. J. Respir. Cell Mol. Biol. 2010, 43, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Dubois, G.R.; Schweizer, R.C.; Versluis, C.; Bruijnzeel-Koomen, C.A.F.M.; Bruijnzeel, P.L.B. Human eosinophils constitutively express a functional interleukin-4 receptor: Interleukin-4-induced priming of chemotactic responses and induction of PI-3 kinase activity. Am. J. Respir. Cell Mol. Biol. 1998, 19, 691–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myrtek, D.; Knoll, M.; Matthiesen, T.; Krause, S.; Lohrmann, J.; Schillinger, D.; Idzko, M.; Virchow, J.C.; Friedrich, K.; Luttmann, W. Expression of interleukin-13 receptor α 1-subunit on peripheral blood eosinophils is regelated by cytokines. Immunology 2004, 112, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Gounni, A.S.; Lamkhioued, B.; Ochial, K.; Tanaka, Y.; Delaporte, E.; Capron, A.; Kinet, J.P.; Capron, M. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature 1994, 367, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, C.; Roy, S.; Li, Z.; Khanna, M.; Jackson, R.J. IL-4 and IL-13 Receptors. In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 2549–2557. ISBN 978-3-319-67199-4. [Google Scholar]
- Ingram, N.; Northwood, E.L.; Perry, S.L.; Marston, G.; Snowden, H.; Taylor, J.C.; Scott, N.; Bishop, D.T.; Coletta, P.L.; Hull, M.A. Reduced type II interleukin-4 receptor signalling drives initiation, but not progression, of colorectal carcinogenesis: Evidence from transgenic mouse models and human case–control epidemiological observations. Carcinogenesis 2013, 34, 2341. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F. The role of thymic stromal lymphopoietin (TSLP) in allergic disorders. Curr. Opin. Immunol. 2010, 22, 795–799. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.H.; Zuo, Y.G. Thymic Stromal Lymphopoietin in Cutaneous Immune-Mediated Diseases. Front. Immunol. 2021, 12, 698522. [Google Scholar] [CrossRef]
- Hui, C.C.K.; Mcnagny, K.M.; Denburg, J.A.; Siracusa, M.C. In situ hematopoiesis: A regulator of TH2 cytokine-mediated immunity and inflammation at mucosal surfaces. Mucosal Immunol. 2015, 8, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.M.; Maric, I.; Shukla, J.; Brown, M.; Santos, C.; Simakova, O.; Khoury, P.; Fay, M.P.; Kozhich, A.; Kolbeck, R.; et al. Interleukin-5 receptor α levels in patients with marked eosinophilia or mastocytosis. J. Allergy Clin. Immunol. 2011, 128, 1086. [Google Scholar] [CrossRef] [Green Version]
- Eberle, J.U.; Radtke, D.; Nimmerjahn, F.; Voehringer, D. Eosinophils Mediate Basophil-Dependent Allergic Skin Inflammation in Mice. J. Investig. Dermatol. 2019, 139, 1957–1965.e2. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lester, P.; Builder, S.; Shire, S.J. Characterization of Complex Formation by Humanized Anti-IgE Monoclonal Antibody and Monoclonal Human IgE. Biochemistry 1995, 34, 10474–10482. [Google Scholar] [CrossRef]
- Kraft, S.; Kinet, J.P. New developments in FcεRI regulation, function and inhibition. Nat. Rev. Immunol. 2007, 7, 365–378. [Google Scholar] [CrossRef]
- Ortega, E.; Lara, M.; Lee, I.; Santana, C.; Martinez, A.M.; Pfeiffer, J.R.; Lee, R.J.; Wilson, B.S.; Oliver, J.M. Lyn Dissociation from Phosphorylated FcεRI Subunits: A New Regulatory Step in the FcεRI Signaling Cascade Revealed by Studies of FcεRI Dimer Signaling Activity. J. Immunol. 1999, 162, 176–185. [Google Scholar]
- Lara, M.; Ortega, E.; Pecht, I.; Pfeiffer, J.R.; Martinez, A.M.; Lee, R.J.; Surviladze, Z.; Wilson, B.S.; Oliver, J.M. Overcoming the Signaling Defect of Lyn-Sequestering, Signal-Curtailing FcεRI Dimers: Aggregated Dimers Can Dissociate from Lyn and Form Signaling Complexes with Syk. J. Immunol. 2001, 167, 4329–4337. [Google Scholar] [CrossRef] [Green Version]
- Hall, I.P. Second messenger, ion channels and pharmacology of aiway smooth muscle. Eur. Respir. J. 2000, 15, 1120–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berridge, M.J. Inositol trisphosphate and calcium signalling. Nature 1993, 361, 315–325. [Google Scholar] [CrossRef]
- Amrani, Y.; Panettieri, R.A. Airway smooth muscle: Contraction and beyond. Int. J. Biochem. Cell Biol. 2003, 35, 272–276. [Google Scholar] [CrossRef]
- Balhara, J.; Redhu, N.S.; Shan, L.; Gounni, A.S. IgE Regulates the Expression of smMLCK in Human Airway Smooth Muscle Cells. PLoS ONE 2014, 9, e93946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, S.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B.; et al. Dupilumab in Persistent Asthma with Elevated Eosinophil Levels. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Todoroki, M.; Misawa, M. Interleukin-4 upregulates RhoA protein via an activation of STAT6 in cultured human bronchial smooth muscle cells. Pharmacol. Res. 2010, 61, 188–192. [Google Scholar] [CrossRef]
- Janssen, L.J.; Killian, K. Airway smooth muscle as a target of asthma therapy: History and new directions. Respir. Res. 2006, 7, 123. [Google Scholar] [CrossRef] [Green Version]
- Nesmith, A.P.; Agarwal, A.; McCain, M.L.; Parker, K.K. Human airway musculature on a chip: An in vitro model of allergic asthmatic bronchoconstriction and bronchodilation. Lab Chip 2014, 14, 3925–3936. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, D.A.; Walseth, T.F.; Panettieri, R.A.; Kannan, M.S. CD38/cyclic ADP-ribose-mediated Ca2+ signaling contributes to airway smooth muscle hyper-responsiveness. FASEB J. 2003, 17, 452–454. [Google Scholar] [CrossRef]
- Deshpande, D.A.; Dogan, S.; Walseth, T.F.; Miller, S.M.; Amrani, Y.; Panettieri, R.A.; Kannan, M.S. Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: Role of CD38/cyclic adenosine diphosphate ribose pathway. Am. J. Respir. Cell Mol. Biol. 2004, 31, 36–42. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; O’Byrne, P.M.; Boulet, L.-P.; Wang, Y.; Cockcroft, D.; Bigler, J.; FitzGerald, J.M.; Boedigheimer, M.; Davis, B.E.; Dias, C.; et al. Effects of an Anti-TSLP Antibody on Allergen-Induced Asthmatic Responses. N. Engl. J. Med. 2014, 370, 2102–2110. [Google Scholar] [CrossRef]
- Ying, S.; O’Connor, B.; Ratoff, J.; Meng, Q.; Mallett, K.; Cousins, D.; Robinson, D.; Zhang, G.; Zhao, J.; Lee, T.H.; et al. Thymic Stromal Lymphopoietin Expression Is Increased in Asthmatic Airways and Correlates with Expression of Th2-Attracting Chemokines and Disease Severity. J. Immunol. 2005, 174, 8183–8190. [Google Scholar] [CrossRef] [PubMed]
- Shikotra, A.; Choy, D.F.; Ohri, C.M.; Doran, E.; Butler, C.; Hargadon, B.; Shelley, M.; Abbas, A.R.; Austin, C.D.; Jackman, J.; et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J. Allergy Clin. Immunol. 2012, 129, 104–111.e1-9. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.G.; Lebold, K.M.; Roth-Carter, Q.R.; Pincus, A.B.; Blum, E.D.; Proskocil, B.J.; Jacoby, D.B.; Fryer, A.D.; Nie, Z. Eosinophil and airway nerve interactions in asthma. J. Leukoc. Biol. 2018, 104, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Puxeddu, E.; Ora, J.; Facciolo, F.; Rogliani, P.; Matera, M.G. Pharmacological characterisation of the interaction between glycopyrronium bromide and indacaterol fumarate in human isolated bronchi, small airways and bronchial epithelial cells. Respir. Res. 2016, 17, 70. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.W.; Ndukwu, I.M.; Ikeda, K.; Arbetter, K.; Leff, A.R. Effect of immune sensitization on stimulated ACh release from trachealis muscle in vitro. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1993, 265, L13–L18. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, M.; Miura, M.; Tomaki, M.; Oyake, T.; Kageyama, N.; Ikarashi, Y.; Maruyama, Y.; Shirato, K. Incubation with IgE increases cholinergic neurotransmission in human airways in vitro. Am. J. Respir. Crit. Care Med. 1996, 154, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Billington, C.K.; Penn, R.B. m3 Muscarinic Acetylcholine Receptor Regulation in the Airway. Am. J. Respir. Cell Mol. Biol. 2012, 26, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Ohtawa, J. Effects of adding omalizumab, an anti-immunoglobulin E antibody, on airway wall thickening in asthma. Respiration 2012, 83, 520–528. [Google Scholar] [CrossRef]
- Roth, M.; Zhong, J.; Zumkeller, C.; S’ng, C.T.; Goulet, S.; Tamm, M. The Role of IgE-Receptors in IgE-Dependent Airway Smooth Muscle Cell Remodelling. PLoS ONE 2013, 8, e56015. [Google Scholar] [CrossRef]
- Roth, M.; Zhao, F.; Zhong, J.; Lardinois, D.; Tamm, M. Serum IgE induced airway smooth muscle cell remodeling is independent of allergens and is prevented by omalizumab. PLoS ONE 2015, 10, e0136549. [Google Scholar]
- Mauri, P.; Riccio, A.M.; Rossi, R.; Di Silvestre, D.; Benazzi, L.; De Ferrari, L.; Negro, R.W.D.; Holgate, S.T.; Canonica, G.W. Proteomics of bronchial biopsies: Galectin-3 as a predictive biomarker of airway remodelling modulation in omalizumab-treated severe asthma patients. Immunol. Lett. 2014, 162, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.H.; Weng, C.M.; Huang, T.T.; Lee, M.J.; Lo, C.Y.; Chen, M.C.; Chou, C.L.; Kuo, H.P. Anti-IgE therapy inhibits chemotaxis, proliferation and transformation of circulating fibrocytes in patients with severe allergic asthma. Respirology 2021, 26, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.M.; Mauri, P.; De Ferrari, L.; Rossi, R.; Di Silvestre, D.; Benazzi, L.; Chiappori, A.; Dal Negro, R.W.; Micheletto, C.; Canonica, G.W. Galectin-3: An early predictive biomarker of modulation of airway remodeling in patients with severe asthma treated with omalizumab for 36 months. Clin. Transl. Allergy 2017, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agusti, A.; Bel, E.; Thomas, M.; Vogelmeier, C.; Brusselle, G.; Holgate, S.; Humbert, M.; Jones, P.; Gibson, P.G.; Vestbo, J.; et al. Treatable traits: Toward precision medicine of chronic airway diseases. Eur. Respir. J. 2016, 47, 410–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bel, E.H.; ten Brinke, A. New Anti-Eosinophil Drugs for Asthma and COPD: Targeting the Trait! Chest 2017, 152, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.G.; Irvin, C.G. Mechanisms of Airway Hyperresponsiveness in Asthma: The Past, Present and Yet to Come. Clin. Exp. Allergy 2015, 45, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelaia, C.; Calabrese, C.; Terracciano, R.; de Blasio, F.; Vatrella, A.; Pelaia, G. Omalizumab, the first available antibody for biological treatment of severe asthma: More than a decade of real-life effectiveness. Ther. Adv. Respir. Dis. 2018, 12, 1753466618810192. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study, Year, and Reference | Study Characteristics | Treatment Duration | Type of Cells, Animals, Donors, or Analyzed Patients | Number of Animals, Donors, or Patients | Drugs, Doses and Regimen of Administration | Mean Age (Years) | Male (%) | Current Smokers (%) | Smoking History (Pack-Years) | Post-Bronchodilator FEV1 (% Predicted) | Investigated Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diver et al., 2021 (CASCADE trial) [42] | RCT | Planned 28 wks | Patients with uncontrolled moderate to severe asthma | 116 | Tezepelumab (210 mg, every 4 wks; SC injection) | 50.4 | 44.0 | 0.0 | NA | 69.1 | AHR to mannitol |
| Sverrild et al., 2021 (UPSTREAM trial) [33] | RCT | 12 wks | Patients with uncontrolled asthma | 40 | Tezepelumab (700 mg, every 4 wks; IV infusion) | 41.0 | 42.0 | 0.0 | NA | 88.7 | AHR to mannitol |
| Calzetta et al., 2020 [25] | Ex vivo, prospective, randomized, negative- and positive- controlled, blind, parallel-group study | Overnight | Passively sensitized subsegmental bronchi (4–6 mm) from patients undergoing lobectomy for lung cancer (with normal serum IgE levels < 100 IU/mL and normal preoperative lung function parameters) | 16 | Benralizumab (1 μg/mL–100 μg/mL) vs. mepolizumab (1 μg/mL–100 μg/mL) | 50.0 | 50.0 | 25.0 | 24.4 | 93.1 | AHR to His, EFS, and QS and assessment of treatment effect on cAMP levels |
| Manson et al., 2020 [18] | Ex vivo and in vitro study | 1 day | Human small airways and primary ASMCs | 33 | Dupilumab (1 μM) | 69.0 | 27.0 | 36.4 | NA | NA | AHR to His and EFS |
| Tajiri et al., 2014 [46] | Prospective, single-arm, observational study | 48 wks | Patients with severe refractory asthma | 31 | OMA (every 2–4 wks, dosing based on body weight and baseline total serum IgE; SC injection) | 55.0 | 32.3 | 0.0 | ≤10 | 93.5 | AHR to methacholine |
| Kang et al., 2010 [43] | In vivo study | 3 months | BALB/c mice challenged with OVA (murine model of chronic asthma) | 10–15 per group | Rat anti-mouse IgE mAb clone R35–92 (100 μg/200 μL in normal saline), once a month from day 38; IV injection) | 8–10 wks | 0.0 | NA | NA | NA | AHR to methacholine |
| Roth et al., 2010 [44] | In vitro study | 24 h | Primary ASMCs isolated from allergic asthma donors | 6 | OMA (0.1, 0.5, 1.0 μg/mL) | 33.3 | 66.7 | NA | NA | 69.0 | IL-4, IL-6, IL-8, and TNF-α secretion and synthesis by ASMCs and IgE receptor expression in ASMCs |
| Haldar et al., 2009 [34] | RCT | 1 year | Patients with refractory eosinophilic asthma and a history of recurrent exacerbations | 61 | Mepolizumab (750 mg every month; IV infusion) vs. PBO | 49.0 | 52.5 | 0.0 | NA | 77.9 | AHR to methacholine |
| Berger et al., 2007 [45] | Ex vivo study | 1 h | Passively sensitized medium bronchi and small airways obtained from non-atopic and non-asthmatic patients | 10 | OMA (60, 120, 180 μg/mL) | 64.4 | 100.0 | NA | 24.5 | 86.9 | ASM contractile responses to His and Dermatophagoides pteronyssinus |
| Prieto et al., 2006 [35] | RCT | 12 wks | Patients with mild to moderate allergic asthma | 34 | OMA (150–300 mg every 4 wks or 225–375 mg every 2 wks; SC injection) vs. PBO | 31.0 | 47.1 | 0.0 | NA | 100.7 | AHR to AMP |
| Djukanovic et al., 2004 [36] | RCT | 4 months | Patients with mild to moderate asthma | 45 | OMA (150–300 mg every 4 wks or 225–375 mg every 2 wks; SC injection) vs. PBO | 26.0 | 46.0 | 0.0 | NA | 85.0 | AHR to methacholine |
| Flood-Page et al., 2003 [37] | RCT on | 20 wks | Patients with mild asthma | 24 | Mepolizumab (750 mg, 3 doses; IV infusion) vs. PBO | 30.5 | 70.8 | 0.0 | NA | 83.5 | AHR to His |
| Noga et al., 2003 [38] | Sub-study conducted as part of a large multicentre RCT [47,48] | 16 wks | Patients with moderate to severe allergic asthma | 35 | OMA (at least 0.016 mg/kg/IgE IU/mL, every 4 wks; SC injection) vs. PBO | 54.3 | 36.5 | NA | NA | 79.5 | AHR to acetylcholine |
| Leckie et al., 2000 [39] | RCT | 1 day | Patients with mild allergic asthma | 24 | SB-240563 (2.5 or 10.0 mg/kg, single dose; IV infusion) vs. PBO | 27.9 | 100.0 | 0.0 | NA | 88.4 | AHR to His |
| Boulet et al., 1997 [40] | RCT | 10 wks | Patients with mild allergic asthma | 20 | rhuMAb-E25 (1.0 mg/kg; IV infusion) vs. PBO | 27.0 | 60.0 | 0.0 | ≤10 | 92.2 | AHR to methacholine |
| Fahy et al., 1997 [41] | RCT | 9 wks | Patients with mild allergic asthma | 18 | rhuMAb-E25 (0.5 mg/kg; IV infusion) vs. PBO | 31.5 | NA | NA | NA | 94.5 | AHR to methacholine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzetta, L.; Aiello, M.; Frizzelli, A.; Bertorelli, G.; Ritondo, B.L.; Rogliani, P.; Chetta, A. The Impact of Monoclonal Antibodies on Airway Smooth Muscle Contractility in Asthma: A Systematic Review. Biomedicines 2021, 9, 1281. https://doi.org/10.3390/biomedicines9091281
Calzetta L, Aiello M, Frizzelli A, Bertorelli G, Ritondo BL, Rogliani P, Chetta A. The Impact of Monoclonal Antibodies on Airway Smooth Muscle Contractility in Asthma: A Systematic Review. Biomedicines. 2021; 9(9):1281. https://doi.org/10.3390/biomedicines9091281
Chicago/Turabian StyleCalzetta, Luigino, Marina Aiello, Annalisa Frizzelli, Giuseppina Bertorelli, Beatrice Ludovica Ritondo, Paola Rogliani, and Alfredo Chetta. 2021. "The Impact of Monoclonal Antibodies on Airway Smooth Muscle Contractility in Asthma: A Systematic Review" Biomedicines 9, no. 9: 1281. https://doi.org/10.3390/biomedicines9091281
APA StyleCalzetta, L., Aiello, M., Frizzelli, A., Bertorelli, G., Ritondo, B. L., Rogliani, P., & Chetta, A. (2021). The Impact of Monoclonal Antibodies on Airway Smooth Muscle Contractility in Asthma: A Systematic Review. Biomedicines, 9(9), 1281. https://doi.org/10.3390/biomedicines9091281







