A Novel Competitive Binding Screening Assay Reveals Sennoside B as a Potent Natural Product Inhibitor of TNF-α
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Cell Lines
2.2. Method Development
2.2.1. Competitive Binding Screening Assay Using Analytical SEC
2.2.2. LC–Tandem MS Analysis
2.3. Cell Viability Assay
2.4. TNF-α Dependent L929 Cytotoxicity Assay
2.5. Western Blot Analysis
2.6. Measurement of PGD2 and PGE2 by LC-high Resolution MS
2.7. Molecular Docking and Dynamic Simulations
2.8. Statistical Analysis
3. Results
3.1. Development of Competitive Binding Screening Assay
3.2. Natural Product Compound Library Screening
3.3. Sennoside B Inhibits TNF-α-Induced L929 Cell Death
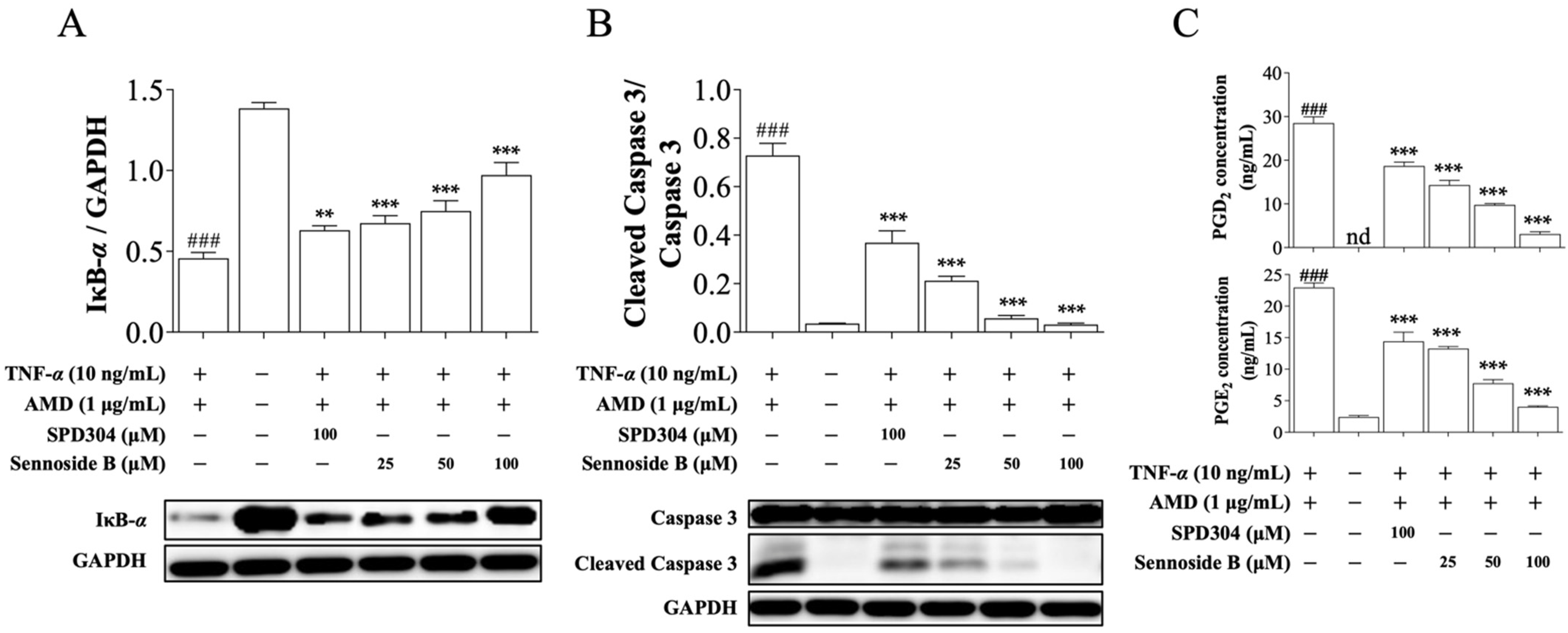
3.4. Sennoside B Blocks TNF-α-Induced Signaling Pathways in Mouse L929 Cells
3.5. Sennoside B Blocks Degradation of IκB-α in HeLa Cells
3.6. Sennoside B-TNF-α Binding Interactions and Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Tracey, D.; Klareskog, L.; Sasso, E.H.; Salfeld, J.G.; Tak, P.P. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol. Ther. 2008, 117, 244–279. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Feldmann, M. Anti-TNF biologic agents: Still the therapy of choice for rheumatoid arthritis. Nat. Rev. Rheumatol. 2009, 5, 578–582. [Google Scholar] [CrossRef]
- Levin, A.D.; Wildenberg, M.E.; van den Brink, G.R. Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease. J. Crohns. Colitis. 2016, 10, 989–997. [Google Scholar] [CrossRef] [Green Version]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef]
- O’Connell, J.; Porter, J.; Kroeplien, B.; Norman, T.; Rapecki, S.; Davis, R.; McMillan, D.; Arakaki, T.; Burgin, A.; Fox Iii, D.; et al. Small molecules that inhibit TNF signalling by stabilising an asymmetric form of the trimer. Nat. Commun. 2019, 10, 5795. [Google Scholar] [CrossRef]
- Rider, P.; Carmi, Y.; Cohen, I. Biologics for Targeting Inflammatory Cytokines, Clinical Uses, and Limitations. Int. J. Cell Biol. 2016, 2016, 9259646. [Google Scholar] [CrossRef] [Green Version]
- Alzani, R.; Cozzi, E.; Corti, A.; Temponi, M.; Trizio, D.; Gigli, M.; Rizzo, V. Mechanism of suramin-induced deoligomerization of tumor necrosis factor alpha. Biochemistry 1995, 34, 6344–6350. [Google Scholar] [CrossRef]
- He, M.M.; Smith, A.S.; Oslob, J.D.; Flanagan, W.M.; Braisted, A.C.; Whitty, A.; Cancilla, M.T.; Wang, J.; Lugovskoy, A.A.; Yoburn, J.C.; et al. Small-molecule inhibition of TNF-alpha. Science 2005, 310, 1022–1025. [Google Scholar] [CrossRef]
- Chan, D.S.; Lee, H.M.; Yang, F.; Che, C.M.; Wong, C.C.; Abagyan, R.; Leung, C.H.; Ma, D.L. Structure-based discovery of natural-product-like TNF-alpha inhibitors. Angew. Chem. Int. Ed. Engl 2010, 49, 2860–2864. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Gong, H.; Zhu, H.; Ji, Q.; Su, P.; Liu, P.; Cao, S.; Yao, J.; Jiang, L.; Han, M.; et al. A novel small-molecule tumor necrosis factor alpha inhibitor attenuates inflammation in a hepatitis mouse model. J. Biol. Chem. 2014, 289, 12457–12466. [Google Scholar] [CrossRef] [Green Version]
- Luzi, S.; Kondo, Y.; Bernard, E.; Stadler, L.K.; Vaysburd, M.; Winter, G.; Holliger, P. Subunit disassembly and inhibition of TNFalpha by a semi-synthetic bicyclic peptide. Protein Eng. Des. Sel. 2015, 28, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Li, Y.H.; Lv, D.Y.; Chen, X.F.; Chen, L.D.; Zhu, Z.Y.; Chai, Y.F.; Zhang, J.P. Identification of a ligand for tumor necrosis factor receptor from Chinese herbs by combination of surface plasmon resonance biosensor and UPLC-MS. Anal. Bioanal. Chem. 2016, 408, 5359–5367. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Feng, Z.; Wang, Y.; Ma, S.; Hu, Z.; Yang, P.; Chai, Y.; Xie, X. Discovery of Novel Ligands for TNF-alpha and TNF Receptor-1 through Structure-Based Virtual Screening and Biological Assay. J. Chem. Inf. Model. 2017, 57, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- McGeary, R.P.; Bennett, A.J.; Tran, Q.B.; Cosgrove, K.L.; Ross, B.P. Suramin: Clinical uses and structure-activity relationships. Mini. Rev. Med. Chem. 2008, 8, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yost, G.S. Metabolic activation of a novel 3-substituted indole-containing TNF-alpha inhibitor: Dehydrogenation and inactivation of CYP3A4. Chem. Res. Toxicol. 2008, 21, 374–385. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Rubin, B.Y.; Smith, L.J.; Hellermann, G.R.; Lunn, R.M.; Richardson, N.K.; Anderson, S.L. Correlation between the anticellular and DNA fragmenting activities of tumor necrosis factor. Cancer Res. 1988, 48, 6006–6010. [Google Scholar]
- Trost, L.C.; Lemasters, J.J. A cytotoxicity assay for tumor necrosis factor employing a multiwell fluorescence scanner. Anal. Biochem. 1994, 220, 149–153. [Google Scholar] [CrossRef]
- Nesbitt, A.; Fossati, G.; Bergin, M.; Stephens, P.; Stephens, S.; Foulkes, R.; Brown, D.; Robinson, M.; Bourne, T. Mechanism of action of certolizumab pegol (CDP870): In vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm. Bowel. Dis. 2007, 13, 1323–1332. [Google Scholar] [CrossRef]
- Shin, J.S.; Peng, L.; Kang, K.; Choi, Y. Direct analysis of prostaglandin-E2 and -D2 produced in an inflammatory cell reaction and its application for activity screening and potency evaluation using turbulent flow chromatography liquid chromatography-high resolution mass spectrometry. J. Chromatogr. A 2016, 1463, 128–135. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Gohlke, H.; Hendlich, M.; Klebe, G. Knowledge-based scoring function to predict protein-ligand interactions. J. Mol. Biol. 2000, 295, 337–356. [Google Scholar] [CrossRef]
- Pronk, S.; Pall, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- Schuttelkopf, A.W.; van Aalten, D.M. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D 2004, 60, 1355–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphreys, D.T.; Wilson, M.R. Modes of L929 cell death induced by TNF-alpha and other cytotoxic agents. Cytokine 1999, 11, 773–782. [Google Scholar] [CrossRef]
- Faustman, D.; Davis, M. TNF receptor 2 pathway: Drug target for autoimmune diseases. Nat. Rev. Drug Discov. 2010, 9, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Van Herreweghe, F.; Festjens, N.; Declercq, W.; Vandenabeele, P. Tumor necrosis factor-mediated cell death: To break or to burst, that’s the question. Cell Mol. Life Sci. CMLS 2010, 67, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Oliveira, M.I.; Sette, L.; Almeida, C.R.; Oliveira, M.J.; Barbosa, M.A.; Santos, S.G. Resveratrol as a natural anti-tumor necrosis factor-alpha molecule: Implications to dendritic cells and their crosstalk with mesenchymal stromal cells. PLoS ONE 2014, 9, e91406. [Google Scholar] [CrossRef]
- Wu, H.; Feng, F.; Jiang, X.; Hu, B.; Qiu, J.; Wang, C.; Xiang, Z. Pharmacokinetic and metabolic profiling studies of sennoside B by UPLC-MS/MS and UPLC-Q-TOF-MS. J. Pharm. Biomed. Anal. 2020, 179, 112938. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chang, C.N.; Hsu, H.C.; Chiou, S.J.; Lee, L.T.; Hseu, T.H. Sennoside B inhibits PDGF receptor signaling and cell proliferation induced by PDGF-BB in human osteosarcoma cells. Life Sci. 2009, 84, 915–922. [Google Scholar] [CrossRef]
- Abdallah, H.M.; El-Halawany, A.M.; Sirwi, A.; El-Araby, A.M.; Mohamed, G.A.; Ibrahim, S.R.M.; Koshak, A.E.; Asfour, H.Z.; Awan, Z.A.; Elfaky, M.A. Repurposing of Some Natural Product Isolates as SARS-COV-2 Main Protease Inhibitors via In Vitro Cell Free and Cell-Based Antiviral Assessments and Molecular Modeling Approaches. Pharmaceuticals 2021, 14, 213. [Google Scholar] [CrossRef] [PubMed]


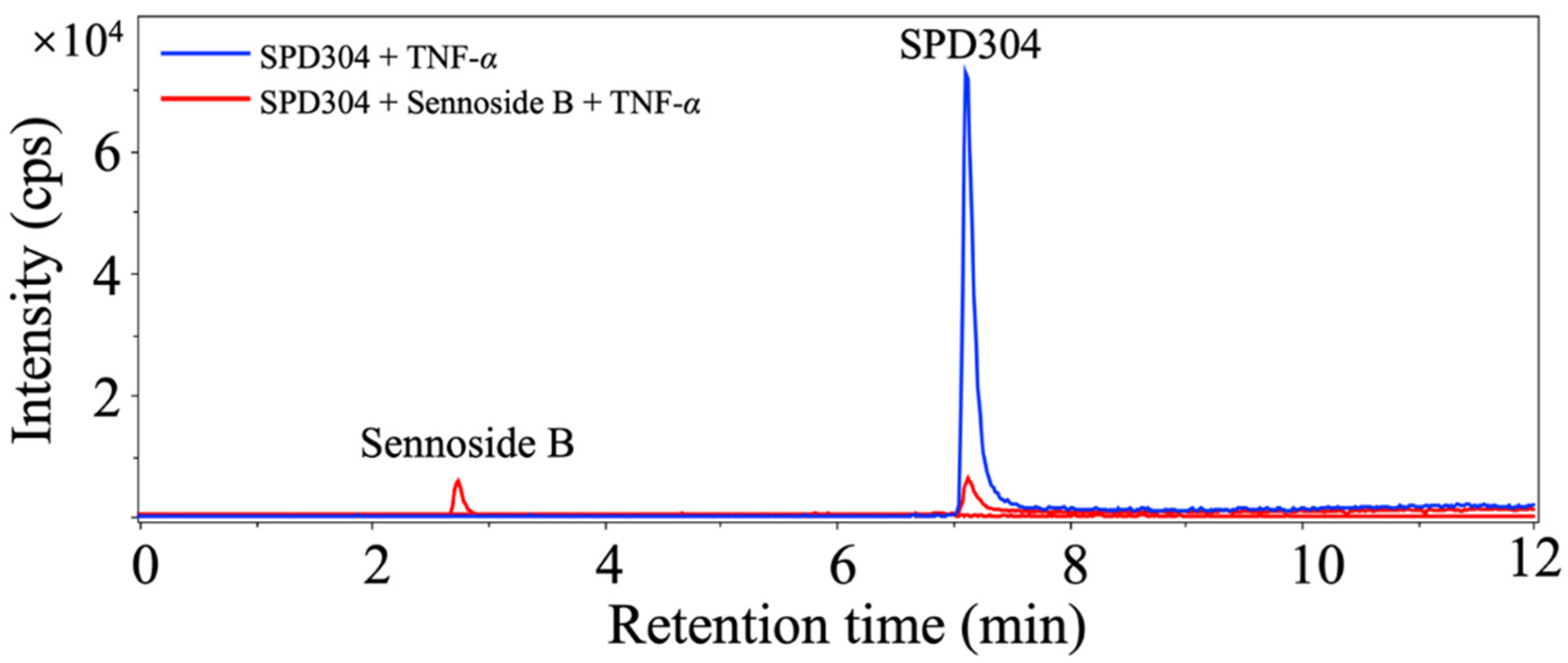



| Sample # | Signal Inhibition a | Name | Sample # | Signal Inhibition a | Name |
|---|---|---|---|---|---|
| 1 | 18.7 | (23)-Hydroxyursolic acid | 19 | 1.3 | Gomisin N |
| 2 | 29.3 | Fraxinellone | 20 | −1.3 | Harpagoside |
| 3 | 20.2 | Limonin | 21 | 21.1 | (+)-Matrine |
| 4 | 33.3 | Obacunone | 22 | 32.5 | Paeoniflorin |
| 5 | 14.7 | Heraclenol | 23 | 89.4 | Sennoside B |
| 6 | 44 | Imperatorin | 24 | 25 | Tanshinone IIA |
| 7 | 25.9 | Dioscin | 25 | 22.6 | Hexahydrocurcumin |
| 8 | 20 | Amygdalin | 26 | 21 | Acutumine |
| 9 | 14.7 | Betaine | 27 | 71.4 | Acutumidine |
| 10 | 17.3 | Decursin | 28 | 35 | Isoliquiritigenin |
| 11 | 6.9 | 6,7-Dimethylesculetin | 29 | 12.5 | Liquiritin |
| 12 | −1.3 | Ephedrine | 30 | 17.4 | (8)-Demethoxyrunanine |
| 13 | 15 | Eugenol | 31 | 32.5 | Chlorogenic acid |
| 14 | 20 | Evodiamine | 32 | 31.2 | Caffeine |
| 15 | 28 | (6)-Gingerol | 33 | 0 | Dihydrofolic acid |
| 16 | 33.9 | Ginsenoside Rb1 | 34 | 23.5 | N-(1-Naphthyl)ethylenediamine dihydrochloride |
| 17 | 17.4 | Ginsenoside Rg1 | 35 | 15.2 | Myricetin |
| 18 | 25.3 | Gomisin A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Durai, P.; Park, K.; Pyo, J.J.; Choi, Y. A Novel Competitive Binding Screening Assay Reveals Sennoside B as a Potent Natural Product Inhibitor of TNF-α. Biomedicines 2021, 9, 1250. https://doi.org/10.3390/biomedicines9091250
Peng L, Durai P, Park K, Pyo JJ, Choi Y. A Novel Competitive Binding Screening Assay Reveals Sennoside B as a Potent Natural Product Inhibitor of TNF-α. Biomedicines. 2021; 9(9):1250. https://doi.org/10.3390/biomedicines9091250
Chicago/Turabian StylePeng, Lei, Prasannavenkatesh Durai, Keunwan Park, Jeong Joo Pyo, and Yongsoo Choi. 2021. "A Novel Competitive Binding Screening Assay Reveals Sennoside B as a Potent Natural Product Inhibitor of TNF-α" Biomedicines 9, no. 9: 1250. https://doi.org/10.3390/biomedicines9091250
APA StylePeng, L., Durai, P., Park, K., Pyo, J. J., & Choi, Y. (2021). A Novel Competitive Binding Screening Assay Reveals Sennoside B as a Potent Natural Product Inhibitor of TNF-α. Biomedicines, 9(9), 1250. https://doi.org/10.3390/biomedicines9091250







