CD44v6 High Membranous Expression Is a Predictive Marker of Therapy Response in Gastric Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Samples, Data Collection, and Tissue Microarray Preparation
2.2. CD44v6 Immunohistochemistry of GC Samples
2.3. Statistical Analysis
3. Results
3.1. Analysis of De Novo CD44v6 Membranous Expression and Its Relationship with GC Patients’ Survival
3.2. Analysis of CD44v6 Expression in Tumor Cells and Its Relationship with Therapy Response in GC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Oba, K.; Paoletti, X.; Bang, Y.J.; Bleiberg, H.; Burzykowski, T.; Fuse, N.; Michiels, S.; Morita, S.; Ohashi, Y.; Pignon, J.P.; et al. Role of chemotherapy for advanced/recurrent gastric cancer: An individual-patient-data meta-analysis. Eur. J. Cancer 2013, 49, 1565–1577. [Google Scholar] [CrossRef]
- Sugano, K. Screening of gastric cancer in Asia. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 895–905. [Google Scholar] [CrossRef]
- Durães, C.; Almeida, G.M.; Seruca, R.; Oliveira, C.; Carneiro, F. Biomarkers for gastric cancer: Prognostic, predictive or targets of therapy? Virchows Arch. 2014, 464, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Cavaliere, A.; Merz, V.; Casalino, S.; Zecchetto, C.; Simionato, F.; Salt, H.L.; Contarelli, S.; Santoro, R.; Melisi, D. Novel Biomarkers for Prediction of Response to Preoperative Systemic Therapies in Gastric Cancer. J. Gastric Cancer 2019, 19, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Yagi, S.; Wakatsuki, T.; Yamamoto, N.; Chin, K.; Takahari, D.; Ogura, M.; Ichimura, T.; Nakayama, I.; Osumi, H.; Shinozaki, E.; et al. Clinical significance of intratumoral HER2 heterogeneity on trastuzumab efficacy using endoscopic biopsy specimens in patients with advanced HER2 positive gastric cancer. Gastric Cancer 2019, 22, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Karapetis, C.S.; Khambata-Ford, S.; Jonker, D.J.; O’Callaghan, C.J.; Tu, D.; Tebbutt, N.C.; Simes, R.J.; Chalchal, H.; Shapiro, J.D.; Robitaille, S.; et al. Kras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 2008, 359, 1757–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Cunha, C.B.; Oliveira, C.; Wen, X.; Gomes, B.; Sousa, S.; Suriano, G.; Grellier, M.; Huntsman, D.G.; Carneiro, F.; Granja, P.L.; et al. De novo expression of CD44 variants in sporadic and hereditary gastric cancer. Lab. Investig. 2010, 90, 1604–1614. [Google Scholar] [CrossRef] [Green Version]
- Prochazka, L.; Tesarik, R.; Turanek, J. Regulation of alternative splicing of CD44 in cancer. Cell Signal. 2014, 26, 2234–2239. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Ferreira, D.; Mendes, N.; Granja, P.L.; Almeida, G.M.; Oliveira, C. Expression of CD44v6-Containing Isoforms Influences Cisplatin Response in Gastric Cancer Cells. Cancers 2020, 12, 858. [Google Scholar] [CrossRef] [Green Version]
- Lobo, S.; Pereira, C.; Oliveira, C.; Almeida, G.M. Skipping Exon-v6 from CD44v6-Containing Isoforms Influences Chemotherapy Response and Self-Renewal Capacity of Gastric Cancer Cells. Cancers 2020, 12, 2378. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Liu, H.G.; Dong, S.Y.; Yang, F.; Wang, Q.X.; Guo, G.L.; Pan, Y.F.; Zhang, X.H. Upregulation of CD44v6 contributes to acquired chemoresistance via the modulation of autophagy in colon cancer SW480 cells. Tumor Biol. 2016, 37, 8811–8824. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, S.; Tsugawa, H.; Matsuzaki, J.; Hirata, K.; Mori, H.; Saya, H.; Kanai, T.; Suzuki, H. Inhibiting xCT Improves 5-Fluorouracil Resistance of Gastric Cancer Induced by CD44 Variant 9 Expression. Anticancer Res. 2018, 38, 6163–6170. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, Z.; Xu, S.; Xu, P. The prognostic value of CD44 expression in gastric cancer: A meta-analysis. Biomed. Pharmacother. 2014, 68, 693–697. [Google Scholar] [CrossRef]
- Xie, J.W.; Chen, P.C.; Zheng, C.H.; Li, P.; Wang, J.B.; Lin, J.X.; Lu, J.; Chen, Q.Y.; Cao, L.L.; Lin, M.; et al. Evaluation of the prognostic value and functional roles of CD44v6 in gastric cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 1809–1817. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Guo, M.; Yang, L.Q.; Zhou, F.; Yu, C.; Wang, A.; Pang, T.H.; Wu, H.Y.; Zou, X.P.; Zhang, W.J.; et al. Regulation of CD44v6 expression in gastric carcinoma by the IL-6/STAT3 signaling pathway and its clinical significance. Oncotarget 2017, 8, 45848–45861. [Google Scholar] [CrossRef]
- Rodrigues, M.; Vitó, I.; Santos, R.; Paiva, J.; Pontes, P.; Silva, P.; Carneiro, F. Establishment of a Tumour Bank: The experience of the Department of Pathology of Hospital S. João (Porto, Portugal). Cell Tissue Bank. 2009, 10, 75–77. [Google Scholar] [CrossRef]
- Lopes, N.; Bergsland, C.; Bruun, J.; Bjornslett, M.; Vieira, A.F.; Mesquita, P.; Pinto, R.; Gomes, R.; Cavadas, B.; Bennett, E.; et al. A panel of intestinal differentiation markers (CDX2, GPA33, and LI-cadherin) identifies gastric cancer patients with favourable prognosis. Gastric Cancer 2020, 23, 811–823. [Google Scholar] [CrossRef]
- Fang, M.; Wu, J.; Lai, X.; Ai, H.; Tao, Y.; Zhu, B.; Huang, L. CD44 and CD44v6 are Correlated with Gastric Cancer Progression and Poor Patient Prognosis: Evidence from 42 Studies. Cell. Physiol. Biochem. 2016, 40, 567–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Su, W.Y.; Lin, Y.W.; Xiong, H.; Chen, Y.X.; Xu, J.; Fang, J.Y. CD44v6 overexpression related to metastasis and poor prognosis of colorectal cancer: A meta-analysis. Oncotarget 2017, 8, 12866–12876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendardaf, R.; Lamlum, H.; Ristamäki, R.; Pyrhönen, S. CD44 variant 6 expression predicts response to treatment in advanced colorectal cancer. Oncol. Rep. 2004, 11, 41–45. [Google Scholar] [CrossRef] [PubMed]
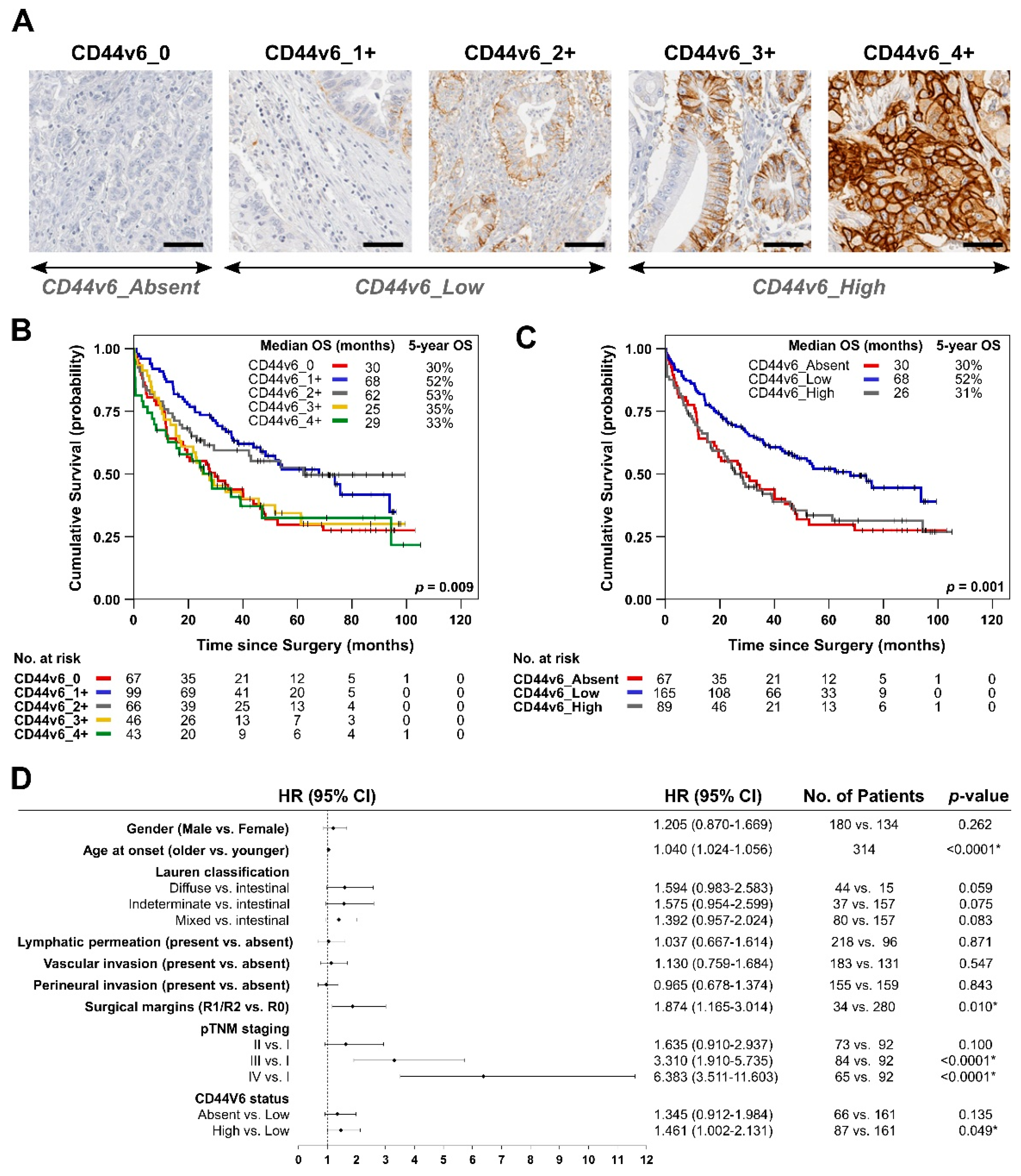
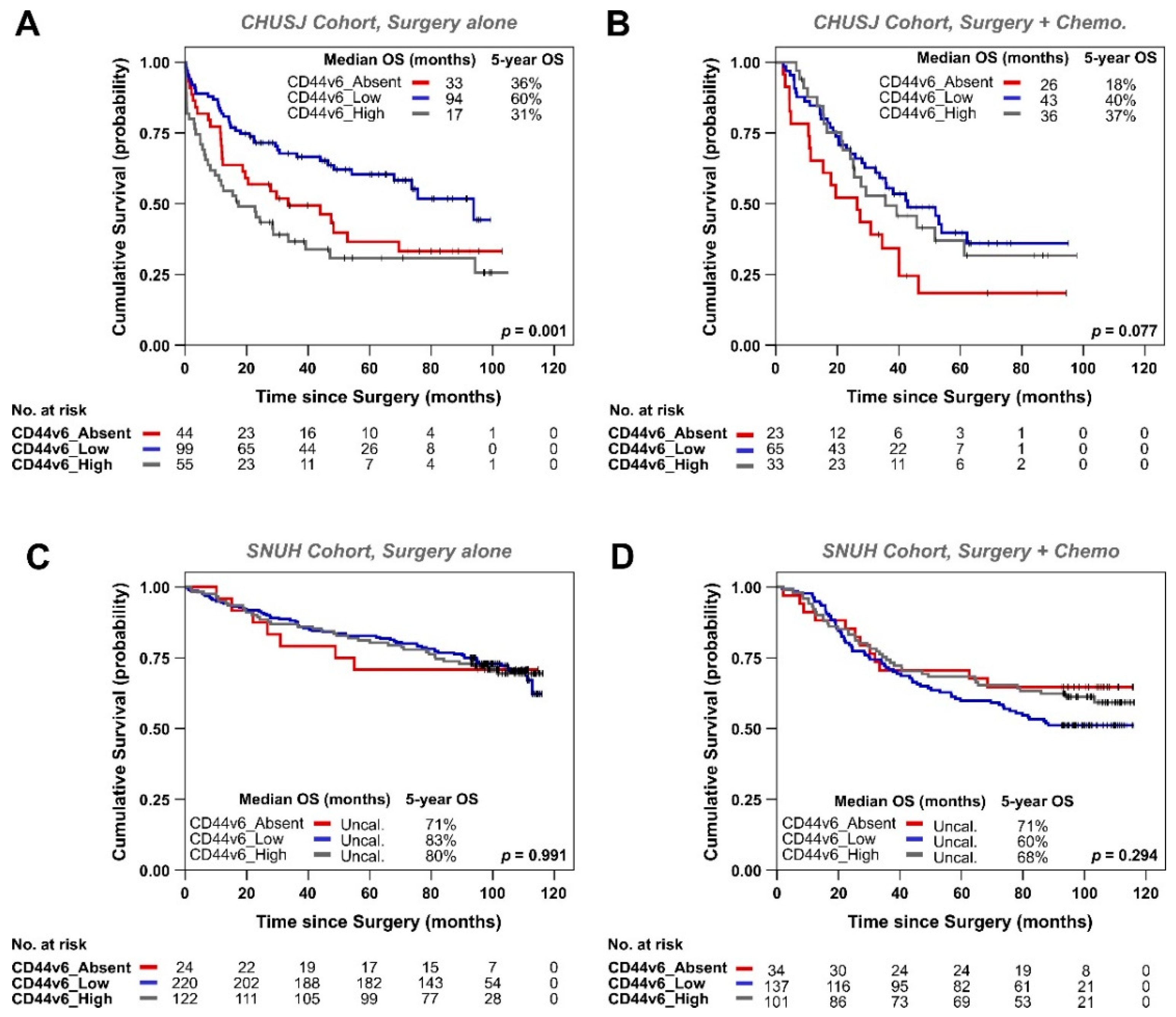
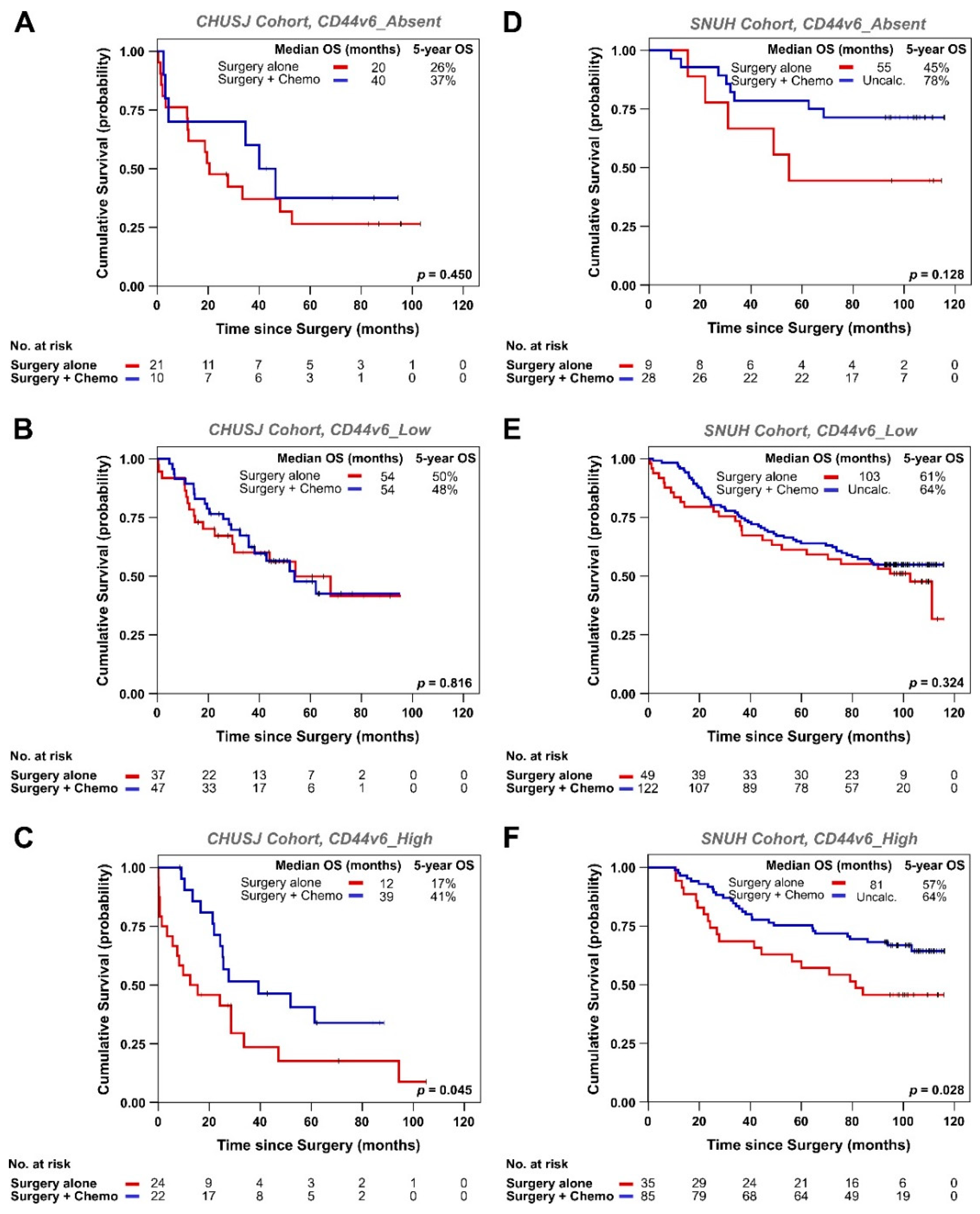
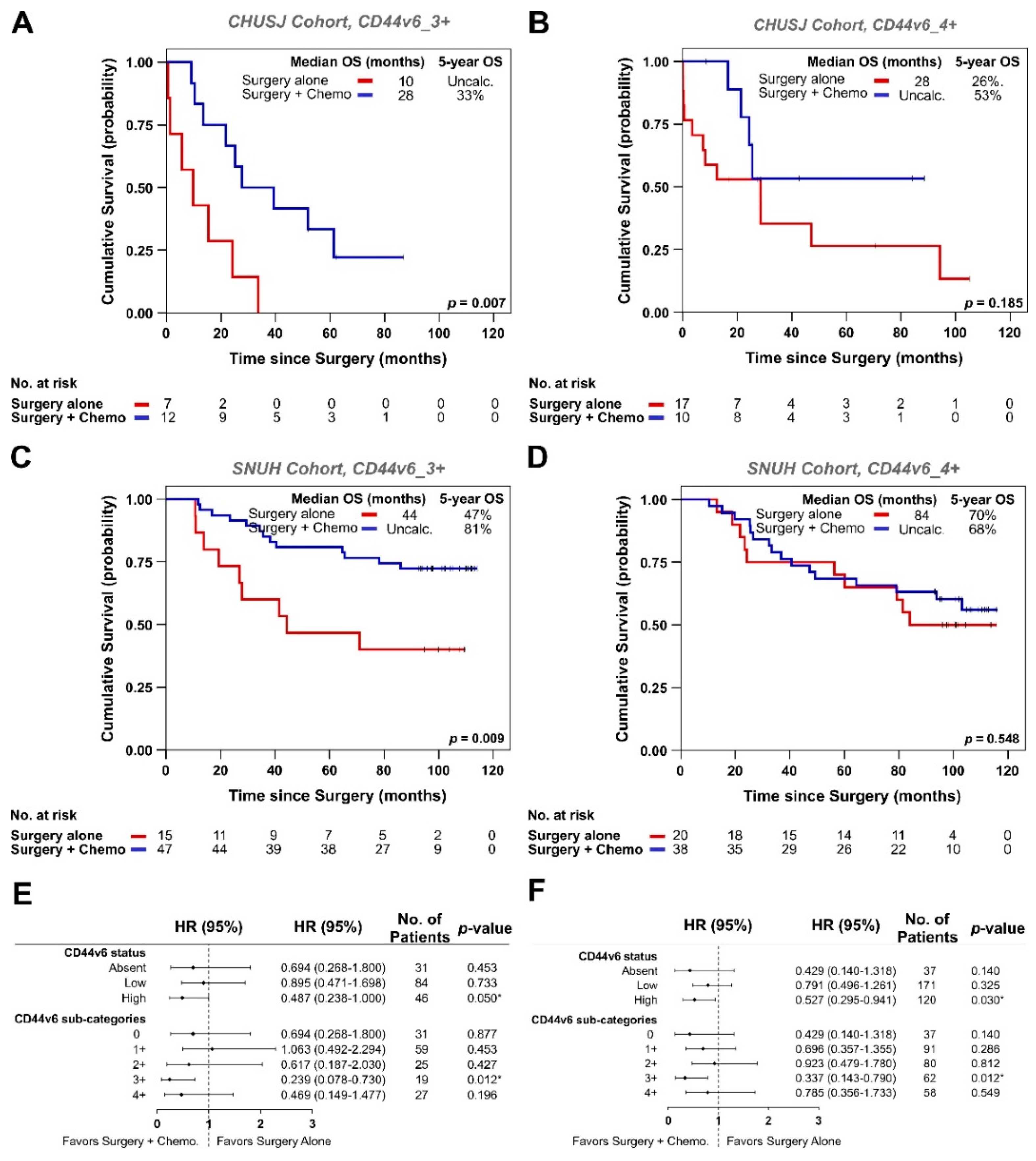
| Variables | Total No. Patients n = 326 | CD44v6_0 n = 68/326 (21%) | CD44v6_1+ n = 101/326 (31%) | CD44v6_2+ n = 68/326 (21%) | CD44v6_3+ n = 46/326 (14%) | CD44v6_4+ n = 43/326 (13%) | p-Value |
|---|---|---|---|---|---|---|---|
| Age (years) | >0.05 | ||||||
| Mean | 67.7 | 68.0 | 67.5 | 66.2 | 69.9 | 69.9 | |
| SD | 11.8 | 12.3 | 10.5 | 13.5 | 11.9 | 11.9 | |
| Gender | 0.005 | ||||||
| Male | 185 (56.7%) | 42 (61.8%) | 65 (64.4%) | 35 (51.5%) | 29 (63.0%) | 14 (32.6%) | |
| Female | 141 (43.3%) | 26 (38.2%) | 36 (35.6%) | 33 (48.5%) | 17 (37.0%) | 29 (67.4%) | |
| M:F ratio | 1.3:1 | 1.6:1 | 1.8:1 | 1.1:1 | 1.7:1 | 0.5:1 | |
| Laurén classification | >0.05 | ||||||
| Intestinal | 163 (50.0%) | 31 (45.6%) | 56 (55.4%) | 35 (51.5%) | 24 (52.2%) | 17 (39.5%) | |
| Diffuse | 44 (13.5%) | 12 (17.6%) | 11 (10.9%) | 9 (13.2%) | 5 (10.9%) | 7 (16.3%) | |
| Mixed | 84 (25.8%) | 18 (26.5%) | 18 (17.8%) | 18 (26.5%) | 15 (32.6%) | 15 (34.9%) | |
| Indeterminate | 35 (10.7%) | 7 (10.3%) | 16 (15.8%) | 6 (8.8%) | 2 (4.3%) | 4 (9.3%) | |
| Growth pattern | >0.05 | ||||||
| Expansive | 60 (18.4%) | 13 (19.1%) | 19 (18.8%) | 12 (17.6%) | 10 (21.7%) | 6 (14.0%) | |
| Infiltrative | 252 (77.3%) | 51 (75.0%) | 77 (76.2%) | 53 (77.9%) | 35 (76.1%) | 36 (83.7%) | |
| Unclassified | 14 (4.3%) | 4 (5.9%) | 5 (5.0%) | 3 (4.4%) | 1 (2.2%) | 1 (2.3%) | |
| Lymphatic permeation *1 | >0.05 | ||||||
| Absent | 99 (30.5%) | 21 (30.9%) | 31 (31.0%) | 26 (38.8%) | 14 (30.4%) | 7 (16.3%) | |
| Present | 225 (69.2%) | 47 (69.1%) | 69 (69.0%) | 41 (61.2%) | 32 (69.6%) | 36 (83.7%) | |
| Perineural invasion *1 | 0.034 | ||||||
| Absent | 166 (51.1%) | 34 (50.0%) | 59 (58.4%) | 38 (55.9%) | 22 (48.9%) | 13 (30.2%) | |
| Present | 159 (48.9%) | 34 (50.0%) | 42 (41.6%) | 30 (44.1%) | 23 (51.1%) | 30 (69.8%) | |
| Vascular invasion *1 | 0.005 | ||||||
| Absent | 131 (40.6%) | 25 (37.3%) | 43 (42.6%) | 38 (56.7%) | 16 (35.6%) | 9 (20.9%) | |
| Present | 192 (59.4%) | 42 (62.7%) | 58 (57.4%) | 29 (43.3%) | 29 (64.4%) | 34 (79.1%) | |
| Surgical margins *1 | 0.007 | ||||||
| R0 | 290 (89.2%) | 58 (85.3%) | 98 (98.0%) | 61 (89.7%) | 37 (80.4%) | 36 (83.7%) | |
| R1/R2 | 35 (10.8%) | 10 (14.7%) | 2 (2.0%) | 7 (10.3%) | 9 (19.6%) | 7 (16.3%) | |
| Depth of invasion (T) | 0.010 | ||||||
| pT1 | 74 (22.7%) | 14 (20.6%) | 24 (23.8%) | 24 (35.3%) | 9 (19.6%) | 3 (7.0%) | |
| pT2 | 41 (12.6%) | 6 (8.8%) | 14 (13.9%) | 41 (60.3%) | 10 (21.7%) | 8 (18.6%) | |
| pT3–T4 | 211 (64.7%) | 48 (70.6%) | 63 (62.4%) | 3 (4.4%) | 27 (58.7%) | 32 (74.4%) | |
| Lymph node metastases (N) *1 | >0.05 | ||||||
| Absent (pN0) | 126 (38.8%) | 27 (39.7%) | 39 (39.0%) | 29 (42.6%) | 17 (37.0%) | 14 (32.6%) | |
| Present (pN+) | 199 (61.2%) | 41 (60.3%) | 61 (61.0%) | 39 (57.4%) | 29 (63.0%) | 29 (67.4%) | |
| Distant metastases (M) | >0.05 | ||||||
| Absent | 257 (78.8%) | 48 (70.6%) | 89 (88.1%) | 51 (75.0%) | 35 (76.1%) | 34 (79.1%) | |
| Present | 69 (21.2%) | 20 (29.4%) | 12 (11.9%) | 17 (25.0%) | 11 (23.9%) | 9 (20.9%) | |
| TNM Staging | >0.05 | ||||||
| I | 95 (29.1%) | 17 (25.0%) | 30 (29.7%) | 25 (36.8%) | 16 (34.8%) | 7 (16.3%) | |
| II | 74 (22.7%) | 16 (23.5%) | 27 (26.7%) | 11 (16.2%) | 7 (15.2%) | 13 (30.2%) | |
| III | 88 (27.0%) | 15 (22.1%) | 32 (31.7%) | 15 (22.1%) | 12 (26.1%) | 14 (32.6%) | |
| IV | 69 (21.2%) | 20 (29.4%) | 12 (11.9%) | 17 (25.0%) | 11 (23.9%) | 9 (20.9%) |
| Variables | Total No. Patients n = 638 | CD44v6_0 n = 58/638 (9%) | CD44v6_1+ n = 163/638 (26%) | CD44v6_2+ n = 194/638 (30%) | CD44v6_3+ n = 136/638 (21%) | CD44v6_4+ n = 87/638 (14%) | p-Value |
|---|---|---|---|---|---|---|---|
| Age (years) | >0.05 | ||||||
| Mean | 60.8 | 59.5 | 60.8 | 60.2 | 60.7 | 63.3 | |
| SD | 12.3 | 12.8 | 12.5 | 11.9 | 12.1 | 12.8 | |
| Gender | 0.009 | ||||||
| Male | 420 (65.8%) | 39 (67.2%) | 120 (73.6%) | 131 (67.5%) | 73 (53.7%) | 57 (65.5%) | |
| Female | 218 (34.2%) | 19 (32.8%) | 43 (26.4%) | 63 (32.5%) | 63 (46.3%) | 30 (34.5%) | |
| M:F ratio | 1.9:1 | 2.1:1 | 2.8:1 | 2.1:1 | 1.2:1 | 1.9:1 | |
| Laurén classification *1 | >0.05 | ||||||
| Intestinal | 299 (47.3%) | 26 (44.8%) | 67 (41.9%) | 102 (52.8%) | 64 (47.4%) | 40 (46.5%) | |
| Diffuse | 239 (37.8%) | 29 (50.0%) | 68 (42.5%) | 58 (30.1%) | 51 (37.8%) | 33 (32.5%) | |
| Mixed | 88 (13.9%) | 2 (3.4%) | 22 (13.8%) | 33 (17.1%) | 18 (13.3%) | 13 (15.1%) | |
| Indeterminate *2 | 6 (0.9%) | 1 (1.7%) | 3 (1.9%) | 0 (0.0%) | 2 (1.5%) | 0 (0.0%) | |
| Growth pattern | >0.05 | ||||||
| Expansive | 65 (10.2%) | 6 (10.3%) | 17 (10.4%) | 20 (10.3%) | 13 (9.6%) | 9 (10.3%) | |
| Infiltrative | 573 (89.8%) | 52 (89.7%) | 146 (89.6%) | 174 (89.7%) | 123 (90.4%) | 78 (89.7%) | |
| Lymphatic permeation | 0.001 | ||||||
| Absent | 309 (48.4%) | 28 (48.3%) | 82 (50.3%) | 99 (51.0%) | 76 (55.9%) | 24 (27.6%) | |
| Present | 329 (51.6%) | 30 (51.7%) | 81 (49.7%) | 95 (49.0%) | 60 (44.1%) | 63 (72.4%) | |
| Perineural invasion | >0.05 | ||||||
| Absent | 405 (63.5%) | 27 (46.6%) | 103 (63.2%) | 130 (67.0%) | 88 (64.7%) | 57 (65.5%) | |
| Present | 233 (36.5%) | 31 (53.4%) | 60 (36.8%) | 64 (33.0%) | 48 (35.3%) | 30 (34.5%) | |
| Vascular invasion | >0.05 | ||||||
| Absent | 536 (84.0%) | 48 (82.8%) | 133 (81.6%) | 168 (86.6%) | 114 (83.8%) | 73 (83.9%) | |
| Present | 102 (16.0%) | 10 (17.2%) | 30 (18.4%) | 26 (13.4%) | 22 (16.2%) | 14 (16.1%) | |
| Surgical margins *3 | |||||||
| R0 | 638 (100%) | 58 (100%) | 163 (100%) | 194 (100%) | 136 (100%) | 87 (100%) | |
| R1/R2 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Depth of invasion (T) | 0.0001 | ||||||
| pT1 | 212 (33.2%) | 11 (19.0%) | 53 (32.5%) | 83 (42.8%) | 45 (33.1%) | 20 (23.0%) | |
| pT2 | 118 (18.5%) | 7 (12.1%) | 23 (14.1%) | 39 (20.1%) | 33 (24.3%) | 16 (18.4%) | |
| pT3–T4 | 308 (48.3%) | 40 (69.0%) | 87 (53.4%) | 72 (37.1%) | 58 (42.6%) | 51 (58.6%) | |
| Lymph node metastases (N) | 0.008 | ||||||
| Absent (pN0) | 324 (50.8%) | 25 (43.1%) | 79 (48.5%) | 113 (58.2%) | 75 (55.1%) | 32 (36.8%) | |
| Present (pN+) | 314 (49.2%) | 33 (56.9%) | 84 (51.5%) | 81 (41.8%) | 61 (44.9%) | 55 (63.2%) | |
| Distant metastases(M) | >0.05 | ||||||
| Absent | 596 (93.4%) | 52 (89.7%) | 153 (93.9%) | 186 (95.9%) | 125 (91.9%) | 80 (92.0%) | |
| Present | 42 (6.6%) | 6 (10.3%) | 10 (6.1%) | 8 (4.1%) | 11 (8.1%) | 7 (8.0%) | |
| TNM Staging | 0.001 | ||||||
| I | 269(42.2%) | 15 (25.9%) | 62 (38.0%) | 106 (54.6%) | 63 (46.3%) | 23 (26.4%) | |
| II | 158 (24.8%) | 16 (27.6%) | 45 (27.6%) | 36 (18.6%) | 34 (25.0%) | 27 (31.0%) | |
| III | 170 (26.6%) | 21 (36.2%) | 46 (28.2%) | 44 (22.7%) | 28 (20.6%) | 31 (35.6%) | |
| IV | 41 (6.4%) | 6 (10.3%) | 10 (6.1%) | 8 (4.1%) | 11 (8.1%) | 6 (6.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, G.M.; Pereira, C.; Park, J.-H.; Lemos, C.; Campelos, S.; Gullo, I.; Martins, D.; Gonçalves, G.; Leitão, D.; Neto, J.L.; et al. CD44v6 High Membranous Expression Is a Predictive Marker of Therapy Response in Gastric Cancer Patients. Biomedicines 2021, 9, 1249. https://doi.org/10.3390/biomedicines9091249
Almeida GM, Pereira C, Park J-H, Lemos C, Campelos S, Gullo I, Martins D, Gonçalves G, Leitão D, Neto JL, et al. CD44v6 High Membranous Expression Is a Predictive Marker of Therapy Response in Gastric Cancer Patients. Biomedicines. 2021; 9(9):1249. https://doi.org/10.3390/biomedicines9091249
Chicago/Turabian StyleAlmeida, Gabriela M, Carla Pereira, Ji-Hyeon Park, Carolina Lemos, Sofia Campelos, Irene Gullo, Diana Martins, Gilza Gonçalves, Dina Leitão, João Luís Neto, and et al. 2021. "CD44v6 High Membranous Expression Is a Predictive Marker of Therapy Response in Gastric Cancer Patients" Biomedicines 9, no. 9: 1249. https://doi.org/10.3390/biomedicines9091249
APA StyleAlmeida, G. M., Pereira, C., Park, J.-H., Lemos, C., Campelos, S., Gullo, I., Martins, D., Gonçalves, G., Leitão, D., Neto, J. L., André, A., Borges, C., Almeida, D., Lee, H.-J., Kong, S.-H., Kim, W. H., Carneiro, F., Almeida, R., Yang, H.-K., & Oliveira, C. (2021). CD44v6 High Membranous Expression Is a Predictive Marker of Therapy Response in Gastric Cancer Patients. Biomedicines, 9(9), 1249. https://doi.org/10.3390/biomedicines9091249







