RIOK2 Inhibitor NSC139021 Exerts Anti-Tumor Effects on Glioblastoma via Inducing Skp2-Mediated Cell Cycle Arrest and Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines and Culture Conditions
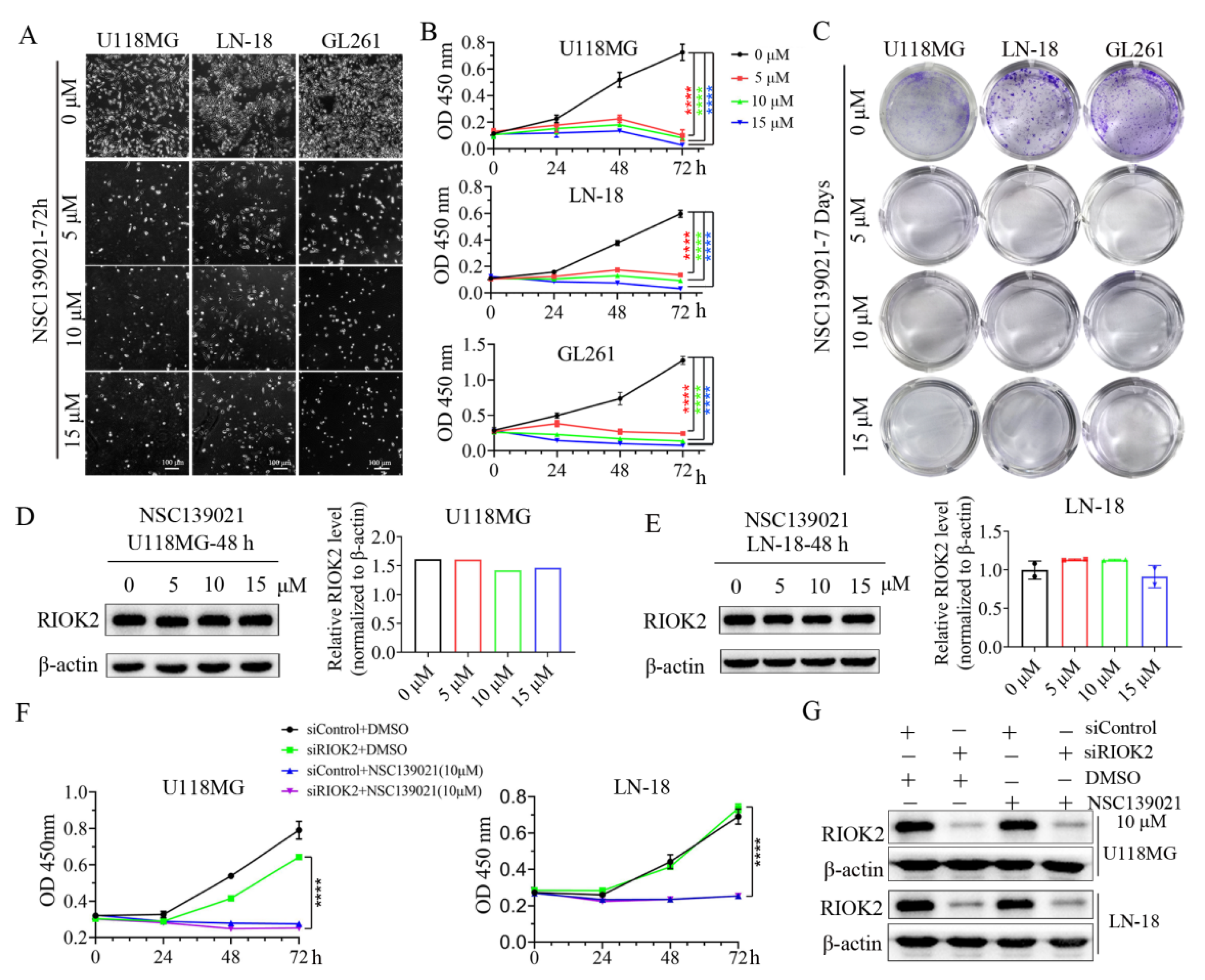
2.3. Proliferation Assays
2.4. Transfection and siRNAs
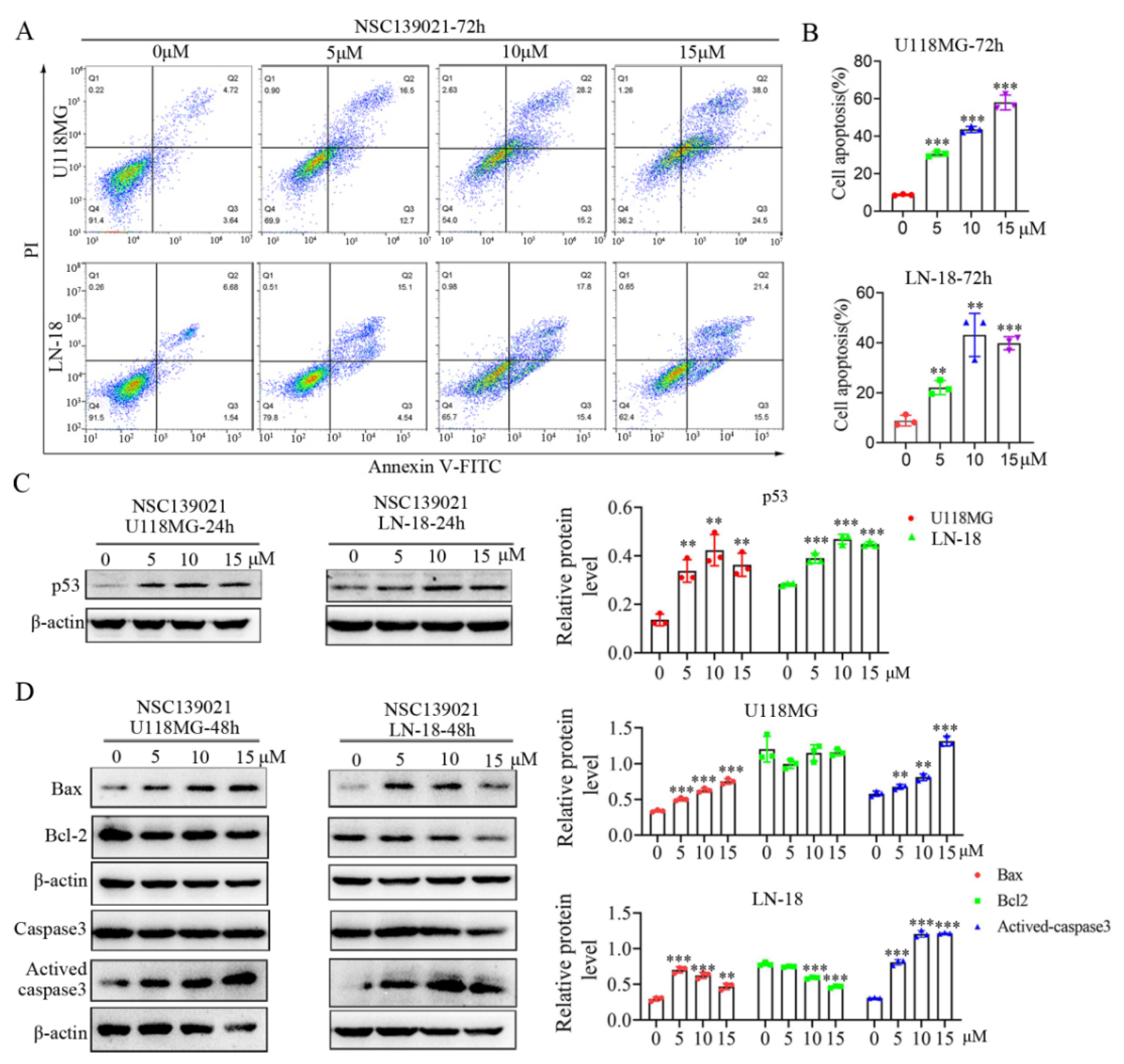
2.5. Flow Cytometry Analysis of Cell Cycle and Apoptosis
2.6. Total RNA Isolation and Real-Time PCR
- β-actin: 5′-GCAAAGACCTGTACGCCAAC-3′ (forward)
- and 5′-GATCTTCATTGTGCTGGGTGC-3′ (reverse);
- Skp2: 5′-AGACTGGATGAGCTGAACCTCTCC-3′ (forward)
- and 5′-GGTGATGGTCTCTGACACATGCG-3′ (reverse);
- p27: 5′-ACTGAGGCGGAGACGAAGGTG-3′ (forward)
- and 5′-CGCTGTTTGTCTTGGAGGAGGATC-3′ (reverse);
- RIOK2: 5′-TCCAGGGCTATCGGTTGACAAATG-3′ (forward)
- and 5′-TTGCCAACACCCATCTGGTTTCC-3′ (reverse).
2.7. Western Blot Analysis
2.8. Mouse Tumor Model and Treatment
2.9. Quantification and Statistical Analysis
3. Results
3.1. NSC139021 Inhibits Glioblastoma Cell Proliferation through a RIOK2-Independent Mechanism
3.2. NSC139021 Induces Cell Cycle Arrest in G0/G1 Phase
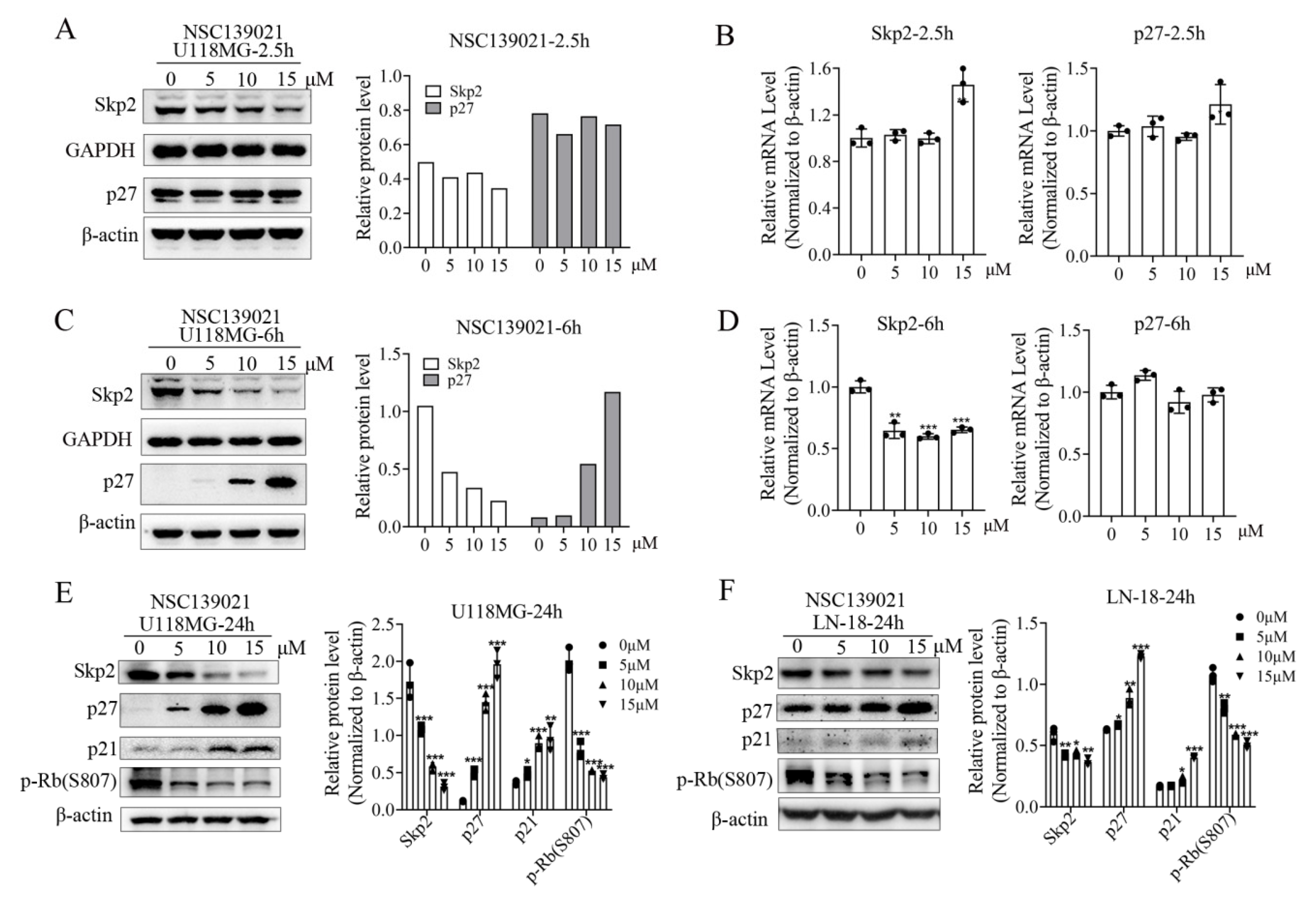
3.3. NSC139021 Arrests Cell Cycle by Regulating the Skp2-p27/p21-CDK2-Rb Signaling Pathway
3.4. NSC139021 Activates p53 Signaling Pathway and Triggers Apoptosis of Glioblastoma Cells
3.5. NSC139021 Suppresses the Proliferation of Glioblastoma In Vivo
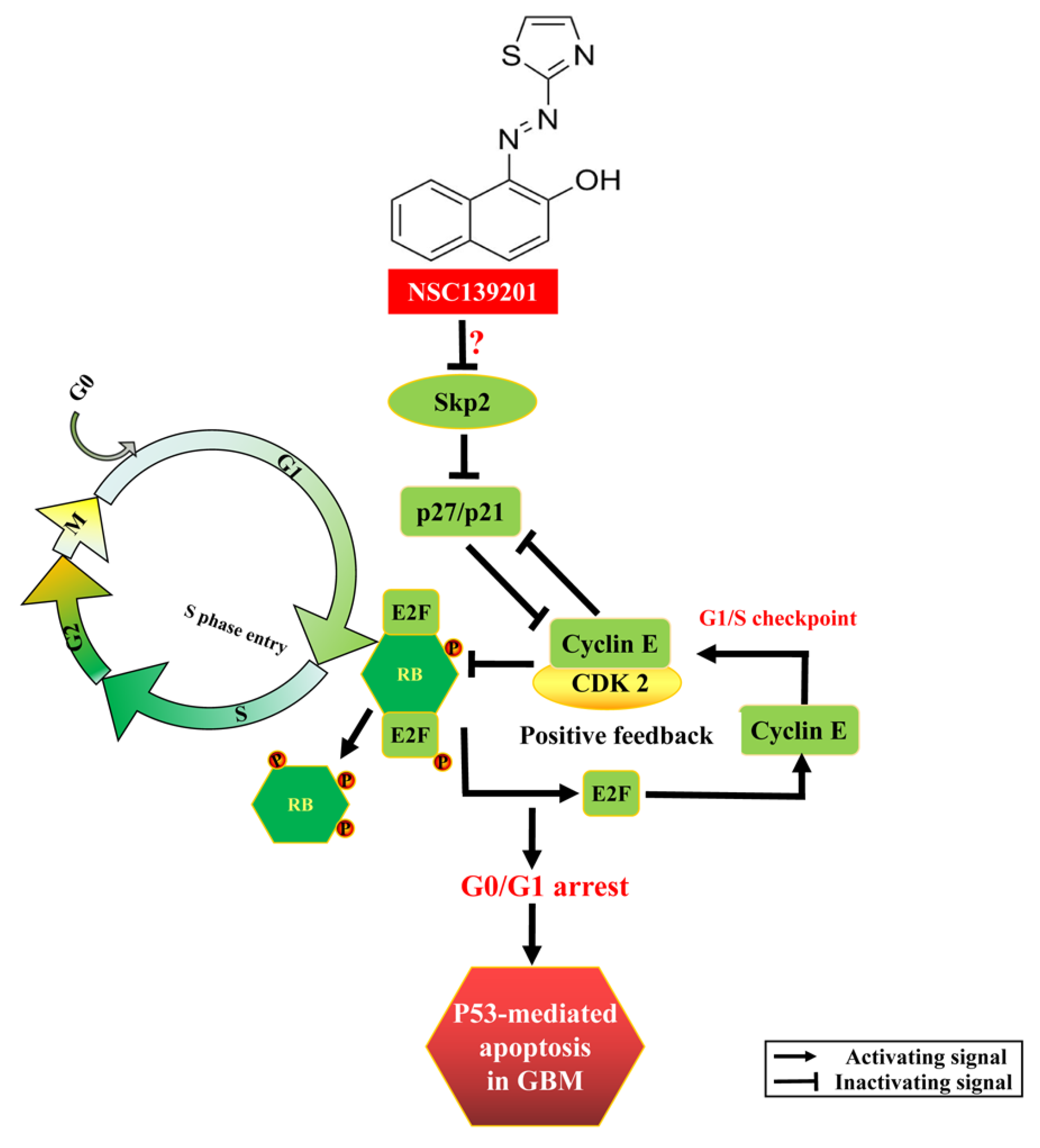
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shergalis, A.; Bankhead, A., 3rd; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef] [Green Version]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Nishikawa, R. Standard therapy for glioblastoma--a review of where we are. Neurol. Med.-Chir. 2010, 50, 713–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Meir, E.G.; Hadjipanayis, C.G.; Norden, A.D.; Shu, H.K.; Wen, P.Y.; Olson, J.J. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. CA Cancer J. Clin. 2010, 60, 166–193. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro-Oncology 2016, 18, v1–v75. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.E.; Mazor, T.; Hong, C.; Barnes, M.; Aihara, K.; McLean, C.Y.; Fouse, S.D.; Yamamoto, S.; Ueda, H.; Tatsuno, K.; et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 2014, 343, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Friedman, H.S.; Kerby, T.; Calvert, H. Temozolomide and treatment of malignant glioma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 2585–2597. [Google Scholar]
- Schreck, K.C.; Grossman, S.A. Role of Temozolomide in the Treatment of Cancers Involving the Central Nervous System. Oncology 2018, 32, 555–569. [Google Scholar] [PubMed]
- Chen, J.; Li, Y.; Yu, T.S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.A.; Xavier, C.P.; Sukumar, G.; Tan, S.H.; Ravindranath, L.; Seraj, N.; Kumar, V.; Sreenath, T.; McLeod, D.G.; Petrovics, G.; et al. Identification of a Small Molecule That Selectively Inhibits ERG-Positive Cancer Cell Growth. Cancer Res. 2018, 78, 3659–3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaRonde-LeBlanc, N.; Wlodawer, A. A family portrait of the RIO kinases. J. Biol. Chem. 2005, 280, 37297–37300. [Google Scholar] [CrossRef] [Green Version]
- Read, R.D.; Fenton, T.R.; Gomez, G.G.; Wykosky, J.; Vandenberg, S.R.; Babic, I.; Iwanami, A.; Yang, H.; Cavenee, W.K.; Mischel, P.S.; et al. A kinome-wide RNAi screen in Drosophila Glia reveals that the RIO kinases mediate cell proliferation and survival through TORC2-Akt signaling in glioblastoma. PLoS Genet. 2013, 9, e1003253. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Li, C.; Jin, L.; Xing, J.; Sha, Z.; Zhang, T.; Ji, D.; Yu, R.; Gao, S. RIOK2 is negatively regulated by miR-4744 and promotes glioma cell migration/invasion through epithelial-mesenchymal transition. J. Cell. Mol. Med. 2020, 24, 4494–4509. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Ji, D.; Wang, P.; Liang, D.; Jin, L.; Shi, H.; Liu, X.; Meng, Q.; Yu, R.; Gao, S. The atypical protein kinase RIOK3 contributes to glioma cell proliferation/survival, migration/invasion and the AKT/mTOR signaling pathway. Cancer Lett. 2018, 415, 151–163. [Google Scholar] [CrossRef]
- Carrano, A.C.; Eytan, E.; Hershko, A.; Pagano, M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1999, 1, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.K.; Gervais, J.L.; Zhang, H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21CIP1/WAF1 and cyclin D proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 11324–11329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamura, T.; Hara, T.; Kotoshiba, S.; Yada, M.; Ishida, N.; Imaki, H.; Hatakeyama, S.; Nakayama, K.; Nakayama, K.I. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc. Natl. Acad. Sci. USA 2003, 100, 10231–10236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Moten, A.; Peng, D.; Hsu, C.C.; Pan, B.S.; Manne, R.; Li, H.Y.; Lin, H.K. The Skp2 Pathway: A Critical Target for Cancer Therapy. Semin. Cancer Biol. 2020, 67, 16–33. [Google Scholar] [CrossRef]
- Lee, Y.; Lim, H.S. Skp2 Inhibitors: Novel Anticancer Strategies. Curr. Med. Chem. 2016, 23, 2363–2379. [Google Scholar] [CrossRef]
- Wu, X.; Yu, M.; Zhang, Z.; Leng, F.; Ma, Y.; Xie, N.; Lu, F. DDB2 regulates DNA replication through PCNA-independent degradation of CDT2. Cell Biosci. 2021, 11, 34. [Google Scholar] [CrossRef]
- Chang, J.; Mancuso, M.R.; Maier, C.; Liang, X.; Yuki, K.; Yang, L.; Kwong, J.W.; Wang, J.; Rao, V.; Vallon, M.; et al. Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat. Med. 2017, 23, 450–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, S.I. Control of the G1/S transition. Cancer Surv. 1997, 29, 7–23. [Google Scholar] [PubMed]
- Frescas, D.; Pagano, M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: Tipping the scales of cancer. Nat. Rev. Cancer 2008, 8, 438–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedesco, D.; Lukas, J.; Reed, S.I. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCFSkp2. Genes Dev. 2002, 16, 2946–2957. [Google Scholar] [CrossRef] [Green Version]
- Tsvetkov, L.M.; Yeh, K.H.; Lee, S.J.; Sun, H.; Zhang, H. p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Current Biol. CB 1999, 9, 661–664. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Li, X.; Yan, W.; Zhang, X.; Sun, Y.; Zhang, F. SKP2 Promotes Hepatocellular Carcinoma Progression Through Nuclear AMPK-SKP2-CARM1 Signaling Transcriptionally Regulating Nutrient-Deprived Autophagy Induction. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 47, 2484–2497. [Google Scholar] [CrossRef]
- Byun, W.S.; Jin, M.; Yu, J.; Kim, W.K.; Song, J.; Chung, H.J.; Jeong, L.S.; Lee, S.K. A novel selenonucleoside suppresses tumor growth by targeting Skp2 degradation in paclitaxel-resistant prostate cancer. Biochem. Pharmacol. 2018, 158, 84–94. [Google Scholar] [CrossRef]
- Wu, J.; Su, H.K.; Yu, Z.H.; Xi, S.Y.; Guo, C.C.; Hu, Z.Y.; Qu, Y.; Cai, H.P.; Zhao, Y.Y.; Zhao, H.F.; et al. Skp2 modulates proliferation, senescence and tumorigenesis of glioma. Cancer Cell Int. 2020, 20, 71. [Google Scholar] [CrossRef] [Green Version]
- Masuda, T.A.; Inoue, H.; Sonoda, H.; Mine, S.; Yoshikawa, Y.; Nakayama, K.; Nakayama, K.; Mori, M. Clinical and biological significance of S-phase kinase-associated protein 2 (Skp2) gene expression in gastric carcinoma: Modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res. 2002, 62, 3819–3825. [Google Scholar]
- Westermann, F.; Henrich, K.O.; Wei, J.S.; Lutz, W.; Fischer, M.; Konig, R.; Wiedemeyer, R.; Ehemann, V.; Brors, B.; Ernestus, K.; et al. High Skp2 expression characterizes high-risk neuroblastomas independent of MYCN status. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 4695–4703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.K.; Chen, Z.; Wang, G.; Nardella, C.; Lee, S.W.; Chan, C.H.; Yang, W.L.; Wang, J.; Egia, A.; Nakayama, K.I.; et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature 2010, 464, 374–379. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Hu, X.; Yan, J.; Wang, Y.; Lu, F.; Chang, J. RIOK2 Inhibitor NSC139021 Exerts Anti-Tumor Effects on Glioblastoma via Inducing Skp2-Mediated Cell Cycle Arrest and Apoptosis. Biomedicines 2021, 9, 1244. https://doi.org/10.3390/biomedicines9091244
Yu M, Hu X, Yan J, Wang Y, Lu F, Chang J. RIOK2 Inhibitor NSC139021 Exerts Anti-Tumor Effects on Glioblastoma via Inducing Skp2-Mediated Cell Cycle Arrest and Apoptosis. Biomedicines. 2021; 9(9):1244. https://doi.org/10.3390/biomedicines9091244
Chicago/Turabian StyleYu, Min, Xiaoyan Hu, Jingyu Yan, Ying Wang, Fei Lu, and Junlei Chang. 2021. "RIOK2 Inhibitor NSC139021 Exerts Anti-Tumor Effects on Glioblastoma via Inducing Skp2-Mediated Cell Cycle Arrest and Apoptosis" Biomedicines 9, no. 9: 1244. https://doi.org/10.3390/biomedicines9091244





