Promising Biomarkers of Radiation-Induced Lung Injury: A Review
Abstract
:1. Introduction
2. Molecular Mechanisms of Radiation-Induced Lung Injury
3. Preclinical Animal Models of Radiation-Induced Lung Injury
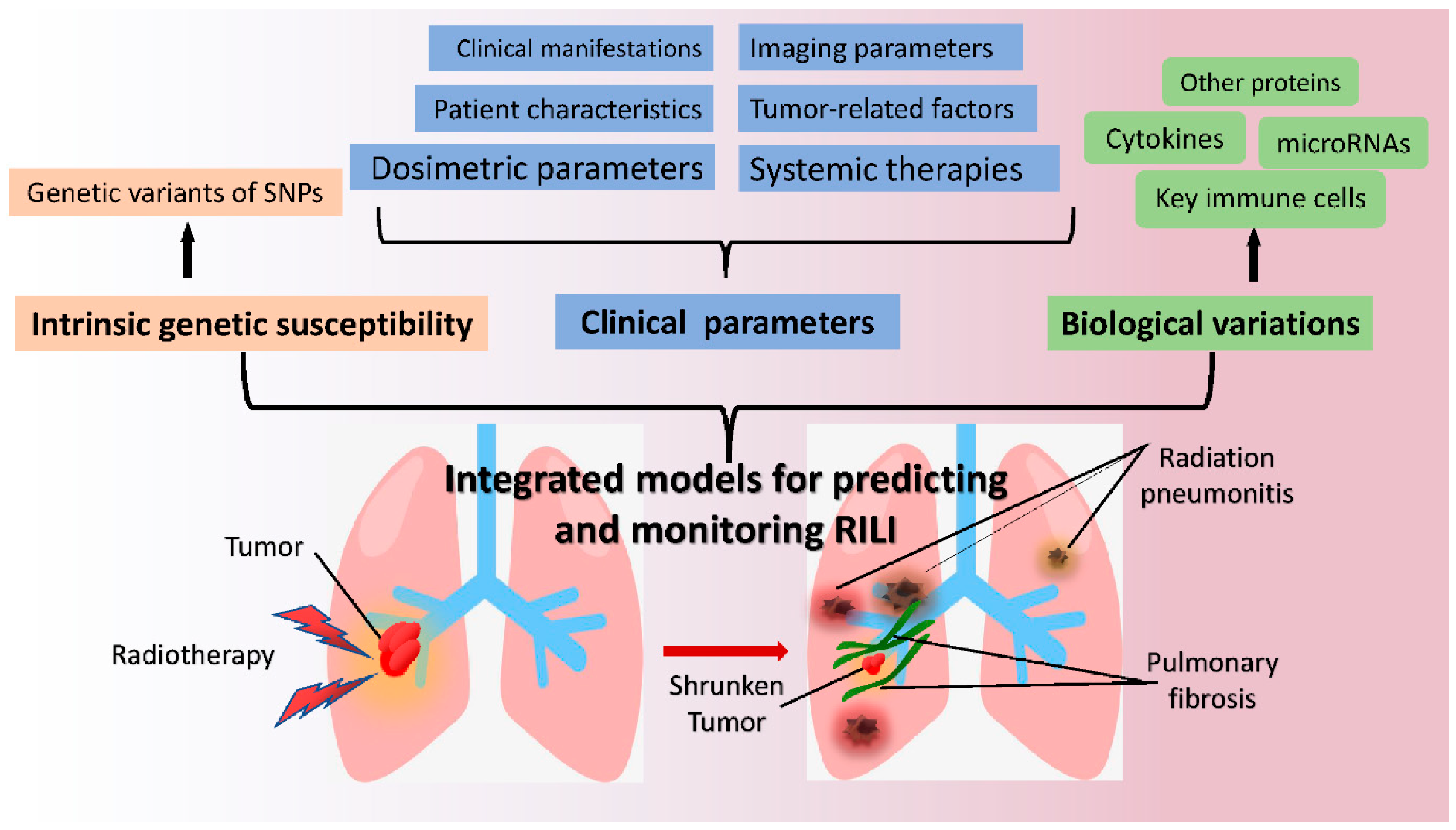
4. Potential Biomarkers for Monitoring Radiation-Induced Lung Injury
4.1. Key Immune Cells
4.2. Cytokines and Proteins
4.3. MicroRNAs
4.4. Genetic Characteristics
4.5. Imaging Based Biomarkers and Others
5. Predicting Models of Radiation-Induced Lung Injury
6. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, W.R.; Gabani, P.; Nikitas, J.; Robinson, C.G.; Bradley, J.D.; Roach, M.C. Repeat Stereotactic Body Radiation Therapy (Sbrt) for Salvage of Isolated Local Recurrence after Definitive Lung Sbrt. Radiother. Oncol. 2020, 142, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Luo, W.; Xu, H.; Wang, W.; Zhou, S.; Tang, X.; Li, Z.; Zhou, C.; Yang, H. Adaptive Intensity-Modulated Radiotherapy with Simultaneous Integrated Boost for Stage Iii Non-Small Cell Lung Cancer: Is a Routine Adaptation Beneficial? Radiother. Oncol. 2021, 158, 118–124. [Google Scholar] [CrossRef]
- van Houdt, P.J.; Yang, Y.; van der Heide, U.A. Quantitative Magnetic Resonance Imaging for Biological Image-Guided Adaptive Radiotherapy. Front. Oncol. 2020, 10, 615643. [Google Scholar] [CrossRef]
- Liao, Z.X.; Komaki, R.R.; Thames, H.D., Jr.; Liu, H.H.; Tucker, S.L.; Mohan, R.; Martel, M.K.; Wei, X.; Yang, K.; Kim, E.S.; et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.G.; Hu, C.; Choy, H.; Komaki, R.U.; Timmerman, R.D.; Schild, S.E.; Bogart, J.A.; Dobelbower, M.C.; Bosch, W.; Galvin, J.M.; et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J. Clin. Oncol. 2017, 35, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Senan, S.; Tsujino, K.; Barriger, R.B.; Rengan, R.; Moreno, M.; Bradley, J.D.; Kim, T.H.; Ramella, S.; Marks, L.B.; et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 444–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueki, N.; Matsuo, Y.; Togashi, Y.; Kubo, T.; Shibuya, K.; Iizuka, Y.; Mizowaki, T.; Togashi, K.; Mishima, M.; Hiraoka, M. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J. Thorac. Oncol. 2015, 10, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Kirkland, R.S.; Kole, A.J.; Batra, H.; Boggs, D.H.; Spencer, S.A.; Dobelbower, M.C.; Willey, C.D.; Thachuthara-George, J.T.; Wei, B.; McDonald, A.M.; et al. Predictors of in-Hospital Death in Patients with Lung Cancer Admitted for Acute Radiation Pneumonitis: A Healthcare Cost and Utilization Project (Hcup) Analysis. Clin. Lung Cancer 2021. [Google Scholar] [CrossRef]
- Ding, X.; Ji, W.; Li, J.; Zhang, X.; Wang, L. Radiation recall pneumonitis induced by chemotherapy after thoracic radiotherapy for lung cancer. Radiat. Oncol. 2011, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Antonia, S.J. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. Reply. N. Engl. J. Med. 2019, 380, 990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Song, Y.; Sun, Z.; Qiu, J.; Shi, L. Dosimetric Factors and Radiomics Features within Different Regions of Interest in Planning CT Images for Improving the Prediction of Radiation Pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, L.; Qin, W.; Yi, M.X.; Liu, B.; Yuan, X. Impact of genetic variant of HIPK2 on the risk of severe radiation pneumonitis in lung cancer patients treated with radiation therapy. Radiat. Oncol. 2020, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, L.; Qin, W.; Yi, M.; Liu, B.; Yuan, X. Validation study of the association between genetic variant of IL4 and severe radiation pneumonitis in lung cancer patients treated with radiation therapy. Radiother. Oncol. 2019, 141, 86–94. [Google Scholar] [CrossRef]
- Sliwinska-Mosson, M.; Wadowska, K.; Trembecki, L.; Bil-Lula, I. Markers Useful in Monitoring Radiation-Induced Lung Injury in Lung Cancer Patients: A Review. J. Pers. Med. 2020, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yoo, Y.; Kim, Y.; Kim, Y.; Cho, J.; Lee, Y.S. Radiation-Induced Lung Fibrosis: Preclinical Animal Models and Therapeutic Strategies. Cancers 2020, 12, 1561. [Google Scholar] [CrossRef]
- Fleckenstein, K.; Gauter-Fleckenstein, B.; Jackson, I.L.; Rabbani, Z.; Anscher, M.; Vujaskovic, Z. Using biological markers to predict risk of radiation injury. Semin Radiat. Oncol. 2007, 17, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Motevaseli, E.; Shirazi, A.; Geraily, G.; Rezaeyan, A.; Norouzi, F.; Rezapoor, S.; Abdollahi, H. Mechanisms of inflammatory responses to radiation and normal tissues toxicity: Clinical implications. Int. J. Radiat. Biol. 2018, 94, 335–356. [Google Scholar] [CrossRef]
- Yarosz, E.L.; Chang, C.H. The Role of Reactive Oxygen Species in Regulating T Cell-mediated Immunity and Disease. Immune Netw. 2018, 18, e14. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yuan, D.; Xiao, L.; Tu, W.; Dong, C.; Liu, W.; Shao, C. The crosstalk between alpha-irradiated Beas-2B cells and its bystander U937 cells through MAPK and NF-kB signaling pathways. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2016, 783, 1–8. [Google Scholar] [CrossRef]
- Moeller, B.J.; Cao, Y.; Li, C.Y.; Dewhirst, M.W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004, 5, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Fleckenstein, K.; Zgonjanin, L.; Chen, L.; Rabbani, Z.; Jackson, I.L.; Thrasher, B.; Kirkpatrick, J.; Foster, W.M.; Vujaskovic, Z. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 196–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Yang, G.; Deng, S.; Wang, Q. Oxidative stress levels and dynamic changes in mitochondrial gene expression in a radiation-induced lung injury model. J. Radiat. Res. 2019, 60, 204–214. [Google Scholar] [CrossRef]
- Hansel, C.; Jendrossek, V.; Klein, D. Cellular Senescence in the Lung: The Central Role of Senescent Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 3279. [Google Scholar] [CrossRef]
- Singh, S.; Vaughan, C.A.; Rabender, C.; Mikkelsen, R.; Deb, S.; Palit Deb, S. DNA replication in progenitor cells and epithelial regeneration after lung injury requires the oncoprotein MDM2. JCI Insight 2019, 4, e128194. [Google Scholar] [CrossRef] [PubMed]
- Yahyapour, R.; Shabeeb, D.; Cheki, M.; Musa, A.E.; Farhood, B.; Rezaeyan, A.; Amini, P.; Fallah, H.; Najafi, M. Radiation Protection and Mitigation by Natural Antioxidants and Flavonoids: Implications to Radiotherapy and Radiation Disasters. Curr. Mol. Pharmacol. 2018, 11, 285–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Zheng, L.; Zhu, Q.; Xu, C.; Li, M.; Li, H.; Yi, P.Q.; Xu, F.F.; Cao, L.; Chen, J.Y. Glycyrrhizin mitigates radiation-induced acute lung injury by inhibiting the HMGB1/TLR4 signalling pathway. J. Cell. Mol. Med. 2020, 24, 214–226. [Google Scholar] [CrossRef] [Green Version]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In Vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Wu, G.; Xiong, W.; Gu, W.; Wang, C.Y. Macrophages: Friend or foe in idiopathic pulmonary fibrosis? Respir. Res. 2018, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.J.; Zhang, L.; Hagood, J.S.; Owen, C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 585–600. [Google Scholar] [CrossRef] [Green Version]
- Rahal, O.M.; Wolfe, A.R.; Mandal, P.K.; Larson, R.; Tin, S.; Jimenez, C.; Zhang, D.; Horton, J.; Reuben, J.M.; McMurray, J.S.; et al. Blocking Interleukin (IL)4- and IL13-Mediated Phosphorylation of STAT6 (Tyr641) Decreases M2 Polarization of Macrophages and Protects Against Macrophage-Mediated Radioresistance of Inflammatory Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 1034–1043. [Google Scholar] [CrossRef] [Green Version]
- Groves, A.M.; Johnston, C.J.; Misra, R.S.; Williams, J.P.; Finkelstein, J.N. Effects of IL-4 on pulmonary fibrosis and the accumulation and phenotype of macrophage subpopulations following thoracic irradiation. Int. J. Radiat. Biol. 2016, 92, 754–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Leve, S.; Wirsdorfer, F.; Cappuccini, F.; Schutze, A.; Meyer, A.V.; Rock, K.; Thompson, L.F.; Fischer, J.W.; Stuschke, M.; Jendrossek, V. Loss of CD73 prevents accumulation of alternatively activated macrophages and the formation of prefibrotic macrophage clusters in irradiated lungs. FASEB J. 2017, 31, 2869–2880. [Google Scholar] [CrossRef] [Green Version]
- Amini, P.; Kolivand, S.; Saffar, H.; Rezapoor, S.; Motevaseli, E.; Najafi, M.; Nouruzi, F.; Shabeeb, D.; Musa, A.E. Protective Effect of Selenium-L-methionine on Radiation-Induced Acute Pneumonitis and Lung Fibrosis in Rat. Curr. Clin. Pharmacol. 2019, 14, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Qiu, J.; Ji, Y.; Li, W.; Ding, Z.; Suo, C.; Chang, J.; Wang, J.; He, R.; Qian, Y.; et al. IL-17-producing ST2+ group 2 innate lymphoid cells play a pathogenic role in lung inflammation. J. Allergy Clin. Immunol. 2019, 143, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, H.; Lv, X.; Tang, Q.; Li, W.; Zhu, H.; Long, Y. Blockade of Aquaporin 4 Inhibits Irradiation-Induced Pulmonary Inflammation and Modulates Macrophage Polarization in Mice. Inflammation 2018, 41, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Li, Z.; Yang, H.Z.; Liu, H.; Wang, J.P.; Ma, Y.G.; Wang, X.X.; Liu, H.Z.; Sun, W.; Hu, Z.W. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J. Immunol. 2011, 187, 3003–3014. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zou, L.; Ni, J.; Zhou, Y.; Ye, L.; Yang, X.; Zhu, Z. Regulatory T Cells: An Emerging Player in Radiation-Induced Lung Injury. Front. Immunol. 2020, 11, 1769. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhang, W.; Yu, F.; Gao, F. The Cellular and Molecular Mechanism of Radiation-Induced Lung Injury. Med. Sci. Monit. 2017, 23, 3446–3450. [Google Scholar] [CrossRef] [Green Version]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Cheng, H.; Wang, Z.; Chen, H.; Suo, C.; Zhang, H.; Zhang, J.; Yang, Y.; Geng, L.; Gu, M.; et al. Bortezomib attenuates renal interstitial fibrosis in kidney transplantation via regulating the EMT induced by TNF-alpha-Smurf1-Akt-mTOR-P70S6K pathway. J. Cell. Mol. Med. 2019, 23, 5390–5402. [Google Scholar] [CrossRef] [PubMed]
- Lovisa, S.; LeBleu, V.S.; Tampe, B.; Sugimoto, H.; Vadnagara, K.; Carstens, J.L.; Wu, C.C.; Hagos, Y.; Burckhardt, B.C.; Pentcheva-Hoang, T.; et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med. 2015, 21, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef]
- Lei, X.; Ma, N.; Liang, Y.; Liu, J.; Zhang, P.; Han, Y.; Chen, W.; Du, L.; Qu, B. Glucosamine protects against radiation-induced lung injury via inhibition of epithelial-mesenchymal transition. J. Cell. Mol. Med. 2020, 24, 11018–11023. [Google Scholar] [CrossRef]
- Wang, L.K.; Wu, T.J.; Hong, J.H.; Chen, F.H.; Yu, J.; Wang, C.C. Radiation Induces Pulmonary Fibrosis by Promoting the Fibrogenic Differentiation of Alveolar Stem Cells. Stem Cells Int. 2020, 2020, 6312053. [Google Scholar] [CrossRef]
- Nagarajan, D.; Melo, T.; Deng, Z.; Almeida, C.; Zhao, W. ERK/GSK3beta/Snail signaling mediates radiation-induced alveolar epithelial-to-mesenchymal transition. Free. Radic. Biol. Med. 2012, 52, 983–992. [Google Scholar] [CrossRef] [Green Version]
- Park, H.R.; Jo, S.K.; Jung, U. Ionizing Radiation Promotes Epithelial-to-Mesenchymal Transition in Lung Epithelial Cells by TGF-beta-producing M2 Macrophages. In Vivo 2019, 33, 1773–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, Y.; Han, J.; Mei, H.; Yu, D.; Ding, Q.; Zhang, T.; Wu, G.; Peng, G.; Lin, Z. Metformin Attenuates Radiation-Induced Pulmonary Fibrosis in a Murine Model. Radiat. Res. 2017, 188, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, N.; Li, F.; Sun, L.; Du, J.; Chen, Y.; Cheng, F.; Li, Y.; Tian, S.; Jiang, Q.; et al. Repeated radon exposure induced lung injury and epithelial-mesenchymal transition through the PI3K/AKT/mTOR pathway in human bronchial epithelial cells and mice. Toxicol. Lett. 2020, 334, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.H.; Han, S.Y.; Lee, Y.S.; Cho, J.; Kim, J.M. LXA4-FPR2 signaling regulates radiation-induced pulmonary fibrosis via crosstalk with TGF-beta/Smad signaling. Cell Death Dis. 2020, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Lin, Z.; Lin, X.; Lu, J.; Wang, N.; Huang, S.; Wang, Y.; Zhu, Y.; Shen, Y.; Jiang, J.; et al. IGFBP7 contributes to epithelial-mesenchymal transition of HPAEpiC cells in response to radiation. J. Cell. Biochem. 2019, 120, 12500–12507. [Google Scholar] [CrossRef]
- Shi, M.; Zhu, J.; Wang, R.; Chen, X.; Mi, L.; Walz, T.; Springer, T.A. Latent TGF-beta structure and activation. Nature 2011, 474, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, T.L.; Liu, M.N.; Zhang, Q.; Feng, W.; Yu, W.; Fu, X.L.; Cai, X.W. The positive role of vitronectin in radiation induced lung toxicity: The in vitro and in vivo mechanism study. J. Transl. Med. 2018, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Dabjan, M.B.; Buck, C.M.; Jackson, I.L.; Vujaskovic, Z.; Marples, B.; Down, J.D. A survey of changing trends in modelling radiation lung injury in mice: Bringing out the good, the bad, and the uncertain. Lab. Investig. 2016, 96, 936–949. [Google Scholar] [CrossRef]
- Williams, J.P.; Brown, S.L.; Georges, G.E.; Hauer-Jensen, M.; Hill, R.P.; Huser, A.K.; Kirsch, D.G.; Macvittie, T.J.; Mason, K.A.; Medhora, M.M.; et al. Animal models for medical countermeasures to radiation exposure. Radiat. Res. 2010, 173, 557–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Z.Y.; Lee, C.G.; Shim, H.S.; Lee, E.J.; Song, K.H.; Choi, B.W.; Cho, J.; Story, M.D. Time, Dose, and Volume Responses in a Mouse Pulmonary Injury Model Following Ablative Irradiation. Lung 2016, 194, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, R.; Lindsay, P.E.; Ansell, S.; Wilson, G.; Jelveh, S.; Hill, R.P.; Jaffray, D.A. Characterization of image quality and image-guidance performance of a preclinical microirradiator. Med. Phys. 2011, 38, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Kodym, R.; Seliounine, S.; Richardson, J.A.; Solberg, T.D.; Story, M.D. High dose-per-fraction irradiation of limited lung volumes using an image-guided, highly focused irradiator: Simulating stereotactic body radiotherapy regimens in a small-animal model. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 895–902. [Google Scholar] [CrossRef]
- Liao, Z.X.; Travis, E.L.; Tucker, S.L. Damage and morbidity from pneumonitis after irradiation of partial volumes of mouse lung. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 1359–1370. [Google Scholar] [CrossRef]
- Jin, H.; Kang, G.Y.; Jeon, S.; Kim, J.M.; Park, Y.N.; Cho, J.; Lee, Y.S. Identification of molecular signatures involved in radiation-induced lung fibrosis. J. Mol. Med. 2019, 97, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Szapiel, S.V.; Elson, N.A.; Fulmer, J.D.; Hunninghake, G.W.; Crystal, R.G. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am. Rev. Respir. Dis. 1979, 120, 893–899. [Google Scholar] [CrossRef]
- Roberts, R.; McCune, S.K. Animal studies in the development of medical countermeasures. Clin. Pharmacol. Ther. 2008, 83, 918–920. [Google Scholar] [CrossRef]
- Fox, J.; Haston, C.K. CXC receptor 1 and 2 and neutrophil elastase inhibitors alter radiation-induced lung disease in the mouse. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 215–222. [Google Scholar] [CrossRef]
- Meziani, L.; Mondini, M.; Petit, B.; Boissonnas, A.; Thomas de Montpreville, V.; Mercier, O.; Vozenin, M.C.; Deutsch, E. CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. Eur. Respir. J. 2018, 51, 1702120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijerathne, H.; Langston, J.C.; Yang, Q.; Sun, S.; Miyamoto, C.; Kilpatrick, L.E.; Kiani, M.F. Mechanisms of radiation-induced endothelium damage: Emerging models and technologies. Radiother. Oncol. 2021, 158, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Jarzebska, N.; Karetnikova, E.S.; Markov, A.G.; Kasper, M.; Rodionov, R.N.; Spieth, P.M. Scarred Lung. An Update on Radiation-Induced Pulmonary Fibrosis. Front. Med. 2020, 7, 585756. [Google Scholar] [CrossRef]
- Wahsner, J.; Desogere, P.; Abston, E.; Graham-O’Regan, K.A.; Wang, J.; Rotile, N.J.; Schirmer, M.D.; Santos Ferreira, D.D.; Sui, J.; Fuchs, B.C.; et al. 68Ga-NODAGA-Indole: An Allysine-Reactive Positron Emission Tomography Probe for Molecular Imaging of Pulmonary Fibrogenesis. J. Am. Chem. Soc. 2019, 141, 5593–5596. [Google Scholar] [CrossRef]
- Desogere, P.; Tapias, L.F.; Hariri, L.P.; Rotile, N.J.; Rietz, T.A.; Probst, C.K.; Blasi, F.; Day, H.; Mino-Kenudson, M.; Weinreb, P.; et al. Type I collagen-targeted PET probe for pulmonary fibrosis detection and staging in preclinical models. Sci. Transl. Med. 2017, 9, eaaf4696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.; Luo, W.; Wang, W.; Zhou, C.; Zhou, S.; Tang, X.; Hou, L.; Kong, F.S.; Yang, H. Intermediate Dose-Volume Parameters, Not Low-Dose Bath, Is Superior to Predict Radiation Pneumonitis for Lung Cancer Treated With Intensity-Modulated Radiotherapy. Front. Oncol. 2020, 10, 584756. [Google Scholar] [CrossRef]
- Katsui, K.; Ogata, T.; Watanabe, K.; Katayama, N.; Soh, J.; Kuroda, M.; Kiura, K.; Maeda, Y.; Toyooka, S.; Kanazawa, S. Dose-volume parameters predict radiation pneumonitis after induction chemoradiotherapy followed by surgery for non-small cell lung cancer: A retrospective analysis. BMC Cancer 2019, 19, 1144. [Google Scholar] [CrossRef]
- Bai, L.; Zhou, B.S.; Zhao, Y.X. Dynamic changes in T-cell subsets and C-reactive protein after radiation therapy in lung cancer patients and correlation with symptomatic radiation pneumonitis treated with steroid therapy. Cancer Manag. Res. 2019, 11, 7925–7931. [Google Scholar] [CrossRef] [Green Version]
- Alam, R.; Gorska, M. 3. Lymphocytes. J. Allergy Clin. Immunol. 2003, 111, S476–S485. [Google Scholar] [CrossRef]
- Paun, A.; Bergeron, M.E.; Haston, C.K. The Th1/Th17 balance dictates the fibrosis response in murine radiation-induced lung disease. Sci. Rep. 2017, 7, 11586. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012, 11, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Choi, H.S.; Jeong, H.; Kang, K.M.; Song, J.H.; Lee, W.S.; Lee, G.W.; Song, H.N.; Kim, H.G.; Kang, M.H.; et al. Neutrophil-lymphocyte ratio and a dosimetric factor for predicting symptomatic radiation pneumonitis in non-small-cell lung cancer patients treated with concurrent chemoradiotherapy. Clin. Respir. J. 2018, 12, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocana, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, W.; Yao, Y.; Xu, B.; Wei, X.; Wang, L.; Yin, X.; Barman, A.K.; Zhang, F.; Zhang, C.; et al. Naringenin Ameliorates Radiation-Induced Lung Injury by Lowering IL-1beta Level. J. Pharmacol. Exp. Ther. 2018, 366, 341–348. [Google Scholar] [CrossRef]
- Deng, Y.; Qiu, T.; Patel, N.; Zhou, S.; Xue, T.; Zhang, H. Clinical Management of Risk of Radiation Pneumonia with Serum Markers During the Radiotherapy for Patients with Thoracic Malignant Tumors. Cancer Manag. Res. 2019, 11, 10249–10256. [Google Scholar] [CrossRef] [Green Version]
- Siva, S.; MacManus, M.; Kron, T.; Best, N.; Smith, J.; Lobachevsky, P.; Ball, D.; Martin, O. A pattern of early radiation-induced inflammatory cytokine expression is associated with lung toxicity in patients with non-small cell lung cancer. PLoS ONE 2014, 9, e109560. [Google Scholar] [CrossRef]
- Kong, F.M.; Ao, X.; Wang, L.; Lawrence, T.S. The use of blood biomarkers to predict radiation lung toxicity: A potential strategy to individualize thoracic radiation therapy. Cancer Control 2008, 15, 140–150. [Google Scholar] [CrossRef]
- Arpin, D.; Perol, D.; Blay, J.Y.; Falchero, L.; Claude, L.; Vuillermoz-Blas, S.; Martel-Lafay, I.; Ginestet, C.; Alberti, L.; Nosov, D.; et al. Early variations of circulating interleukin-6 and interleukin-10 levels during thoracic radiotherapy are predictive for radiation pneumonitis. J. Clin. Oncol. 2005, 23, 8748–8756. [Google Scholar] [CrossRef]
- Yang, L.; Herrera, J.; Gilbertsen, A.; Xia, H.; Smith, K.; Benyumov, A.; Bitterman, P.B.; Henke, C.A. IL-8 mediates idiopathic pulmonary fibrosis mesenchymal progenitor cell fibrogenicity. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L127–L136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Wu, H.; Wang, W.; Jolly, S.; Jin, J.Y.; Hu, C.; Kong, F.S. Machine Learning to Build and Validate a Model for Radiation Pneumonitis Prediction in Patients with Non-Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 4343–4350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Campbell, J.; Stenmark, M.H.; Zhao, J.; Stanton, P.; Matuszak, M.M.; Ten Haken, R.K.; Kong, F.S. Plasma Levels of IL-8 and TGF-beta1 Predict Radiation-Induced Lung Toxicity in Non-Small Cell Lung Cancer: A Validation Study. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Castanares, C.; Redondo-Horcajo, M.; Magan-Marchal, N.; ten Dijke, P.; Lamas, S.; Rodriguez-Pascual, F. Signaling by ALK5 mediates TGF-beta-induced ET-1 expression in endothelial cells: A role for migration and proliferation. J. Cell Sci. 2007, 120, 1256–1266. [Google Scholar] [CrossRef] [Green Version]
- Shi-wen, X.; Kennedy, L.; Renzoni, E.A.; Bou-Gharios, G.; du Bois, R.M.; Black, C.M.; Denton, C.P.; Abraham, D.J.; Leask, A. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor beta in human lung fibroblasts. Arthritis Rheum. 2007, 56, 4189–4194. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Shibamoto, Y.; Baba, F.; Sugie, C.; Ogino, H.; Murata, R.; Yanagi, T.; Otsuka, S.; Kosaki, K.; Murai, T.; et al. Correlation between the serum KL-6 level and the grade of radiation pneumonitis after stereotactic body radiotherapy for stage I lung cancer or small lung metastasis. Radiother. Oncol. 2011, 101, 267–270. [Google Scholar] [CrossRef]
- Ishikawa, N.; Hattori, N.; Yokoyama, A.; Kohno, N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir. Investig. 2012, 50, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Lee, E.Y.; Ha, Y.J.; Kang, E.H.; Lee, Y.J.; Song, Y.W. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res. Ther. 2019, 21, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.K.; Vaughan, D.E. PAI-1 in tissue fibrosis. J. Cell Physiol. 2012, 227, 493–507. [Google Scholar] [CrossRef] [Green Version]
- Aso, S.; Navarro-Martin, A.; Castillo, R.; Padrones, S.; Castillo, E.; Montes, A.; Martinez, J.I.; Cubero, N.; Lopez, R.; Rodriguez, L.; et al. Severity of radiation pneumonitis, from clinical, dosimetric and biological features: A pilot study. Radiat. Oncol. 2020, 15, 246. [Google Scholar] [CrossRef] [PubMed]
- Shioya, S.; Masuda, T.; Senoo, T.; Horimasu, Y.; Miyamoto, S.; Nakashima, T.; Iwamoto, H.; Fujitaka, K.; Hamada, H.; Hattori, N. Plasminogen activator inhibitor-1 serves an important role in radiation-induced pulmonary fibrosis. Exp. Ther. Med. 2018, 16, 3070–3076. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.; Schmetter, A.; Imsak, R.; Wirsdorfer, F.; Unger, K.; Jastrow, H.; Stuschke, M.; Jendrossek, V. Therapy with Multipotent Mesenchymal Stromal Cells Protects Lungs from Radiation-Induced Injury and Reduces the Risk of Lung Metastasis. Antioxid. Redox Signal. 2016, 24, 53–69. [Google Scholar] [CrossRef] [Green Version]
- Vu, T.N.; Chen, X.; Foda, H.D.; Smaldone, G.C.; Hasaneen, N.A. Interferon-gamma enhances the antifibrotic effects of pirfenidone by attenuating IPF lung fibroblast activation and differentiation. Respir. Res. 2019, 20, 206. [Google Scholar] [CrossRef] [Green Version]
- Gamez, A.S.; Gras, D.; Petit, A.; Knabe, L.; Molinari, N.; Vachier, I.; Chanez, P.; Bourdin, A. Supplementing defect in club cell secretory protein attenuates airway inflammation in COPD. Chest 2015, 147, 1467–1476. [Google Scholar] [CrossRef]
- Groves, A.M.; Williams, J.P.; Hernady, E.; Reed, C.; Fenton, B.; Love, T.; Finkelstein, J.N.; Johnston, C.J. A Potential Biomarker for Predicting the Risk of Radiation-Induced Fibrosis in the Lung. Radiat. Res. 2018, 190, 513–525. [Google Scholar] [CrossRef]
- Sasaki, R.; Soejima, T.; Matsumoto, A.; Maruta, T.; Yamada, K.; Ota, Y.; Kawabe, T.; Nishimura, H.; Sakai, E.; Ejima, Y.; et al. Clinical significance of serum pulmonary surfactant proteins a and d for the early detection of radiation pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 301–307. [Google Scholar] [CrossRef]
- Bao, E.T.; Zhao, W.G.; Mou, M.; Liu, X.F. MicroRNA-21 mediates bone marrow mesenchymal stem cells protection of radiation-induced lung injury during the acute phase by regulating polarization of alveolar macrophages. Transl. Cancer Res. 2020, 9, 231–239. [Google Scholar] [CrossRef]
- Duru, N.; Zhang, Y.; Gernapudi, R.; Wolfson, B.; Lo, P.K.; Yao, Y.; Zhou, Q. Loss of miR-140 is a key risk factor for radiation-induced lung fibrosis through reprogramming fibroblasts and macrophages. Sci. Rep. 2016, 6, 39572. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Pu, X.; Ye, Y.; Lu, C.; Chang, J.Y.; Wu, X. Association between Genetic Variants in DNA Double-Strand Break Repair Pathways and Risk of Radiation Therapy-Induced Pneumonitis and Esophagitis in Non-Small Cell Lung Cancer. Cancers 2016, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, Z.; Zhang, J.; Li, H.; Qiao, Y.; Huang, C.; Li, B. Genetic Variants in MTHFR Gene Predict ≥ 2 Radiation Pneumonitis in Esophageal Squamous Cell Carcinoma Patients Treated with Thoracic Radiotherapy. PLoS ONE 2017, 12, e0169147. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, L.; Yu, J.; Jiang, M.; Zhang, S.; Cai, X.; Zhu, M. Genetic variations in DNA repair gene NEIL1 associated with radiation pneumonitis risk in lung cancer patients. Mol. Genet. Genomic. Med. 2021, e1698. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, B.; Li, J.; Wu, H.; Yang, J.; Zhou, X.; Yi, M.; Li, Q.; Yu, S.; Yuan, X. Genetic variants in PI3K/AKT pathway are associated with severe radiation pneumonitis in lung cancer patients treated with radiation therapy. Cancer Med. 2016, 5, 24–32. [Google Scholar] [CrossRef]
- Wen, J.; Liu, H.; Wang, L.; Wang, X.; Gu, N.; Liu, Z.; Xu, T.; Gomez, D.R.; Komaki, R.; Liao, Z.; et al. Potentially Functional Variants of ATG16L2 Predict Radiation Pneumonitis and Outcomes in Patients with Non-Small Cell Lung Cancer after Definitive Radiotherapy. J. Thorac. Oncol. 2018, 13, 660–675. [Google Scholar] [CrossRef] [Green Version]
- Yi, M.; Tang, Y.; Liu, B.; Li, Q.; Zhou, X.; Yu, S.; Fu, S.; Cai, Y.; Yuan, X. Genetic variants in the ITGB6 gene is associated with the risk of radiation pneumonitis in lung cancer patients treated with thoracic radiation therapy. Tumor Biol. 2016, 37, 3469–3477. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yi, M.; Tang, Y.; Liu, Q.; Qiu, H.; Zou, Y.; Peng, P.; Zhang, L.; Hu, C.; Yuan, X. MMP-1 promoter polymorphism is associated with risk of radiation-induced lung injury in lung cancer patients treated with radiotherapy. Oncotarget 2016, 7, 70175–70184. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, L.; Yan, D.; Huang, C.; Chen, G.; Wang, Z.; Zhong, L.; Luo, W.; Chen, D.; Chun, C.; et al. Early variations in lymphocytes and T lymphocyte subsets are associated with radiation pneumonitis in lung cancer patients and experimental mice received thoracic irradiation. Cancer Med. 2020, 9, 3437–3444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Zhou, Z.; Fei, L.; Sun, H.; Wang, R.; Chen, Y.; Chen, H.; Wang, J.; Tang, H.; Ge, W.; et al. Construction of a human cell landscape at single-cell level. Nature 2020, 581, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, T.; Sun, K.H.; Wetter, J.B.; Wilson-Kanamori, J.R.; Hazelwood, L.A.; Henderson, N.C.; Adams, T.S.; Schupp, J.C.; Poli, S.D.; Rosas, I.O.; et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat. Commun. 2020, 11, 1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagnoli, J.W.; Ziegenhain, C.; Janjic, A.; Wange, L.E.; Vieth, B.; Parekh, S.; Geuder, J.; Hellmann, I.; Enard, W. Sensitive and powerful single-cell RNA sequencing using mcSCRB-seq. Nat. Commun. 2018, 9, 2937. [Google Scholar] [CrossRef] [Green Version]
- Squillaro, T.; Finicelli, M.; Alessio, N.; Del Gaudio, S.; Di Bernardo, G.; Melone, M.A.B.; Peluso, G.; Galderisi, U. A rapid, safe, and quantitative in vitro assay for measurement of uracil-DNA glycosylase activity. J. Mol. Med. 2019, 97, 991–1001. [Google Scholar] [CrossRef]
- Wu, D.; Waalkes, A.; Penewit, K.; Salipante, S.J. Ultrasensitive Detection of Chimerism by Single-Molecule Molecular Inversion Probe Capture and High-Throughput Sequencing of Copy Number Deletion Polymorphisms. Clin. Chem. 2018, 64, 938–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Wang, M.; Pang, Z.; Jiang, F.; Chen, J.; Zhang, J. Locally instilled tumor necrosis factor alpha antisense oligonucleotide contributes to inhibition of TH2-driven pulmonary fibrosis via induced CD4+ CD25+ Foxp3+ regulatory T cells. J. Gene Med. 2013, 15, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Refahi, S.; Pourissa, M.; Zirak, M.R.; Hadadi, G. Modulation expression of tumor necrosis factor alpha in the radiation-induced lung injury by glycyrrhizic acid. J. Med. Phys. 2015, 40, 95–101. [Google Scholar] [CrossRef]
- Zhang, M.; Qian, J.; Xing, X.; Kong, F.M.; Zhao, L.; Chen, M.; Lawrence, T.S. Inhibition of the tumor necrosis factor-alpha pathway is radioprotective for the lung. Clin. Cancer Res. 2008, 14, 1868–1876. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Chen, S.H.; Lu, H.J.; Tan, Y. Predictive values of TNF-alpha, IL-6, IL-10 for radiation pneumonitis. Int. J. Radiat. Res. 2016, 14, 173–179. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, W.; Yan, X.; Guo, T.; Wang, B.; Xia, H.; Ye, L.; Xiong, J.; Jiang, Z.; Liu, Y.; et al. Prognostic Value of C-Reactive Protein in Patients With Coronavirus 2019. Clin. Infect. Dis. 2020, 71, 2174–2179. [Google Scholar] [CrossRef]
- Avan, A.; Tavakoly Sany, S.B.; Ghayour-Mobarhan, M.; Rahimi, H.R.; Tajfard, M.; Ferns, G. Serum C-reactive protein in the prediction of cardiovascular diseases: Overview of the latest clinical studies and public health practice. J. Cell Physiol. 2018, 233, 8508–8525. [Google Scholar] [CrossRef]
- Anscher, M.S.; Marks, L.B.; Shafman, T.D.; Clough, R.; Huang, H.; Tisch, A.; Munley, M.; Herndon, J.E.; Garst, J.; Crawford, J.; et al. Risk of long-term complications after TFG-beta1-guided very-high-dose thoracic radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 988–995. [Google Scholar] [CrossRef]
- Zhao, L.; Sheldon, K.; Chen, M.; Yin, M.S.; Hayman, J.A.; Kalemkerian, G.P.; Arenberg, D.; Lyons, S.E.; Curtis, J.L.; Davis, M.; et al. The predictive role of plasma TGF-beta1 during radiation therapy for radiation-induced lung toxicity deserves further study in patients with non-small cell lung cancer. Lung Cancer 2008, 59, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, L.; Ji, W.; Wang, X.; Zhu, X.; Hayman, J.A.; Kalemkerian, G.P.; Yang, W.; Brenner, D.; Lawrence, T.S.; et al. Elevation of plasma TGF-beta1 during radiation therapy predicts radiation-induced lung toxicity in patients with non-small-cell lung cancer: A combined analysis from Beijing and Michigan. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1385–1390. [Google Scholar] [CrossRef]
- Aliasgharzadeh, A.; Farhood, B.; Amini, P.; Saffar, H.; Motevaseli, E.; Rezapoor, S.; Nouruzi, F.; Shabeeb, D.H.; Eleojo Musa, A.; Mohseni, M.; et al. Melatonin Attenuates Upregulation of Duox1 and Duox2 and Protects against Lung Injury following Chest Irradiation in Rats. Cell J. 2019, 21, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Doucet, C.; Brouty-Boye, D.; Pottin-Clemenceau, C.; Canonica, G.W.; Jasmin, C.; Azzarone, B. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J. Clin. Investig. 1998, 101, 2129–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karsdal, M.A.; Nielsen, S.H.; Leeming, D.J.; Langholm, L.L.; Nielsen, M.J.; Manon-Jensen, T.; Siebuhr, A.; Gudmann, N.S.; Ronnow, S.; Sand, J.M.; et al. The good and the bad collagens of fibrosis—Their role in signaling and organ function. Adv. Drug Deliv. Rev. 2017, 121, 43–56. [Google Scholar] [CrossRef]
- Klinkhammer, B.M.; Floege, J.; Boor, P. PDGF in organ fibrosis. Mol. Asp. Med. 2018, 62, 44–62. [Google Scholar] [CrossRef]
- Bonner, J.C. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004, 15, 255–273. [Google Scholar] [CrossRef]
- Munk, H.L.; Fakih, D.; Christiansen, L.; Tan, Q.; Christensen, A.F.; Ejstrup, L.; Loft, A.G.; Junker, K.; Kyvik, K.O.; Jounblat, R.; et al. Surfactant protein-D, a potential mediator of inflammation in axial spondyloarthritis. Rheumatology 2018, 57, 1861–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Ivashkiv, L.B. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity 2009, 31, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Bushati, N.; Cohen, S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007, 23, 175–205. [Google Scholar] [CrossRef]
- Chen, L.; Heikkinen, L.; Wang, C.; Yang, Y.; Sun, H.; Wong, G. Trends in the development of miRNA bioinformatics tools. Brief. Bioinform. 2019, 20, 1836–1852. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Y.; Dou, Y.; Chen, L.; Wang, J.; Jiang, N.; Guo, C.; Yao, Q.; Wang, C.; Liu, L.; et al. Degradation of unmethylated miRNA/miRNA*s by a DEDDy-type 3′ to 5′ exoribonuclease Atrimmer 2 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E6659–E6667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Gao, S.; Lindberg, D.; Panja, D.; Wakabayashi, Y.; Li, K.; Kleinman, J.E.; Zhu, J.; Li, Z. Temporal dynamics of miRNAs in human DLPFC and its association with miRNA dysregulation in schizophrenia. Transl. Psychiatry 2019, 9, 196. [Google Scholar] [CrossRef]
- Chen, X.; Wu, L.; Li, D.; Xu, Y.; Zhang, L.; Niu, K.; Kong, R.; Gu, J.; Xu, Z.; Chen, Z.; et al. Radiosensitizing effects of miR-18a-5p on lung cancer stem-like cells via downregulating both ATM and HIF-1alpha. Cancer Med. 2018, 7, 3834–3847. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Huang, D.; Huang, X.; Wang, W.; Yang, S.; Chen, S. Exosomal microRNA-26b-5p down-regulates ATF2 to enhance radiosensitivity of lung adenocarcinoma cells. J. Cell. Mol. Med. 2020, 24, 7730–7742. [Google Scholar] [CrossRef]
- Dinh, T.K.; Fendler, W.; Chalubinska-Fendler, J.; Acharya, S.S.; O’Leary, C.; Deraska, P.V.; D’Andrea, A.D.; Chowdhury, D.; Kozono, D. Circulating miR-29a and miR-150 correlate with delivered dose during thoracic radiation therapy for non-small cell lung cancer. Radiat. Oncol. 2016, 11, 61. [Google Scholar] [CrossRef]
- Rogers, C.J.; Lukaszewicz, A.I.; Yamada-Hanff, J.; Micewicz, E.D.; Ratikan, J.A.; Starbird, M.A.; Miller, T.A.; Nguyen, C.; Lee, J.T.; Olafsen, T.; et al. Identification of miRNA signatures associated with radiation-induced late lung injury in mice. PLoS ONE 2020, 15, e0232411. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Shin, H.; Gao, F.; Zhang, J.; Bahamon, B.; Danaee, H.; Melichar, B.; Schilder, R.J.; Coleman, R.L.; Falchook, G.; et al. Aurora A Functional Single Nucleotide Polymorphism (SNP) Correlates With Clinical Outcome in Patients With Advanced Solid Tumors Treated With Alisertib, an Investigational Aurora A Kinase Inhibitor. EBioMedicine 2017, 25, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Kerns, S.L.; Dorling, L.; Fachal, L.; Bentzen, S.; Pharoah, P.D.; Barnes, D.R.; Gomez-Caamano, A.; Carballo, A.M.; Dearnaley, D.P.; Peleteiro, P.; et al. Meta-analysis of Genome Wide Association Studies Identifies Genetic Markers of Late Toxicity Following Radiotherapy for Prostate Cancer. EBioMedicine 2016, 10, 150–163. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.X.; Wang, J.; Huang, L.H.; Li, J.G.; Chen, X. Impact of single-nucleotide polymorphisms on radiation pneumonitis in cancer patients. Mol. Clin. Oncol. 2016, 4, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Bartek, J.; Mailand, N. TOPping up ATR activity. Cell 2006, 124, 888–890. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Yu, W.; Huang, X.; Zhao, N.; Liu, F.; Tong, F.; Zhang, S.; Niu, B.; Liu, X.; Xu, S.; et al. GSTP1 Ile105Val polymorphism might be associated with the risk of radiation pneumonitis among lung cancer patients in Chinese population: A prospective study. J. Cancer 2018, 9, 726–735. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.D.; Pulford, D.J. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol 1995, 30, 445–600. [Google Scholar] [CrossRef] [PubMed]
- Kral-Pointner, J.B.; Schrottmaier, W.C.; Salzmann, M.; Mussbacher, M.; Schmidt, G.J.; Moser, B.; Heber, S.; Birnecker, B.; Paar, H.; Zellner, M.; et al. Platelet PI3K Modulates Innate Leukocyte Extravasation during Acid-Induced Acute Lung Inflammation. Thromb. Haemost. 2019, 119, 1642–1654. [Google Scholar] [CrossRef] [PubMed]
- Villegas, S.N.; Gombos, R.; Garcia-Lopez, L.; Gutierrez-Perez, I.; Garcia-Castillo, J.; Vallejo, D.M.; Da Ros, V.G.; Ballesta-Illan, E.; Mihaly, J.; Dominguez, M. PI3K/Akt Cooperates with Oncogenic Notch by Inducing Nitric Oxide-Dependent Inflammation. Cell Rep. 2018, 22, 2541–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Ma, J.; Zhou, S.; Hubbs, J.L.; Wong, T.Z.; Folz, R.J.; Evans, E.S.; Jaszczak, R.J.; Clough, R.; Marks, L.B. Radiation-induced reductions in regional lung perfusion: 0.1–12 year data from a prospective clinical study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Medhora, M.; Haworth, S.; Liu, Y.; Narayanan, J.; Gao, F.; Zhao, M.; Audi, S.; Jacobs, E.R.; Fish, B.L.; Clough, A.V. Biomarkers for Radiation Pneumonitis Using Noninvasive Molecular Imaging. J. Nucl. Med. 2016, 57, 1296–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farr, K.P.; Khalil, A.A.; Moller, D.S.; Bluhme, H.; Kramer, S.; Morsing, A.; Grau, C. Time and dose-related changes in lung perfusion after definitive radiotherapy for NSCLC. Radiother. Oncol. 2018, 126, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, J.; Audi, S.; Razeghi-Kondelaji, M.H.; Fish, B.L.; Hansen, C.; Narayan, J.; Gao, F.; Sharma, G.; Parchur, A.K.; Banerjee, A.; et al. A Rapid Dynamic In Vivo Near-Infrared Fluorescence Imaging Assay to Track Lung Vascular Permeability after Acute Radiation injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L436–L450. [Google Scholar] [CrossRef]
- Petit, S.F.; van Elmpt, W.J.; Oberije, C.J.; Vegt, E.; Dingemans, A.M.; Lambin, P.; Dekker, A.L.; De Ruysscher, D. [18F]fluorodeoxyglucose uptake patterns in lung before radiotherapy identify areas more susceptible to radiation-induced lung toxicity in non-small-cell lung cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.A.; Binkley, M.S.; Rigdon, J.; Carter, J.N.; Aggarwal, S.; Dudley, S.A.; Qian, Y.; Kumar, K.A.; Hara, W.Y.; Gensheimer, M.; et al. Pre-treatment non-target lung FDG-PET uptake predicts symptomatic radiation pneumonitis following Stereotactic Ablative Radiotherapy (SABR). Radiother. Oncol. 2016, 119, 454–460. [Google Scholar] [CrossRef]
- Abdulla, S.; Salavati, A.; Saboury, B.; Basu, S.; Torigian, D.A.; Alavi, A. Quantitative assessment of global lung inflammation following radiation therapy using FDG PET/CT: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.A.; Arimura, H.; Ninomiya, K.; Yoshitake, T.; Fukunaga, J.I.; Shioyama, Y. Radiomic prediction of radiation pneumonitis on pretreatment planning computed tomography images prior to lung cancer stereotactic body radiation therapy. Sci. Rep. 2020, 10, 20424. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Shi, A.; Zhang, R.; Wen, Q.; Niu, T.; Chen, J.; Qiu, Q.; Wan, Y.; Sun, X.; Xing, L. Cone-beam CT radiomics features might improve the prediction of lung toxicity after SBRT in stage I NSCLC patients. Thorac. Cancer 2020, 11, 964–972. [Google Scholar] [CrossRef]
- Marks, L.B.; Bentzen, S.M.; Deasy, J.O.; Kong, F.M.; Bradley, J.D.; Vogelius, I.S.; El Naqa, I.; Hubbs, J.L.; Lebesque, J.V.; Timmerman, R.D.; et al. Radiation dose-volume effects in the lung. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S70–S76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appelt, A.L.; Vogelius, I.R.; Farr, K.P.; Khalil, A.A.; Bentzen, S.M. Towards individualized dose constraints: Adjusting the QUANTEC radiation pneumonitis model for clinical risk factors. Acta Oncol. 2014, 53, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Defraene, G.; Schuit, E.; De Ruysscher, D. Development and internal validation of a multinomial NTCP model for the severity of acute dyspnea after radiotherapy for lung cancer. Radiother. Oncol. 2019, 136, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.L.; Liu, H.H.; Liao, Z.; Wei, X.; Wang, S.; Jin, H.; Komaki, R.; Martel, M.K.; Mohan, R. Analysis of radiation pneumonitis risk using a generalized Lyman model. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 568–574. [Google Scholar] [CrossRef] [Green Version]
- Jain, V.; Niezink, A.G.H.; Frick, M.; Doucette, A.; Mendes, A.; Simone, C.B., II; Langendijk, J.A.; Wijsman, R.; Feigenberg, S.J.; Levin, W.; et al. Updating Photon-Based Normal Tissue Complication Probability Models for Pneumonitis in Patients With Lung Cancer Treated With Proton Beam Therapy. Pract. Radiat. Oncol. 2020, 10, e330–e338. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.G.; Boonstra, P.S.; Hobson, S.T.; Hearn, J.W.D.; Hayman, J.A.; Ten Haken, R.K.; Matuszak, M.M.; Stanton, P.; Kalemkerian, G.P.; Ramnath, N.; et al. Radiation-induced lung toxicity in non-small-cell lung cancer: Understanding the interactions of clinical factors and cytokines with the dose-toxicity relationship. Radiother. Oncol. 2017, 125, 66–72. [Google Scholar] [CrossRef]
- Yu, H.; Lam, K.O.; Wu, H.; Green, M.; Wang, W.; Jin, J.Y.; Hu, C.; Jolly, S.; Wang, Y.; Kong, F.S. Weighted-Support Vector Machine Learning Classifier of Circulating Cytokine Biomarkers to Predict Radiation-Induced Lung Fibrosis in Non-Small-Cell Lung Cancer Patients. Front. Oncol. 2020, 10, 601979. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tang, Y.; Yi, M.; Liu, Q.; Xiong, H.; Hu, G.; Yuan, X. Genetic variants in the plasminogen activator inhibitor-1 gene are associated with an increased risk of radiation pneumonitis in lung cancer patients. Cancer Med. 2017, 6, 681–688. [Google Scholar] [CrossRef]
- Citrin, D.E.; Prasanna, P.G.S.; Walker, A.J.; Freeman, M.L.; Eke, I.; Barcellos-Hoff, M.H.; Arankalayil, M.J.; Cohen, E.P.; Wilkins, R.C.; Ahmed, M.M.; et al. Radiation-Induced Fibrosis: Mechanisms and Opportunities to Mitigate. Report of an NCI Workshop, September 19, 2016. Radiat. Res. 2017, 188, 1–20. [Google Scholar] [CrossRef]
- Hanania, A.N.; Mainwaring, W.; Ghebre, Y.T.; Hanania, N.A.; Ludwig, M. Radiation-Induced Lung Injury: Assessment and Management. Chest 2019, 156, 150–162. [Google Scholar] [CrossRef] [PubMed]
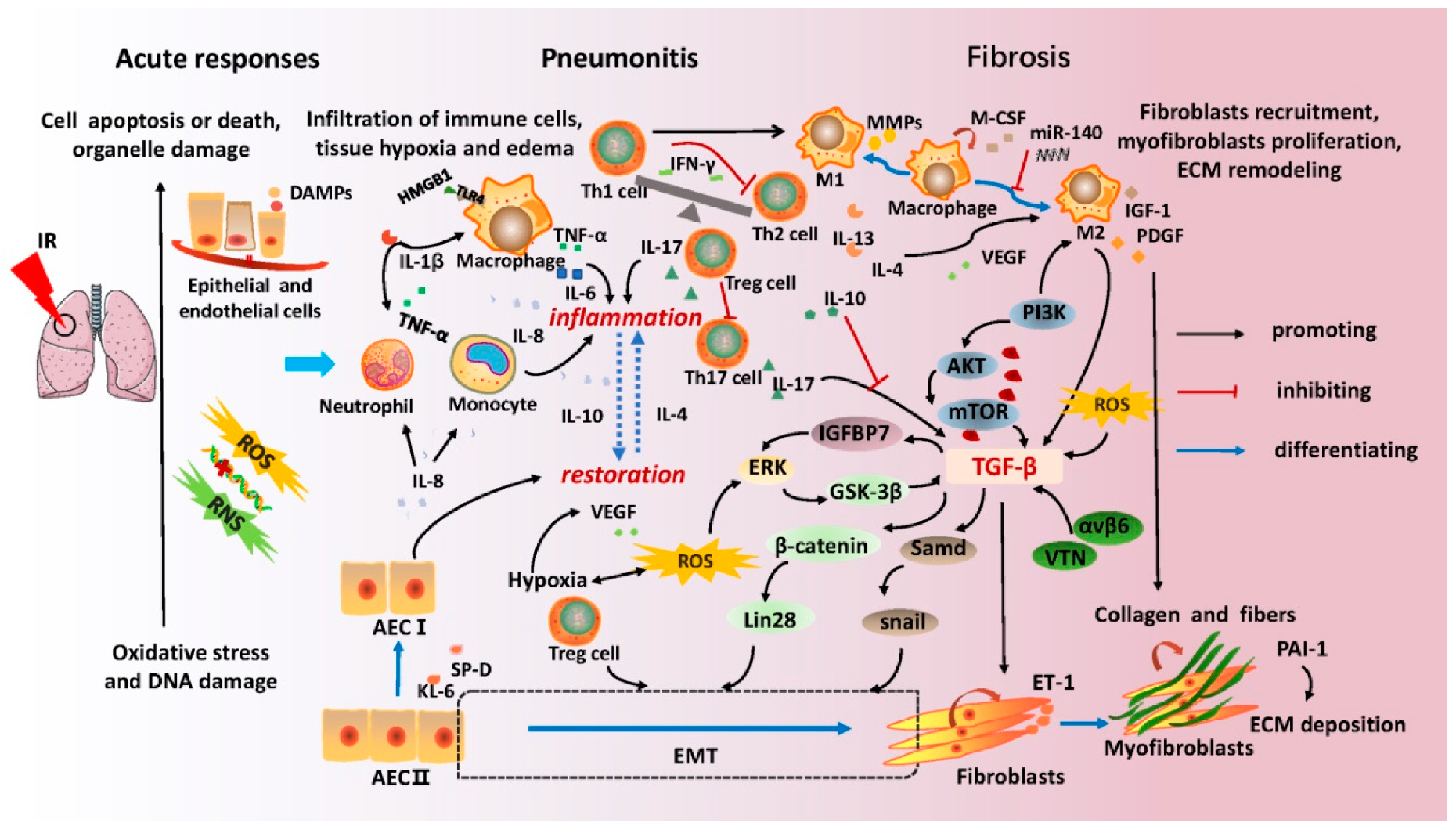
| Categories | Biomarkers | Function | Correlation Research | Ref. |
|---|---|---|---|---|
| Immune cells | T-cell subsets | T cells determine the specificity of immune responses in tissue inflammation, autoimmunity and host defense | Decreased lymphocytes predicted the severity of RP in LC patients | [72,73,74,75] |
| NLR | NLR is an important biomarker of inflammatory status and disease exacerbation | Higher NLR in NSCLC patients with radiological RP predicted the development of symptomatic RP | [76,77] | |
| Inflammation-related factors | IL-1β | IL-1β promotes the recruitment of inflammatory cells and the release of chemokines | IL-1β level was a significant initiator of RILI both in vivo and in vitro studies | [78,79] |
| IL-6 | IL-6 regulates cells proliferation and differentiation, hematopoiesis, angiogenesis and immune reactions | IL-6 level was a potential monitor for RILI development clinically | [80,81,82] | |
| IL-10 | IL-10 is an anti-inflammatory cytokine by blocking the production of pro-inflammatory cytokines and inhibiting the capabilities of antigen-presenting cells | IL-10 level was low throughout the irradiation period in RP patients, various IL-10 levels monitored different RP scales | [83] | |
| CRP | CRP is an acute phase inflammatory protein and elevated after injury, infection or inflammation | CRP level in plasma was a potential predictor for RILI development in LC patients | [72] | |
| IL-8 | IL-8 has an anti-inflammatory effect and mediates pulmonary fibrosis | Lower pre-IL-8 level predicted higher risk of grade 2 RILI in LC patients | [84,85,86] | |
| Fibrosis-related factors | TGF-β | TGF-β promotes the differentiation of fibroblasts into myofibroblasts and synthesis of ECM proteins, and reduces collagen degradation, leading to lung fibrosis | Higher TGF-β1 in plasma monitored symptomatic RILI both in vivo and in vitro studies | [51,86] |
| ET-1 | ET-1 inhibits the proliferation and migration of endothelial cells and promotes ECM production | ET-1 monitored the dynamic changes of PF in mice | [87,88] | |
| KL-6 | KL-6 has chemotactic and anti-apoptotic effects on fibroblasts, leading to lung fibrosis | Increased KL-6 level monitored PF activity and predicted RP severity in patients | [89,90,91] | |
| PAI-1 | PAI-1 inhibits the plasmin system through blocking fibrinolysis and degradation of the ECM | PAI-1 level predicted PF development in patients | [92,93,94] | |
| Chemokines | CCL2/MCP-1 | CCL2, also called MCP-1, is a potent chemokine for monocytes | Lower CCL2 level monitored patients with grade 2 RP | [81,86,95] |
| Other proteins | IFN-γ | IFN-γ is a pleiotropic cytokine with antitumor, antiviral, antibacterial, pro-inflammatory and antifibrotic properties | IFN-γ level indicated the ability to attenuate fibrosis formation in patients | [93,96] |
| SP-D | SP-D works in host defense and regulates immune responses and lung phospholipid levels | Elevated SP-D is a sensitive biomarker for early RILI prediction both in patients and murine models | [97,98,99] | |
| miRNAs | miR-21 | BMSCs inhibit the pro-inflammatory pathway of macrophage 1 in a miR-21 dependent manner | miR-21 over-expressed in BMSCs significantly alleviated alveolitis in RILI rats | [100] |
| miR-140 | miR-140 protects lung tissue from fibrosis through blocking TGF-β1 signaling and inhibiting myofibroblast differentiation | Loss of miR-140 in the lung tissue is a key risk factor for PF murine | [101] |
| SNPs | Year of Publication | Gene Function | Correlation Research | Ref. |
|---|---|---|---|---|
| TOPBP1: rs1051772 | 2016 | DNA repair | decreased risk of RP in NSCLC patients | [102] |
| MTHFR: rs1801131 | 2017 | DNA repair | decreased risk of grade ≥ 2 RP in esophageal squamous cell carcinoma patients | [103] |
| NEIL1: rs4462560 | 2021 | DNA repair | decreased risk of grade ≥ 2 RP in LC patients | [104] |
| NEIL1: rs7402844 | 2021 | DNA repair | higher risk of grade ≥ 2 RP in LC patients | [104] |
| PI3CA: rs9838117 AKT2: rs33933140, rs11880261 | 2016 | Inflammation | higher risk of grade ≥ 3 RP in LC patients | [105] |
| IL4: rs2243250 | 2019 | Inflammation | higher risk of grade ≥ 3 RP in LC patients | [13] |
| ATG16L2: rs10898880 | 2018 | Autophagy | higher risk of RP in NSCLC patients | [106] |
| PAI-1: rs7242 | 2017 | Plasmin system inhibition | higher risk of grade ≥ 3 RP in LC patients | [92] |
| ITGB6: rs4665162 | 2016 | Cell surface adhesion | higher risk of grade ≥ 2 RP in LC patients | [107] |
| MMP-1: rs1144393 | 2018 | Protein degradation | higher risk of grade ≥ 2 RILI in LC patients | [108] |
| HIPK2: rs2030712 | 2020 | Cell apoptosis, proliferation and DNA repair | higher risk of grade ≥ 2 RP in LC patients | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Shao, C.; Fu, J. Promising Biomarkers of Radiation-Induced Lung Injury: A Review. Biomedicines 2021, 9, 1181. https://doi.org/10.3390/biomedicines9091181
Liu X, Shao C, Fu J. Promising Biomarkers of Radiation-Induced Lung Injury: A Review. Biomedicines. 2021; 9(9):1181. https://doi.org/10.3390/biomedicines9091181
Chicago/Turabian StyleLiu, Xinglong, Chunlin Shao, and Jiamei Fu. 2021. "Promising Biomarkers of Radiation-Induced Lung Injury: A Review" Biomedicines 9, no. 9: 1181. https://doi.org/10.3390/biomedicines9091181
APA StyleLiu, X., Shao, C., & Fu, J. (2021). Promising Biomarkers of Radiation-Induced Lung Injury: A Review. Biomedicines, 9(9), 1181. https://doi.org/10.3390/biomedicines9091181







