Genotoxicity of Paragonimus heterotremus Infection in a Rat Model of Simultaneous Pulmonary and Hepatic Paragonimiasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rat Infecting
2.2. Preparation of Single-Cell Suspensions
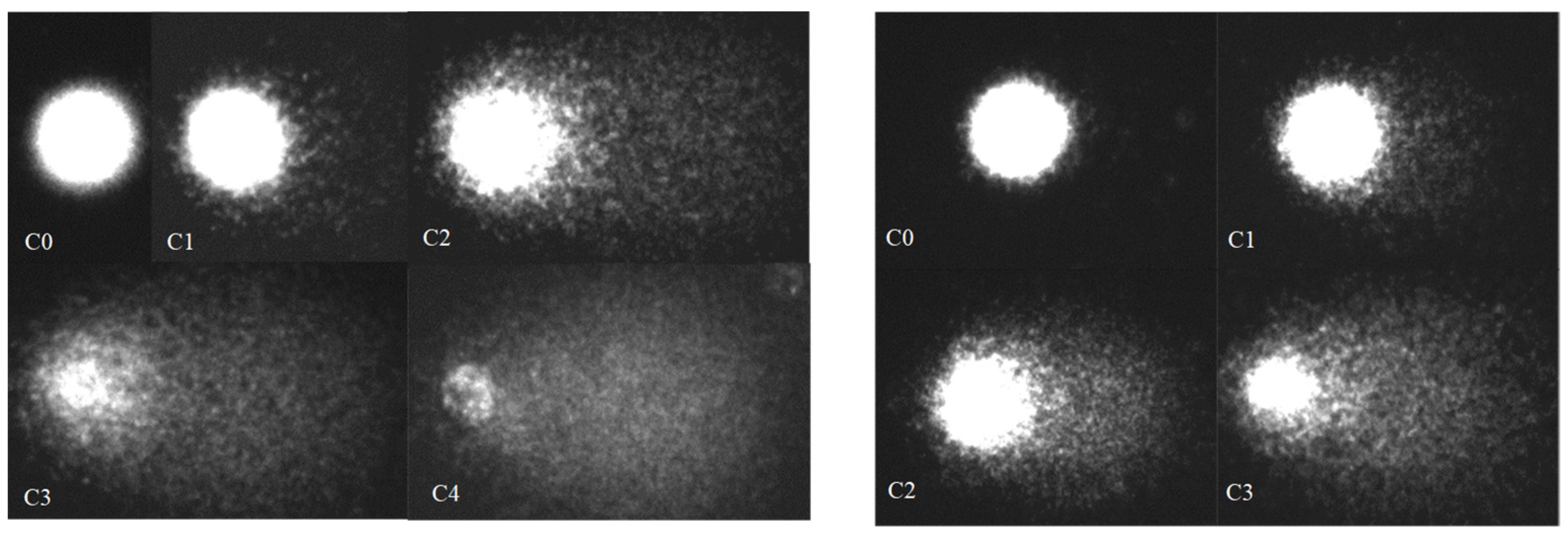
2.3. Alkaline Single-Cell Gel Electrophoresis (Comet) Assay
2.4. Statistical Analysis
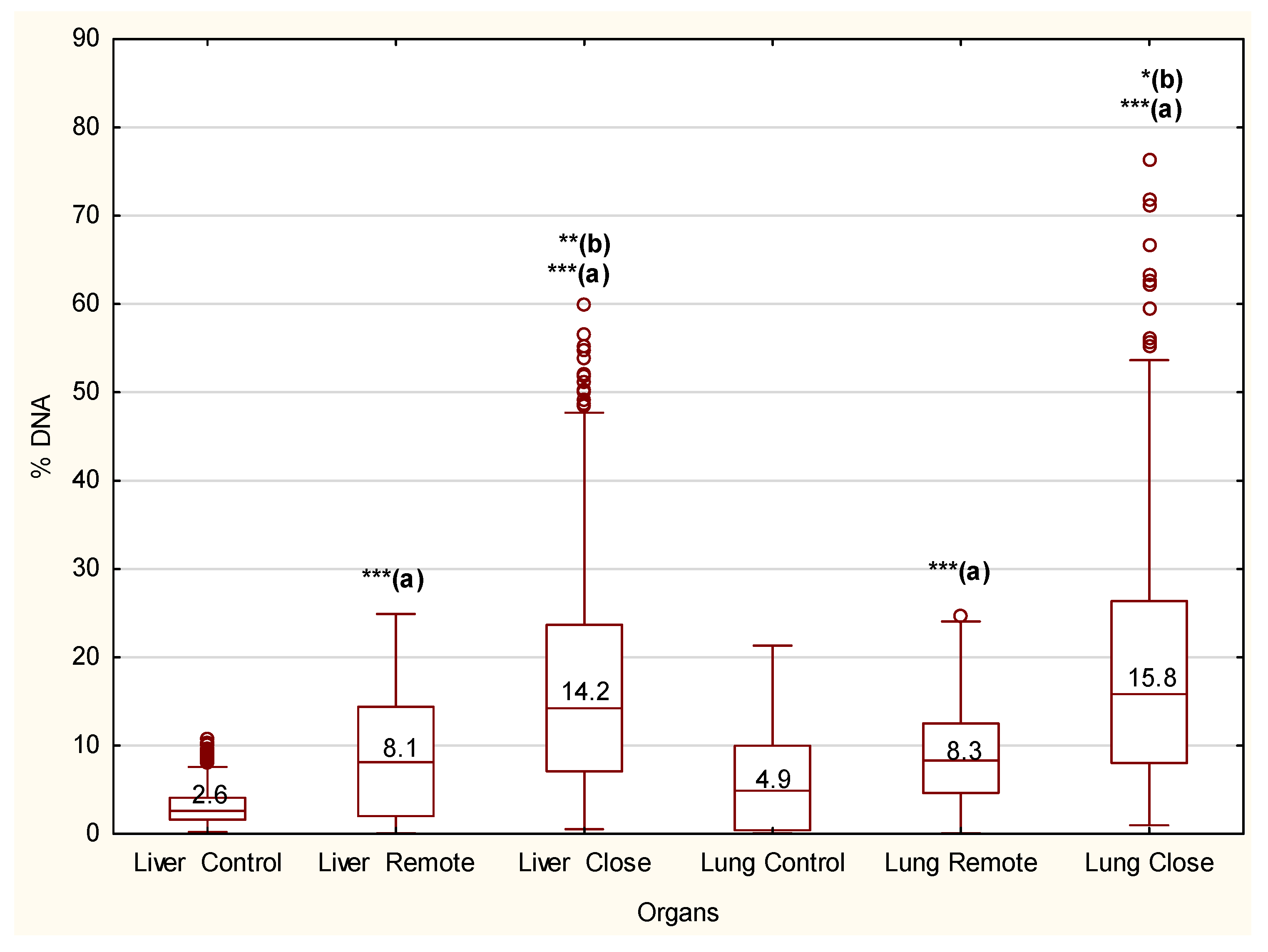
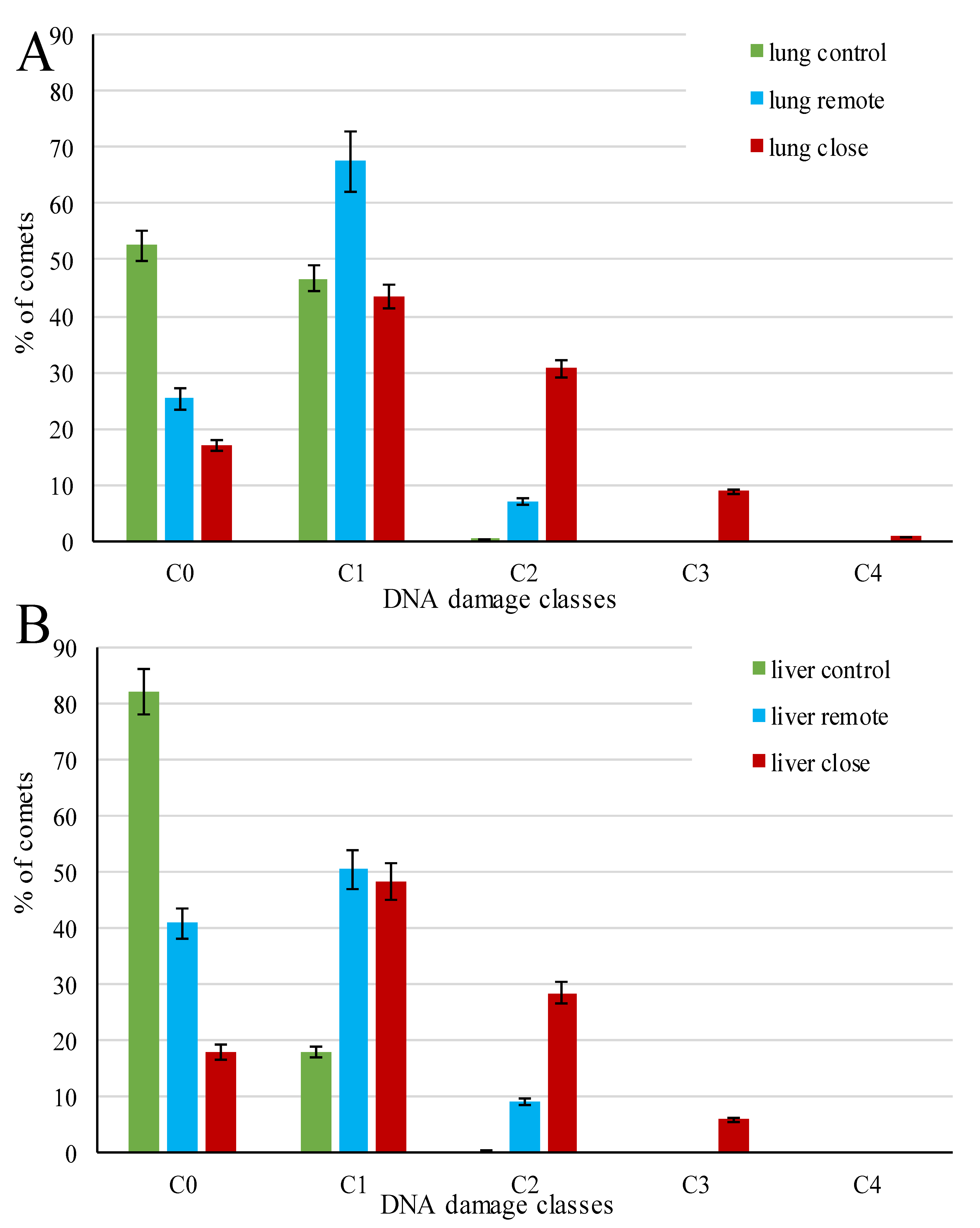
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathi, R.; Jaiswal, N.; Sharma, B.; Malhotra, S.K. Helminth infections mediated DNA damage: Mechanisms and consequences. Single Cell Biol. 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Shareef, P.A.A.; Rehman, A.; Ullah, R.; Rehman, L.; Abidi, S.M.A. Genotoxic potential of Fasciola gigantica infection in experimentally infected rabbits. J. Parasit. Dis. 2016, 41, 423–428. [Google Scholar] [CrossRef]
- Chelomina, G.N. Chapter 66, Clonorchis. In Handbook of Foodborne Diseases; Liu, D., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 723–726. [Google Scholar] [CrossRef]
- Machicado, C.; Marcos, L.A. Carcinogenesis associated with parasites other than Schistosoma, Opisthorchis and Clonorchis: A systematic review. Int. J. Cancer 2016, 138, 2915–2921. [Google Scholar] [CrossRef] [PubMed]
- Callejas, B.E.; Martínez-Saucedo, D.; Terrazas, L.I. Parasites as negative regulators of cancer. Biosci. Rep. 2018, 38, 30266743. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Botelho, M.C. Fasciola hepatica extract induces cell death of mammalian cells. Anti-Infect. Agents 2018, 16, 144–146. [Google Scholar] [CrossRef]
- Pakharukova, M.Y.; Zaparina, O.G.; Kapushchak, Y.K.; Baginskaya, N.V.; Mordvinov, V.A. Opisthorchis felineus infection provokes time-dependent accumulation of oxidative hepatobiliary lesions in the injured hamster liver. PLoS ONE 2019, 14, e0216757. [Google Scholar] [CrossRef]
- Robertson, L.J.; van der Giessen, J.W.B.; Batz, M.B.; Kojima, M.; Cahill, S. Have foodborne parasites finally become a global concern? Trends Parasitol. 2013, 29, 101–103. [Google Scholar] [CrossRef]
- Blair, D. Paragonimiasis, Digenetic Trematodes; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Yoshida, A.; Doanh, P.N.; Maruyama, H. Paragonimus and paragonimiasis in Asia: An update. Acta Trop. 2019, 199, 105074. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Brindley, P.J.; Mulvenna, J.; Laha, T.; Smout, M.; Mairiang, E.; Bethony, J.M.; Loukas, A. The tumorigenic liver fluke Opisthorchis viverrini—Multiple pathways to cancer. Trends Parasitol. 2012, 28, 395–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagonas, K.; Meyer, B.S.; Kaufmann, J.; Lenz, T.L.; Häsler, R.; Eizaguirre, C. Experimental parasite infection causes genome-wide changes in DNA methylation. Mol. Biol. Evol. 2020, 37, 2287–2299. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Interactions 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Salim, E.I.; Morimura, K.; Menesi, A.; El-Lity, M.; Fukushima, S.; Wanibuchi, H. Elevated oxidative stress and DNA damage and repair levels in urinary bladder carcinomas associated with schistosomiasis. Int. J. Cancer 2008, 123, 601–608. [Google Scholar] [CrossRef]
- Morris, I.D.; Ilott, S.; Dixon, L.; Brison, D.R. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum. Reprod. 2002, 17, 990–998. [Google Scholar] [CrossRef]
- Valverde, M.; Rojas, E. Environmental and occupational biomonitoring using the Comet assay. Mutat. Res. Mutat. Res. 2009, 681, 93–109. [Google Scholar] [CrossRef]
- Vodicka, P.; Vodenkova, S.; Opattova, A.; Vodickova, L. DNA damage and repair measured by comet assay in cancer patients. Mutat. Res. Gent. Toxicol. Environ. Mutagen. 2019, 843, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Voronova, A.N.; Besprozvannykh, V.V.; Ngo, H.D.; Plekhova, N.G.; Hung, N.M.; Tatonova, Y.V. Paragonimus heterotremus Chen et Hsia, 1964 (Digenea: Paragonimidae): Species identification based on the biological and genetic criteria, and pathology of infection. Parasitol. Res. 2020, 119, 4073–4088. [Google Scholar] [CrossRef]
- Slobodskova, V.V.; Zhukovskaya, A.F.; Chelomin, V.P. DNA damage in the gill cells of the marine scallop Mizuhopecten yessoensis during anoxic stress and aerobic recovery. Ocean Sci. J. 2012, 47, 95–100. [Google Scholar] [CrossRef]
- Cavas, T.; Konen, S. In vitro genotoxicity testing of the amnesic shellfish poison (domoic acid) in piscine erythrocytes using the micronucleus test and comet assay. Aquat. Toxicol. 2008, 90, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarieh, M.; Schoetzau, A.; Sauter, M.; Grieshaber, M.; Orgül, S.; Golubnitschaja, O.; Flammer, J. Comet assay analysis of single–stranded DNA breaks in circulating leukocytes of glaucoma patients. Mol. Vis. 2008, 14, 1584–1588. [Google Scholar] [PubMed]
- O’Hagan, H.; Mohammad, H.P.; Baylin, S.B. Double strand breaks can initiate gene silencing and SIRT1-dependent onset of DNA methylation in an exogenous promoter CpG island. PLoS Genet. 2008, 4, e1000155. [Google Scholar] [CrossRef] [PubMed]
- Møller, P. Genotoxicity of environmental agents assessed by the alkaline comet assay. Basic Clin. Pharmacol. Toxicol. 2005, 96 (Suppl. 1), 1–42. [Google Scholar] [PubMed]
- Chelomin, V.P.; Slobodskova, V.V.; Zakhartsev, M.; Kukla, S. Genotoxic potential of copper oxide nanoparticles in the bivalve mollusk Mytilus trossulus. J. Ocean Univ. China 2017, 16, 339–345. [Google Scholar] [CrossRef]
- Harba, N.M.; Afifi, A.F. Evaluation of DNA damage by DNA fragmentation and Comet assays in experimental toxoplasmosis with virulent strain. PUJ 2012, 5, 189–198. [Google Scholar]
- Shi, Y.; Wang, P.; Guo, Y.; Liang, X.; Li, Y.; Ding, S. Helicobacter pylori—Induced DNA damage is a potential driver for human gastric cancer AGS cells. DNA Cell Biol. 2019, 38, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Salazar, A.M.; Mendlovic, F.; Cruz-Rivera, M.; Chávez-Talavera, O.; Sordo, M.; Avila, G.; Flisser, A.; Ostrosky-Wegman, P.; Salazar, A.M.; Mendlovic, F.; et al. Genotoxicity induced by Taenia soliumand its reduction by immunization with calreticulin in a hamster model of taeniosis. Environ. Mol. Mutagen. 2013, 54, 347–353. [Google Scholar] [CrossRef]
- Bekish, V.Y.; Durnev, A.D. In vitro genotoxic and cytotoxic effects of protein somatic products from helminths on donor blood lymphocytes. Bull. Exp. Biol. Med. 2004, 138, 174–176. [Google Scholar] [CrossRef]

| Index | Control | Lung | Liver | |||
|---|---|---|---|---|---|---|
| Lung | Liver | Close | Remote | Close | Remote | |
| GDI | 0.48 ± 0.034 | 0.18 ± 0.013 | 1.33 ± 0.093 | 0.82 ± 0.057 | 1.23 ± 0.0161 | 0.68 ± 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelomina, G.N.; Kukla, S.P.; Chelomin, V.P.; Doanh, P.N. Genotoxicity of Paragonimus heterotremus Infection in a Rat Model of Simultaneous Pulmonary and Hepatic Paragonimiasis. Biomedicines 2021, 9, 1180. https://doi.org/10.3390/biomedicines9091180
Chelomina GN, Kukla SP, Chelomin VP, Doanh PN. Genotoxicity of Paragonimus heterotremus Infection in a Rat Model of Simultaneous Pulmonary and Hepatic Paragonimiasis. Biomedicines. 2021; 9(9):1180. https://doi.org/10.3390/biomedicines9091180
Chicago/Turabian StyleChelomina, Galina N., Sergey P. Kukla, Viktor P. Chelomin, and Pham N. Doanh. 2021. "Genotoxicity of Paragonimus heterotremus Infection in a Rat Model of Simultaneous Pulmonary and Hepatic Paragonimiasis" Biomedicines 9, no. 9: 1180. https://doi.org/10.3390/biomedicines9091180
APA StyleChelomina, G. N., Kukla, S. P., Chelomin, V. P., & Doanh, P. N. (2021). Genotoxicity of Paragonimus heterotremus Infection in a Rat Model of Simultaneous Pulmonary and Hepatic Paragonimiasis. Biomedicines, 9(9), 1180. https://doi.org/10.3390/biomedicines9091180






