Association of Caspase 3 Activation and H2AX γ Phosphorylation in the Aging Brain: Studies on Untreated and Irradiated Mice
Abstract
:1. Introduction
2. Materials and Methods
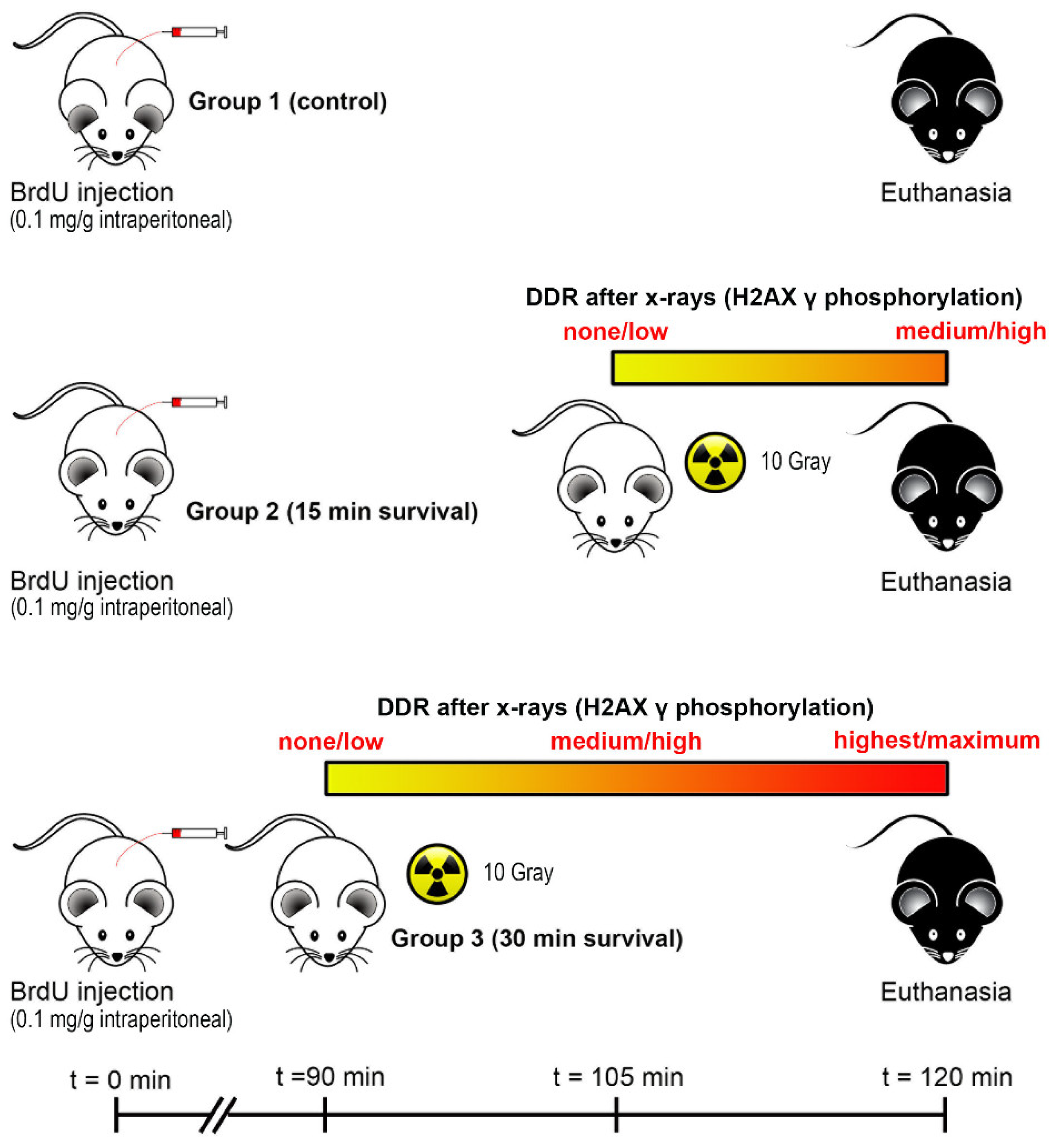
2.1. Animals and X-ray Irradiation
2.2. Brain Sampling and Tissue Processing
2.3. Immunofluorescence (IMF)
2.3.1. Choice of Sections from Series
2.3.2. Single and Double IMF Procedures
2.4. Primary Antibodies and Controls
2.5. Quantitative Studies
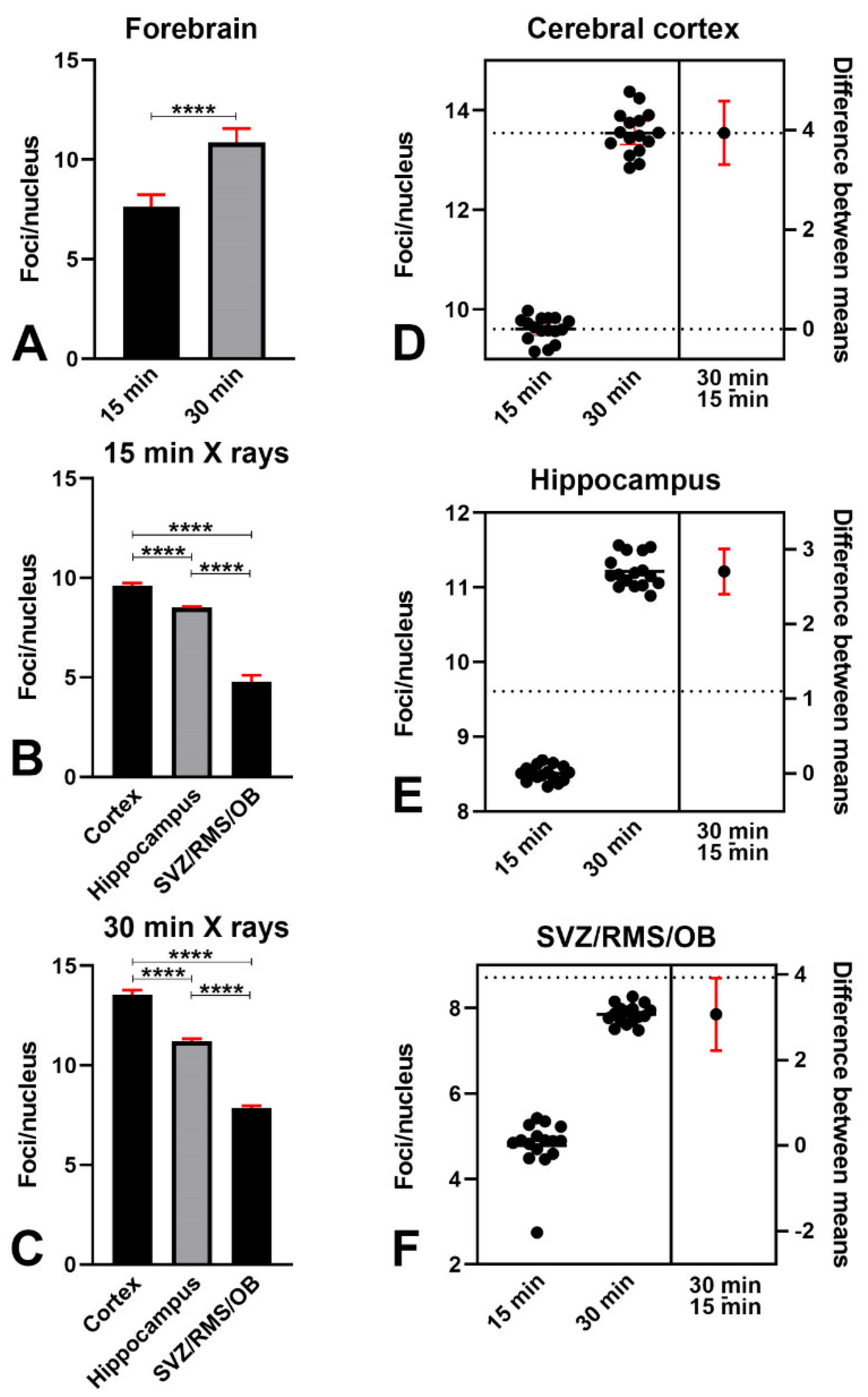
2.5.1. Counting γH2AX-Immunoreactive Nuclear Foci
2.5.2. Counting Immunoreactive Cells
2.5.3. Determination of Volumetric Cellular Densities
2.6. Statistics
3. Results
3.1. X-ray Irradiation Induces a Strong γ Phosphorylation of H2AX and a Change in the Pattern of γH2AX Nuclear Staining
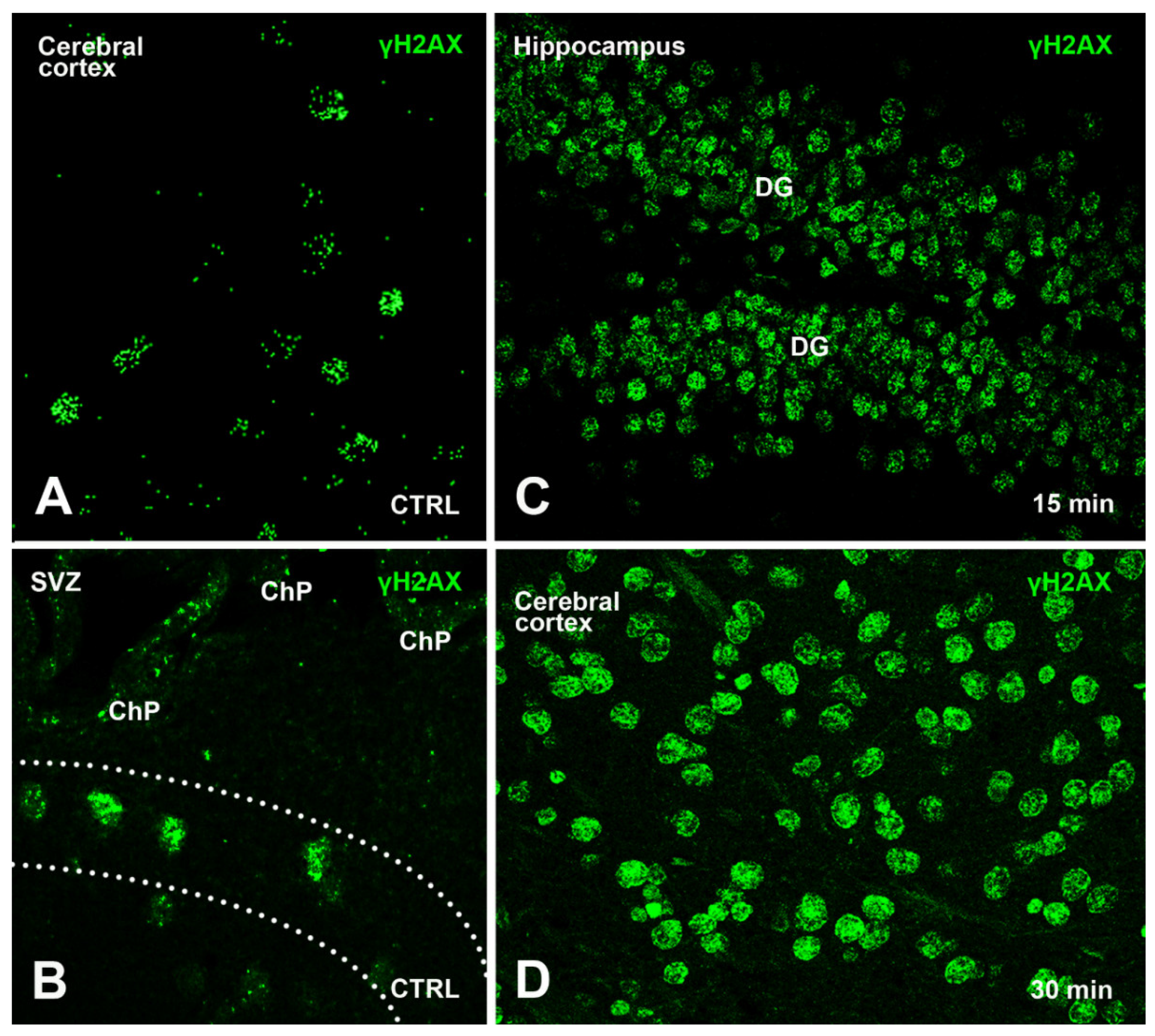
3.1.1. γH2AX Immunoreactivity in Control Mice
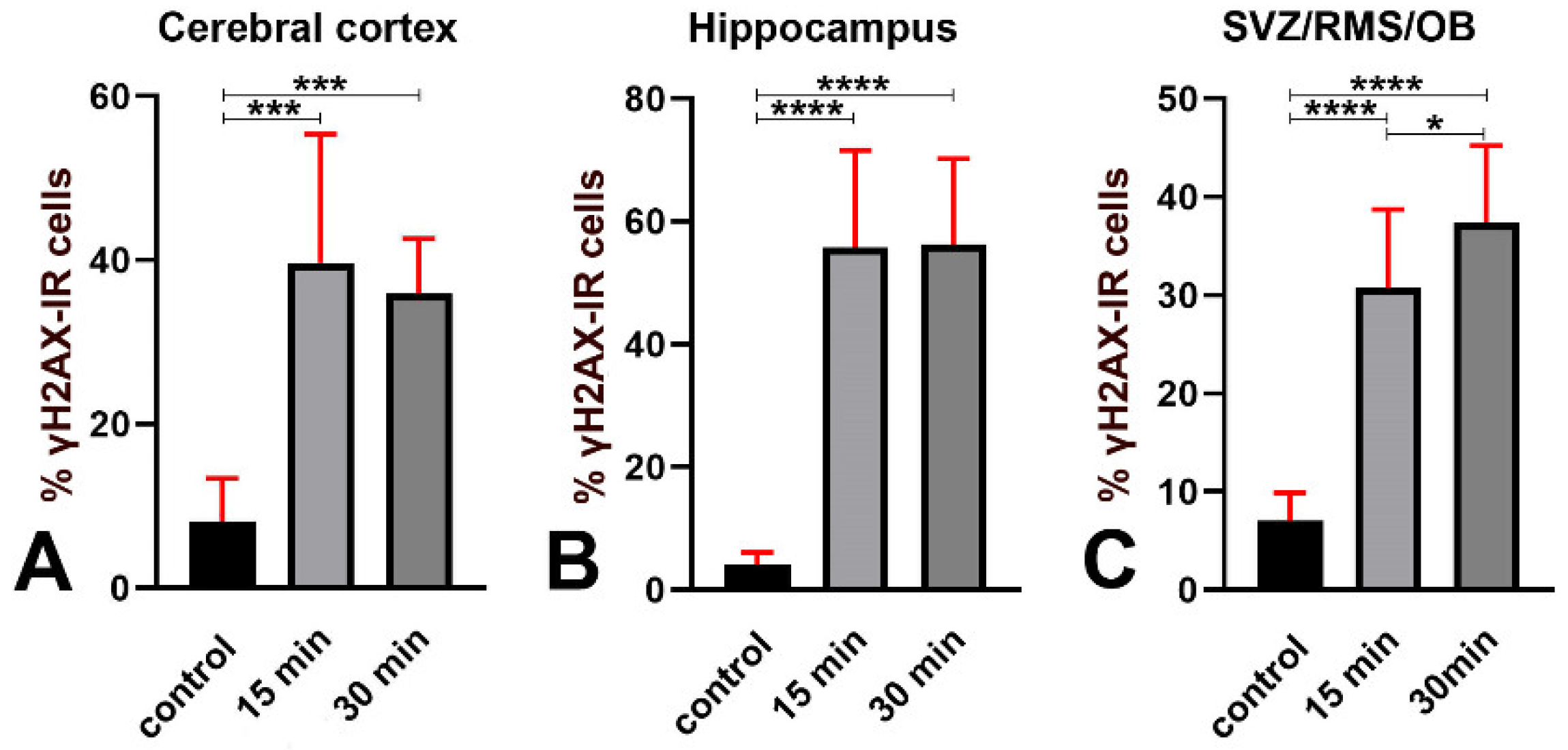
3.1.2. Effects of Irradiation on γ Phosphorylation of H2AX
3.2. Expression of 53BP1 in γH2AX Immunoreactive Cells after X-ray Irradiation
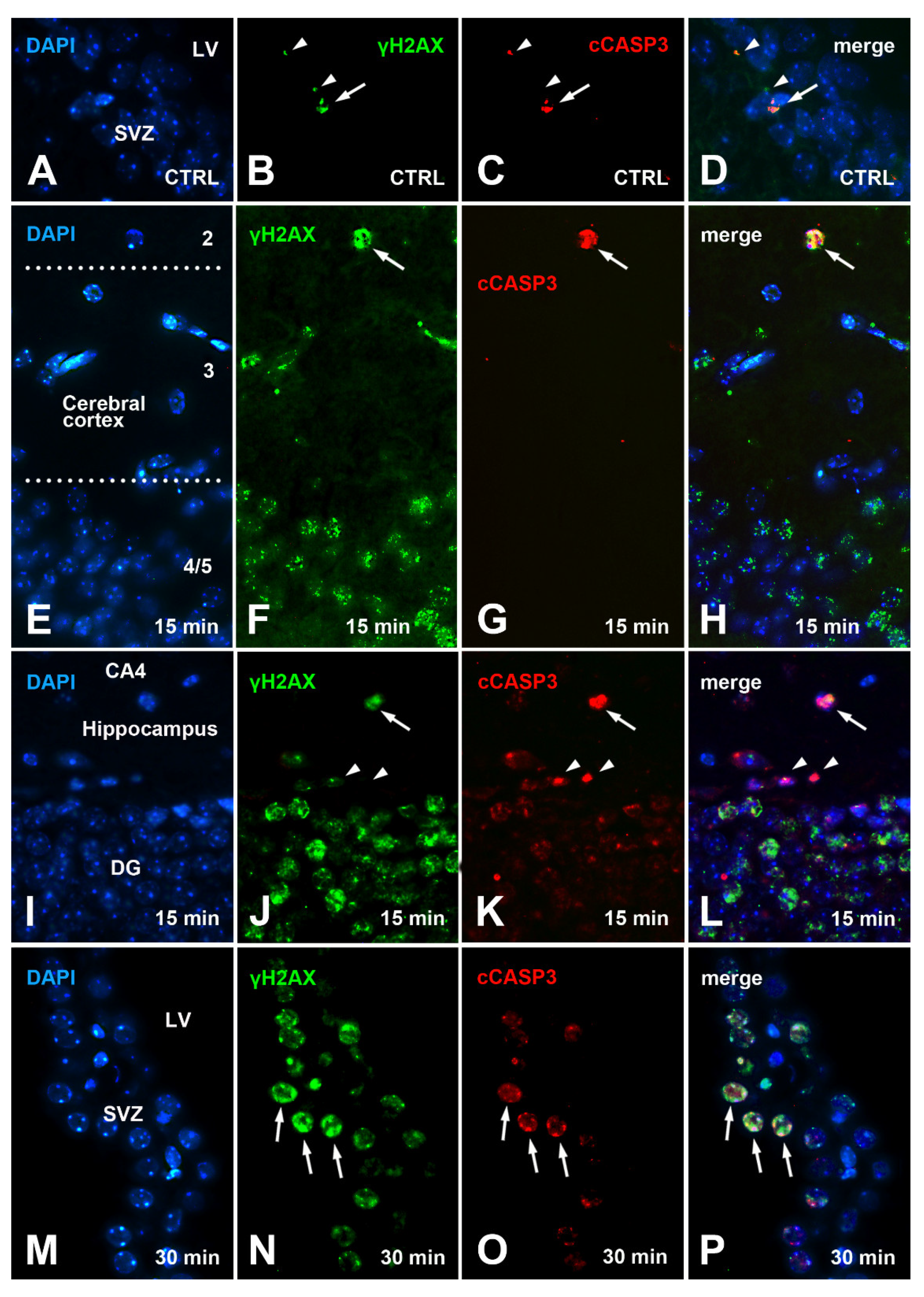
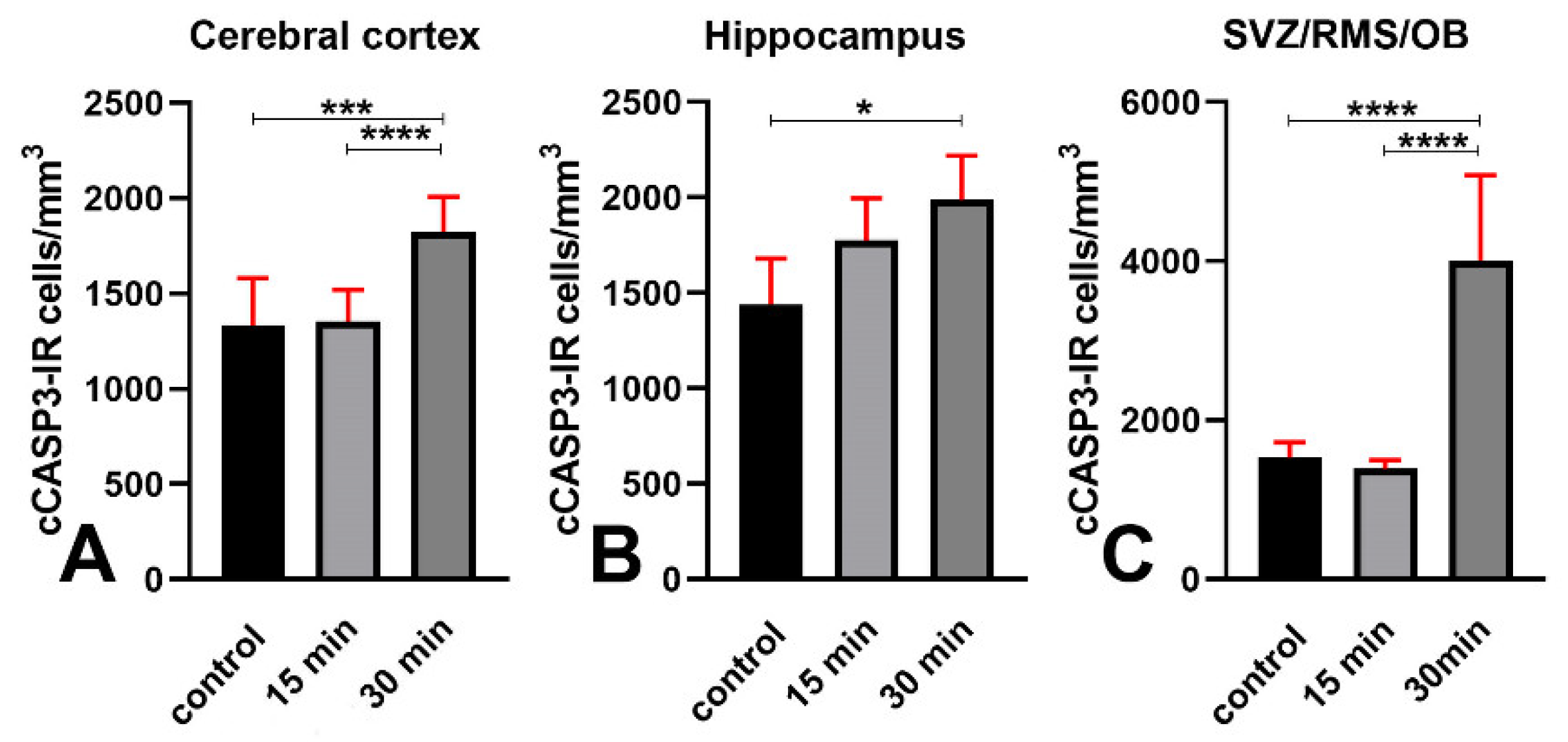
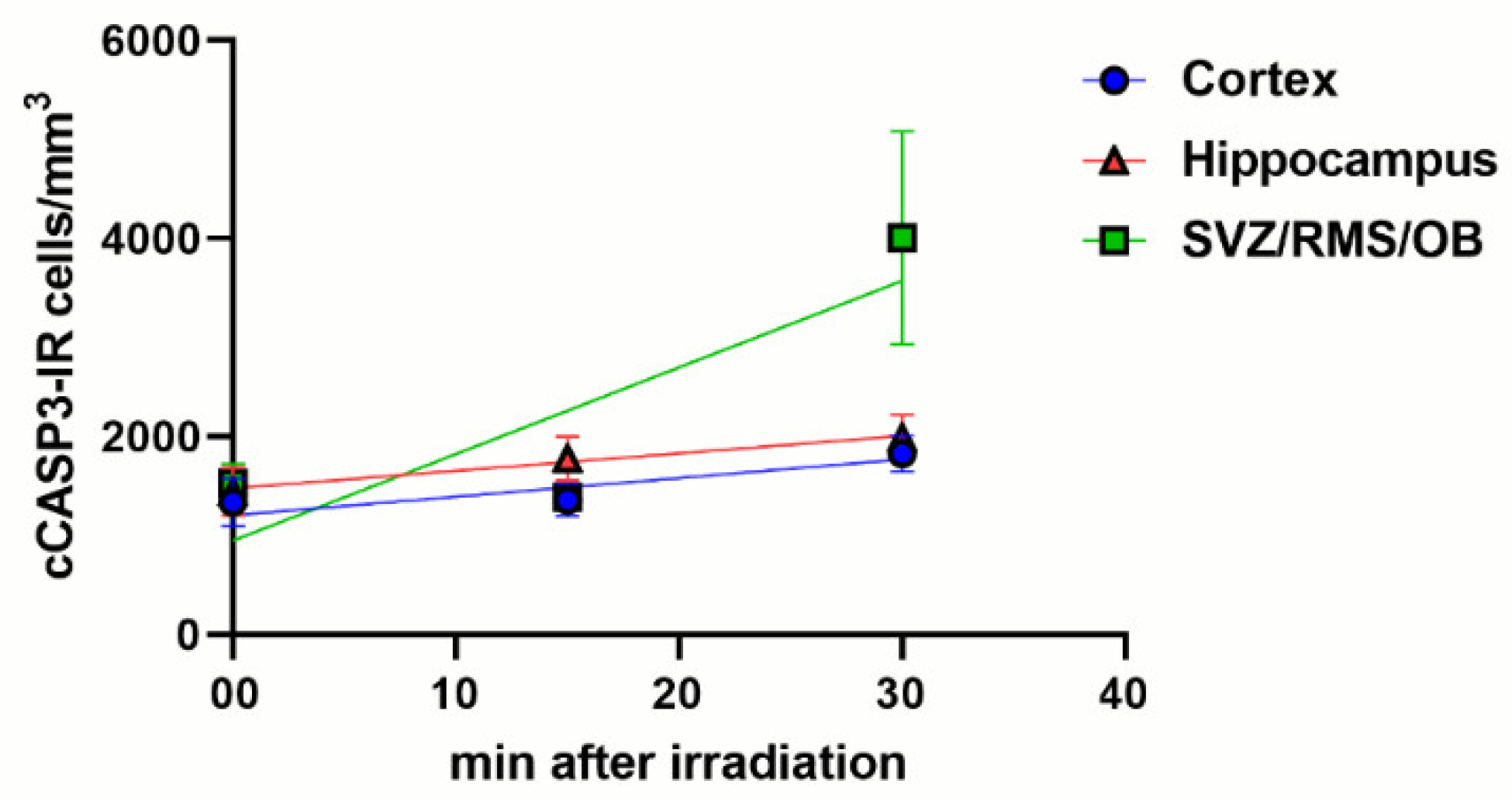
3.3. An Increase of cCASP3 Labeled Cells Accompanies γ Phosphorylation of H2AX after X-ray Irradiation
3.4. Colocalization of γH2AX and cCASP3 in Control and Irradiated Mice
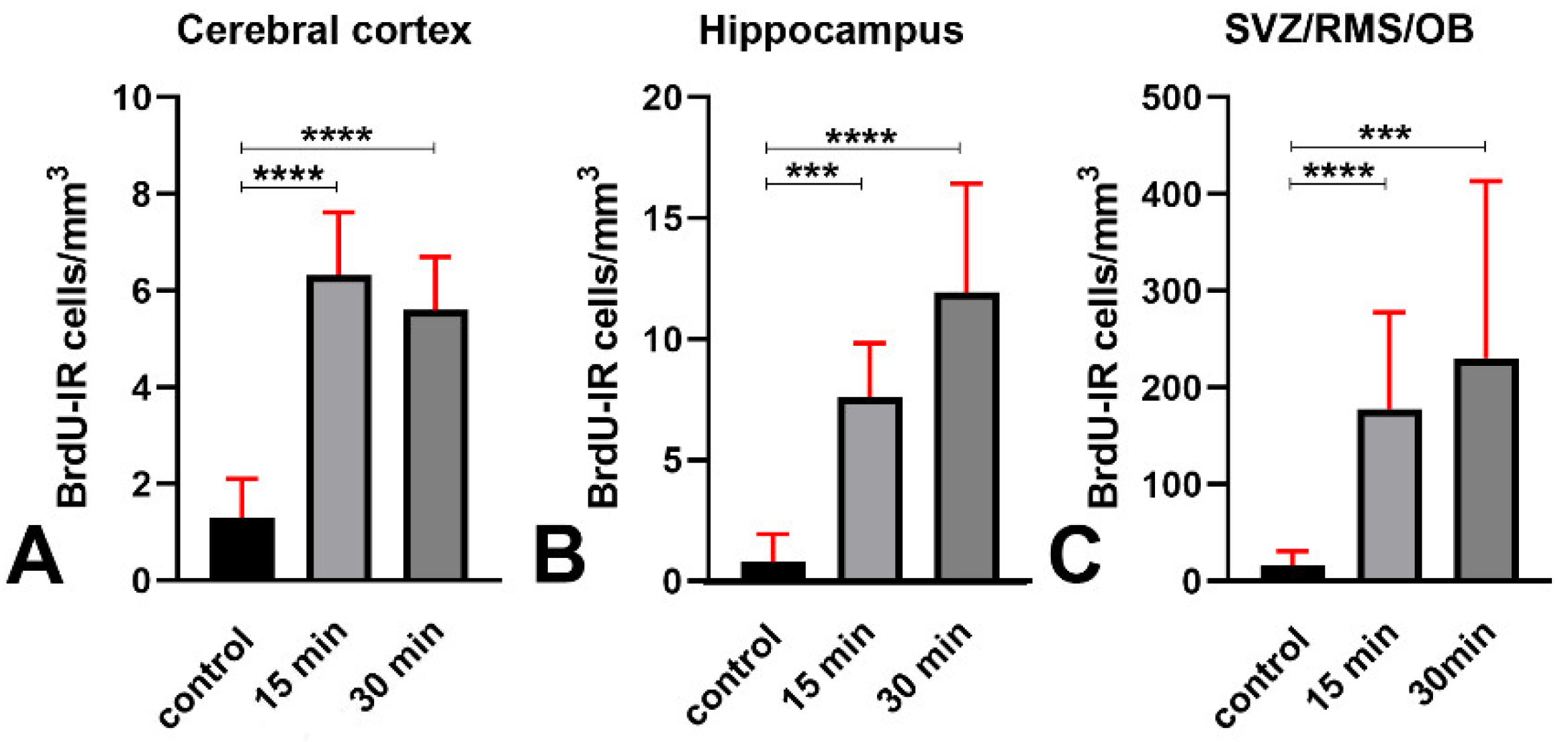
3.5. An Increase in Cellular BrdU Incorporation Accompanies the H2AX Response to X-ray Irradiation
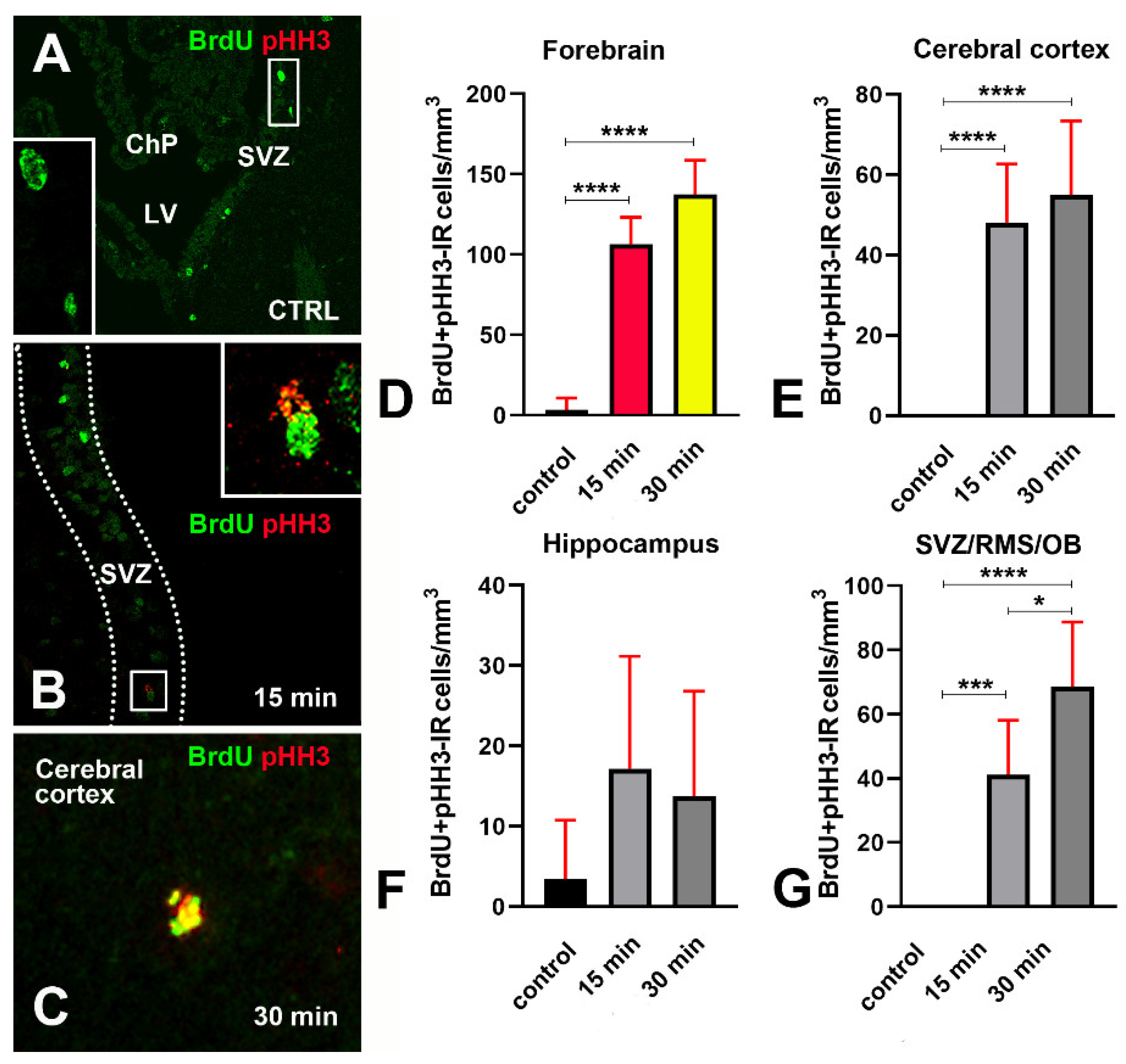
3.6. Increase in Cellular BrdU Incorporation after X-ray Irradiation Occurs Together with Phosphorylation of Histone H3
4. Discussion
4.1. γH2AX/cCASP3 Response to X-ray Irradiation
4.2. γH2AX/cCASP3 in the Normal Aging Brain
4.3. Is There a Different Vulnerability to DNA Damage among Forebrain Areas Related to a Diverse Differentiation State of Neurons?
4.3.1. Cerebral Cortex
4.3.2. Hippocampus and SVZ/MS/OB
- Phosphorylation of H2AX is accompanied by an expression of 53BP1 in both hippocampus and SVZ/RMS/OB (Figure 6F,G) as proof that it is linked to DDR.
- A subpopulation of BrdU immunoreactive cells express pHH3 in the hippocampus but not SVZ/RMS/OB (Figure 15F,G and Figure 16), suggesting that phosphorylation of H2AX in BrdU positive SVZ/RMS/OB cells may not be necessarily linked to true proliferation but rather to the pseudo-proliferative state that is observed in dividing cells entering a senescent state [29].
5. Conclusions
- γH2AX associates with 53BP1 after irradiation and is thus a reliable marker of DDR in vivo.
- Many γH2AX immunoreactive irradiated cells undergo apoptosis with cleavage of CASP3 after failing to repair their genetic material, notwithstanding the incorporation of BrdU as an indicator of de novo DNA synthesis.
- There is a close association of γH2AX, 53BP1, and cCASP3 in untreated mice, indicating that cells in the cerebral cortex, hippocampus, and SVZ/RMS/OB are subjected to some sort of naturally occurring endogenous DNA damage, directly in the form of DSBs or as unrepaired SSBs that are then converted to DSBs.
- The incorporation of BrdU—with or without the concurrent expression of pHH3—may be related to the direct activation of a DDR (in cortical postmitotic neurons) or an aberrant cell cycle reentry with possible evolution into a senescent-like state (in the neurogenic hippocampus and SVZ/RMS/OB), respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 53BP1 | p53-binding protein 1 |
| BER | base excision repair |
| BrdU | 5-bromo-2-deoxyuridine |
| CA | hippocampal cornu Ammonis |
| CASP3 | caspase 3 |
| cCASP3 | cleaved (active) caspase 3 |
| CI | confidence interval |
| DAPI | 4′, 6-diamidino-2-phenylindole dihydrochloride |
| DDR | DNA damage response |
| DSBs | double-stranded DNA breaks |
| GFAP | glial fibrillary acidic protein |
| IMF | immunofluorescence |
| NeuN | neuronal nuclear antigen |
| OB | olfactory bulb |
| PB | phosphate buffer |
| pHH3 | phosphorylated form of histone H3 |
| RMS | rostral migratory stream |
| ROS | reactive oxygen species |
| SSBs | single-stranded DNA breaks |
| SVZ | subventricular zone of the lateral ventricle |
| γH2AX | γ phosphorylated form of histone H2 |
Appendix A
Determination of γH2AX Primary Antibodies Reactiveness
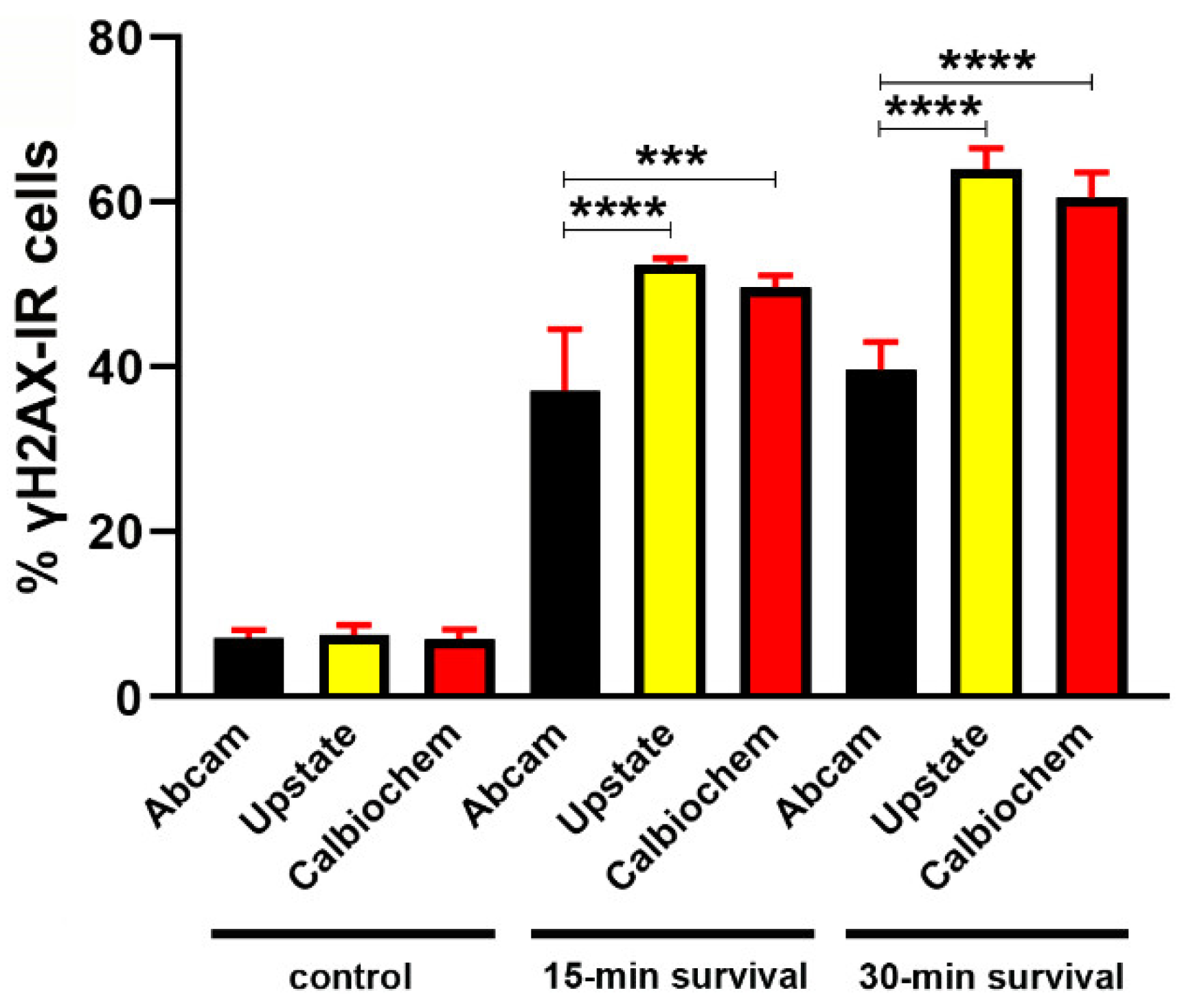
Appendix B
Counting of γH2AX Immunoreactive Foci with the Foci Counter Software
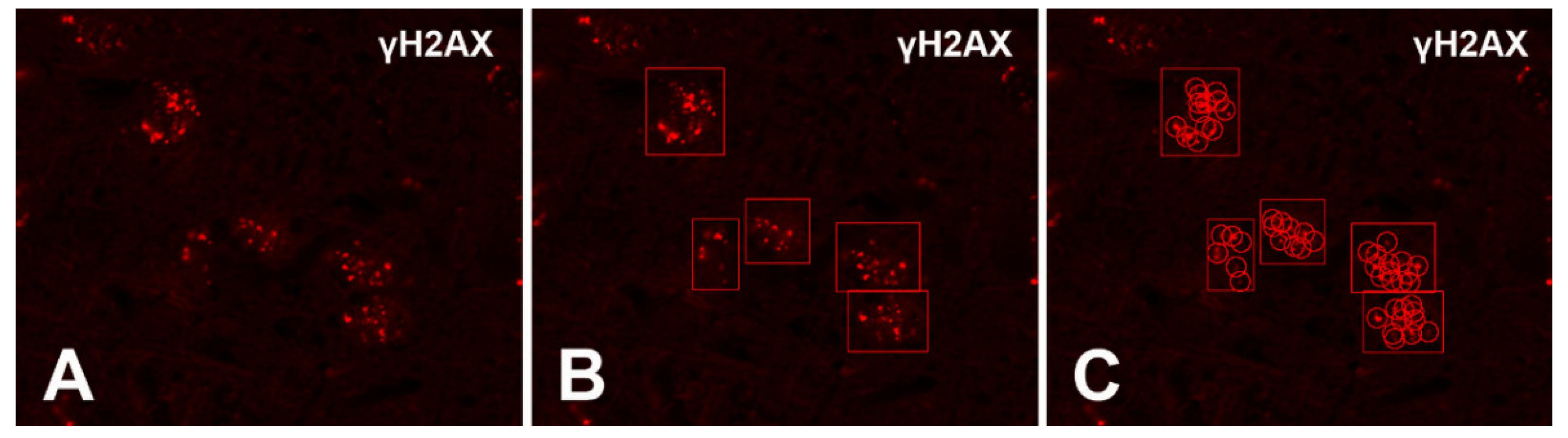
Appendix C
Validation of Cell Counts in Hippocampus
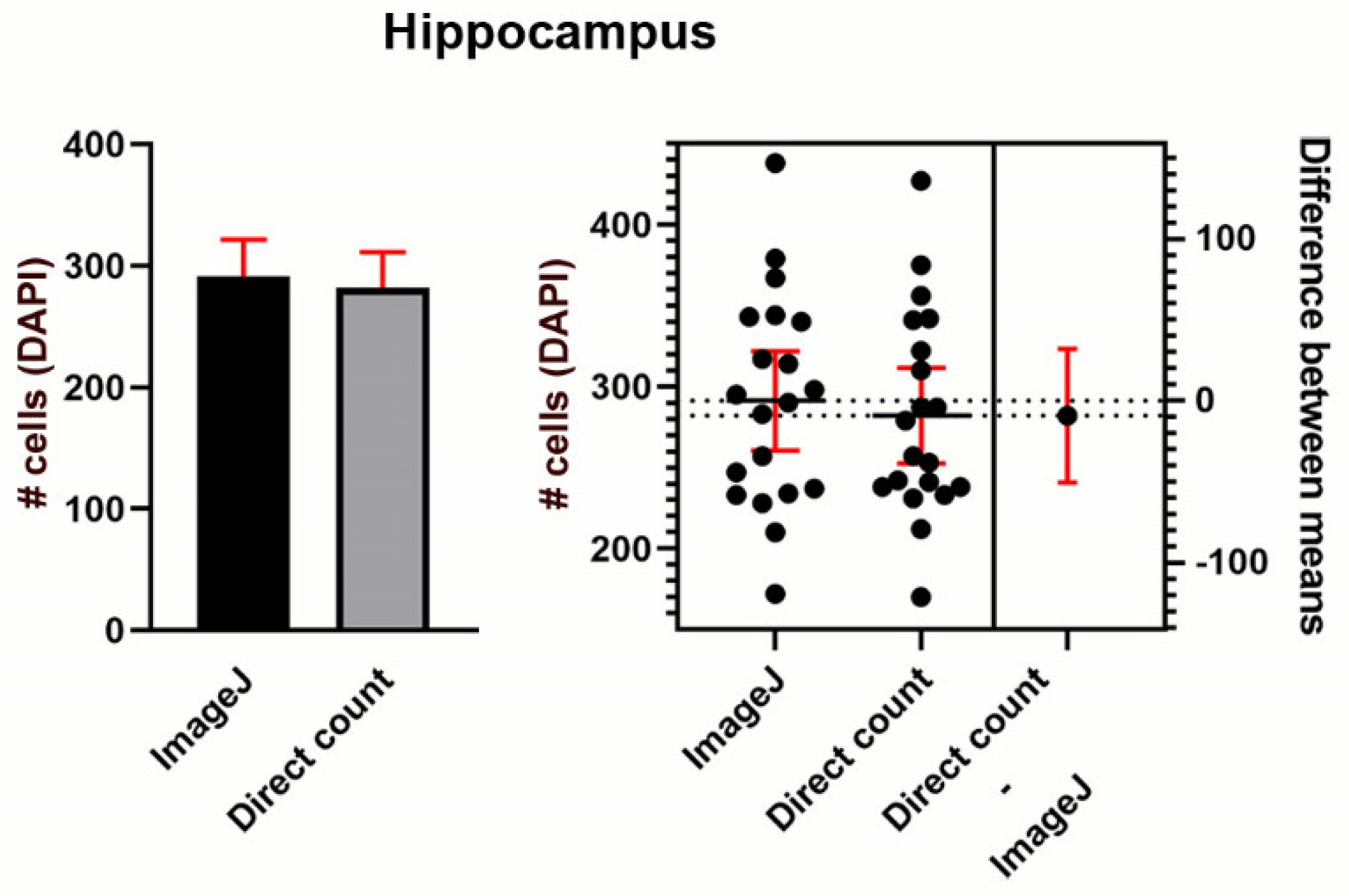
Appendix D
Determination of Cell Densities in Forebrain Areas
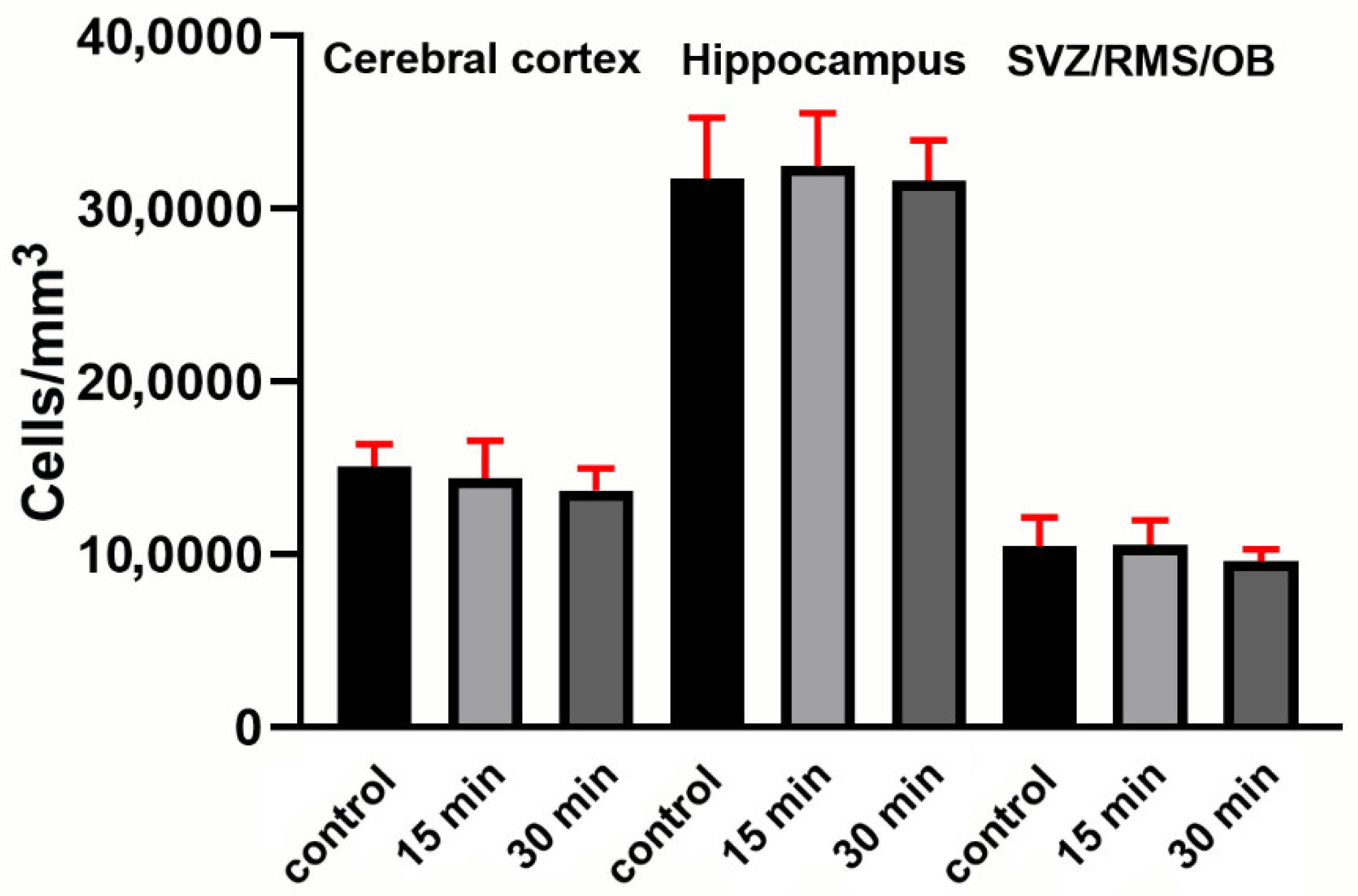
Appendix E
Numerical Data of Statistical Analyses of γH2AX Expression
| Data | Forebrain Area (Anti-γH2AX Antibody) | Mean± CI | Test | Adjusted p Values | ||
| CTRL (A) | 15 min Survival (B) | 30 min Survival (C) | ||||
| Volumetric density of γH2AX-IR cells | Cerebral cortex (Abcam) | 10,760 ± 5914 | 60,634 ± 18,571 | 49,331 ± 7405 | 1 | A vs. B = 0.0002 A vs. C < 0.0001 B vs. C = 0.5407 |
| Cerebral cortex (Upstate) | 12,943 ± 3603 | 74,412 ± 8758 | 85,107 ± 5345 | 1 | A vs. B < 0.0001 A vs. C < 0.0001 B vs. C = 0.1009 | |
| Hippocampus (Abcam) | 14,232 ± 7961 | 179,981 ± 35,115 | 176,697 ± 28,620 | 2 | A vs. B = 0.0004 A vs. C = 0.0001 B vs. C > 0.999 | |
| Hippocampus (Upstate) | 18,351 ± 3036 | 202,375 ± 22,144 | 231,113 ± 11,953 | 1 | A vs. B < 0.0001 A vs. C < 0.0001 B vs. C = 0.0663 | |
| SVZ/RMS/OB (Abcam) | 10,503 ± 4101 | 46,980 ± 16,856 | 36,428 ± 3376 | 2 | A vs. B and A vs. C < 0.0001 B vs. C > 0.9999 | |
| SVZ/RMS/OB (Upstate) | 5505 ± 1074 | 42,374 ± 2494 | 53,833 ± 2344 | 1 | A vs. B and A vs. C < 0.0001 B vs. C < 0.0001. | |
| % γH2AX-IR cells | Cerebral cortex (Abcam) | 8.05 ± 5.31 | 39.65 ± 9.50 | 35.97 ± 3.55 | 2 | A vs. B = 0.0003 A vs. C = 0.0009 B vs. C > 0.9999 |
| Hippocampus (Abcam) | 4.11 ± 1.97 | 55.78 ± 8.42 | 56.22 ± 7.45 | 3 | A vs. B and A vs. C < 0.0001 B vs. C = 0.9952 | |
| SVZ/RMS/OB (Abcam) | 7.04 ± 2.86 | 30.72 ± 4.27 | 37.39 ± 4.18 | 3 | A vs. B and A vs. C < 0.0001 B vs. C = 0.0304 | |
Appendix F
Quantitative Data on 53BP1 Expression and Colocalization with γH2AX

| Data | Forebrain Area (Anti-γH2AX Antibody) | Mean ± CI | Test | Adjusted p Values | ||
|---|---|---|---|---|---|---|
| CTRL (A) | 15 min Survival (B) | 30 min Survival (C) | ||||
| Volumetric density of γH2AX+ 53BP1-IR cells | Whole forebrain (Upstate) | 5605 ± 1135 | 52,653 ± 18,935 | 63,928± 22,613 | 2 | A vs. B and A vs. C < 0.0001 B vs. C = 0.7571 |
| Cerebral cortex (Upstate) | 4827 ± 369 | 36,356 ± 1532 | 42,234 ± 1514 | 1 | A vs. B, A vs. C, and B vs. C < 0.0001 | |
| Hippocampus (Upstate) | 8603 ± 392 | 103,814 ± 3072 | 125,495 ± 1839 | 1 | A vs. B, A vs. C, and B vs. C < 0.0001 | |
| SVZ/RMS/OB (Upstate) | 3387 ± 134 | 17,789 ± 700 | 24,056 ± 2009 | 1 | A vs. B and A vs. C < 0.0001 B vs. C = 0.0008 | |
| % 53BP1-IR cells | Cerebral cortex (Upstate) | 3.885 ± 0.312 | 27.62 ± 0.61 | 32.08 ± 0.73 | 2 | A vs. B, A vs. C, and B vs. C < 0.0001 |
| Hippocampus (Upstate) | 2.883 ± 0.126 | 32.51 ± 1.27 | 38.97 ± 0.57 | 2 | A vs. B, A vs. C, and B vs. C < 0.0001 | |
| SVZ/RMS/OB (Upstate) | 4.448 ± 0.151 | 21.72 ± 0.7 | 29.00 ± 0.50 | 2 | A vs. B, A vs. C, and B vs. C < 0.0001 | |
Appendix G
Numerical Data of Statistical Analyses of cCASP3 Expression
| Data | Forebrain Area | Mean ± CI | Test | Adjusted p Values | ||
|---|---|---|---|---|---|---|
| CTRL (A) | 15 min Survival (B) | 30 min Survival (C) | ||||
| Volumetric density of cCASP3-IR cells | Cerebral cortex | 1335 ± 244 | 1335 ± 184 | 1826 ± 182 | 1 | A vs. B > 0.9999 A vs. C = 0.0007 B vs. C < 0.0001 |
| Hippocampus | 1440 ± 241 | 1776 ± 221 | 1989 ± 231 | 1 | A vs. B = 0.1772 A vs. C = 0.0120 B vs. C = 0.3324 | |
| SVZ/RMS/OB | 1529 ± 194 | 1387 ± 109 | 4008 ± 1074 | 1 | A vs. B > 0.9999 A vs. C and B vs. C < 0.0001. | |
Appendix H
Numerical Data of Statistical Analyses of γH2AX + cCASP3 Colocalization
| Data | Forebrain Area | Mean ± CI | Test | Adjusted p Values | ||
|---|---|---|---|---|---|---|
| CTRL (A) | 15 min Survival (B) | 30 min Survival (C) | ||||
| Volumetric density of γH2AX+ cCASP3-IR cells | Cerebral cortex | 802 ± 364 | 1213 ± 366 | 1729 ± 369 | 1 | A vs. B = 0.4000 A vs. C = 0.0019 B vs. C = 0.0949 |
| Hippocampus | 1022 ± 505 | 1788 ± 350 | 2117 ± 407 | 1 | A vs. B = 0.0791 A vs. C = 0.0031 B vs. C = 0.5790 | |
| SVZ/RMS/OB | 597 ± 301 | 1125 ± 168 | 2176 ± 424 | 1 | A vs. B = 0.1358 A vs. C < 0.0001 B vs. C = 0.0020 | |
Appendix I
Numerical Data of Statistical Analyses of BrdU Expression
| Data | Forebrain Area | Mean ± CI | Test | Adjusted p Values | ||
|---|---|---|---|---|---|---|
| CTRL (A) | 15 min Survival (B) | 30 min Survival (C) | ||||
| Volumetric density of BrdU-IR cells | Cerebral cortex | 1.314 ± 0.79 | 6.321 ± 1.3 | 5.596 ± 1.102 | 1 | A vs. B and A vs. C < 0.0001 B vs. C > 0.9999 |
| Hippocampus | 0.787 ± 1.151 | 7.611 ± 2.232 | 11.93 ± 4.51 | 1 | A vs. B = 0.0003 A vs. C < 0.0001 B vs. C > 0.9999 | |
| SVZ/RMS/OB | 16.28 ± 14.81 | 178.1 ± 99.5 | 230.6 ± 182.9 | 1 | A vs. B < 0.0001 A vs. C = 0.0002 B vs. C > 0.9999 | |
Appendix J
Numerical Data of Statistical Analyses of γH2AX+BrdU Colocalization
| Data | Forebrain Area | Mean ± CI | Test | Adjusted p Values | ||
|---|---|---|---|---|---|---|
| CTRL (A) | 15 min Survival (B) | 30 min Survival (C) | ||||
| Volumetric density of γH2AX+ BrdU-IR cells | Whole forebrain | 3.646 ± 5.356 | 29.59 ± 29.13 | 30.24 ± 25 | 1 | A vs. B and A vs. C < 0.0001 B vs. C > 0.9999 |
| Cerebral cortex | 0.5415 ± 0.3517 | 2.450 ± 0.745 | 2.382 ± 0.553 | 1 | A vs. B = 0.0001 A vs. C = 0.0003 B vs. C > 0.9999 | |
| Hippocampus | 0.4279 ± 0.9121 | 4.878 ± 2.368 | 4.959 ± 2.943 | 1 | A vs. B = 0.0062 A vs. C = 0.0164 B vs. C > 0.9999 | |
| SVZ/RMS/OB | 9.969 ± 16.841 | 81.45 ± 66.45 | 83.37 ± 72.83 | 1 | A vs. B = 0.0047 A vs. C = 0.0055 B vs. C > 0.9999 | |
Appendix K
Numerical Data of Statistical Analyses of BrdU + pHH3 Colocalization
| Data | Forebrain Area | Mean ± CI | Test | Adjusted p Values | ||
|---|---|---|---|---|---|---|
| CTRL (A) | 15 min Survival (B) | 30 min Survival (C) | ||||
| Volumetric density of γH2AX+ BrdU-IR cells | Whole forebrain | 3.434 ± 7316 | 106.5 ± 16.8 | 137.4 ± 21.3 | 1 | A vs. B and A vs. C < 0.0001 B vs. C = 0.0639 |
| Cerebral cortex | 0 | 48.08 ± 14.64 | 54.95 ± 18.51 | 2 | A vs. B and A vs. C < 0.0001 B vs. C = 0.7295 | |
| Hippocampus | 3.434 ± 7316 | 17.17 ± 14.02 | 13.74 ± 13.09 | 1 | A vs. B = 0.2082 A vs. C = 0.3903 B vs. C = 0.9733 | |
| SVZ/RMS/OB | 0 | 41.21 ± 16.9 | 68.68 ± 20 | 2 | A vs. B = 0.0005 A vs. C < 0.0001 B vs. C = 0.0235 | |
References
- Abbotts, R.; Wilson, D.M. Coordination of DNA single strand break repair. Free Radic. Biol. Med. 2017, 107, 228–244. [Google Scholar] [CrossRef]
- Huang, X.; Okafuji, M.; Traganos, F.; Luther, E.; Holden, E.; Darzynkiewicz, Z. Assessment of histone H2AX phosphorylation induced by DNA topoisomerase I and II inhibitors topotecan and mitoxantrone and by the DNA cross-linking agent cisplatin. Cytometry 2004, 58A, 99–110. [Google Scholar] [CrossRef]
- Rulten, S.L.; Caldecott, K.W. DNA strand break repair and neurodegeneration. DNA Repair 2013, 12, 558–567. [Google Scholar] [CrossRef]
- Ma, A.; Dai, X. The relationship between DNA single-stranded damage response and double-stranded damage response. Cell Cycle 2018, 17, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kopp, B.; Khoury, L.; Audebert, M. Validation of the γH2AX biomarker for genotoxicity assessment: A review. Arch. Toxicol. 2019, 93, 2103–2114. [Google Scholar] [CrossRef]
- Solier, S.; Pommier, Y. The apoptotic ring: A novel entity with phosphorylated histones H2AX and H2B, and activated DNA damage response kinases. Cell Cycle 2009, 8, 1853–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grütter, M.G. Caspases: Key players in programmed cell death. Curr. Opin. Struct. Biol. 2000, 10, 649–655. [Google Scholar] [CrossRef]
- Huang, X.; Traganos, F.; Darzynkiewicz, Z. DNA damage induced by DNA topoisomerase I- and topoisomerase II-inhibitors detected by histone H2AX phosphorylation in relation to the cell cycle phase and apoptosis. Cell Cycle 2003, 2, 614–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luczak, M.W.; Zhitkovich, A. Monoubiquitinated γ-H2AX: Abundant product and specific biomarker for non-apoptotic DNA double-strand breaks. Toxicol. Appl. Pharmacol. 2018, 355, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Chwastek, J.; Jantas, D.; Lasoń, W. The ATM kinase inhibitor KU-55933 provides neuroprotection against hydrogen peroxide-induced cell damage via a γH2AX/p-p53/caspase-3-independent mechanism: Inhibition of calpain and cathepsin D. Int. J. Biochem. Cell Biol. 2017, 87, 38–53. [Google Scholar] [CrossRef]
- Cubilla, M.A.; Ramella, M.D.M.; Bachor, T.P.; Suburo, A.M.; Bermudez, V. Mifepristone, a Blocker of Glucocorticoid Receptors, Promotes Photoreceptor Death. Investig. Opthalmol. Vis. Sci. 2013, 54, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frade, J.M.; Ovejero-Benito, M.C. Neuronal cell cycle: The neuron itself and its circumstances. Cell Cycle 2015, 14, 712–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barral, S.; Beltramo, R.; Salio, C.; Aimar, P.; Lossi, L.; Merighi, A. Phosphorylation of Histone H2AX in the Mouse Brain from Development to Senescence. Int. J. Mol. Sci. 2014, 15, 1554–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeglin, E.; Desplancq, D.; Conic, S.; Oulad-Abdelghani, M.; Stoessel, A.; Chiper, M.; Vigneron, M.; Didier, P.; Tora, L.; Weiss, E. Uniform Widespread Nuclear Phosphorylation of Histone H2AX Is an Indicator of Lethal DNA Replication Stress. Cancers 2019, 11, 355. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.R.; Hong, C.J.; Kim, J.Y.; Kim, E.-K.; Sun, W.; Yu, S.-W. Control of adult neurogenesis by programmed cell death in the mammalian brain. Mol. Brain 2016, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Julien, O.; Wells, J.A. Caspases and their substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Rappold, I.; Iwabuchi, K.; Date, T.; Chen, J. Tumor Suppressor P53 Binding Protein 1 (53bp1) Is Involved in DNA Damage–Signaling Pathways. J. Cell Biol. 2001, 153, 613–620. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA Double-stranded Breaks Induce Histone H2AX Phosphorylation on Serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [Green Version]
- Rogakou, E.P.; Boon, C.; Redon, C.; Bonner, W.M. Megabase Chromatin Domains Involved in DNA Double-Strand Breaks in Vivo. J. Cell Biol. 1999, 146, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Keogh, M.-C.; Kim, J.-A.; Downey, M.; Fillingham, J.; Chowdhury, D.; Harrison, J.C.; Onishi, M.; Datta, N.; Galicia, S.; Emili, A.; et al. A phosphatase complex that dephosphorylates γH2AX regulates DNA damage checkpoint recovery. Nat. Cell Biol. 2005, 439, 497–501. [Google Scholar] [CrossRef]
- Ward, I.M.; Minn, K.; van Deursen, J.; Chen, J. p53 Binding Protein 53BP1 Is Required for DNA Damage Responses and Tumor Suppression in Mice. Mol. Cell. Biol. 2003, 23, 2556–2563. [Google Scholar] [CrossRef] [Green Version]
- Ward, I.M.; Minn, K.; Jorda, K.G.; Chen, J. Accumulation of Checkpoint Protein 53BP1 at DNA Breaks Involves Its Binding to Phosphorylated Histone H2AX. J. Biol. Chem. 2003, 278, 19579–19582. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Hunt, C.R.; Chakraborty, S.; Pandita, R.K.; Yordy, J.; Ramnarain, D.B.; Horikoshi, N.; Pandita, T.K. Role of 53BP1 in the Regulation of DNA Double-Strand Break Repair Pathway Choice. Radiat. Res. 2014, 181, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Capetillo, O.; Chen, H.-T.; Celeste, A.; Ward, I.; Romanienko, P.J.; Morales, J.; Naka, K.; Xia, Z.; Camerini-Otero, R.D.; Motoyama, N.; et al. DNA damage-induced G2–M checkpoint activation by histone H2AX and 53BP1. Nat. Cell Biol. 2002, 4, 993–997. [Google Scholar] [CrossRef]
- Gobbel, G.T.; Bellinzona, M.; Vogt, A.R.; Gupta, N.; Fike, J.R.; Chan, P.H. Response of Postmitotic Neurons to X-Irradiation: Implications for the Role of DNA Damage in Neuronal Apoptosis. J. Neurosci. 1998, 18, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Gobbel, G.T.; Chan, P.H. Neuronal death is an active, caspase-dependent process after moderate but not severe DNA damage. J. Neurochem. 2001, 76, 520–531. [Google Scholar] [CrossRef] [Green Version]
- Sabirzhanov, B.; Makarevich, O.; Barrett, J.P.; Jackson, I.L.; Glaser, E.P.; Faden, A.I.; Stoica, B.A. Irradiation-Induced Upregulation of miR-711 Inhibits DNA Repair and Promotes Neurodegeneration Pathways. Int. J. Mol. Sci. 2020, 21, 5239. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Nieves-Neira, W.; Boon, C.; Pommier, Y.; Bonner, W.M. Initiation of DNA Fragmentation during Apoptosis Induces Phosphorylation of H2AX Histone at Serine 139. J. Biol. Chem. 2000, 275, 9390–9395. [Google Scholar] [CrossRef] [Green Version]
- Fielder, E.; von Zglinicki, T.; Jurk, D. The DNA Damage Response in Neurons: Die by Apoptosis or Survive in a Senescence-Like State? J. Alzheimer’s Dis. 2017, 60, S107–S131. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 2007, 53, 198–214. [Google Scholar] [CrossRef]
- Tripathi, K.; Hussein, U.K.; Anupalli, R.; Barnett, R.; Bachaboina, L.; Scalici, J.; Rocconi, R.P.; Owen, L.B.; Piazza, G.; Palle, K. Allyl isothiocyanate induces replication-associated DNA damage response in NSCLC cells and sensitizes to ionizing radiation. Oncotarget 2015, 6, 5237–5252. [Google Scholar] [CrossRef]
- Giunta, S.; Belotserkovskaya, R.; Jackson, S.P. DNA damage signaling in response to double-strand breaks during mitosis. J. Cell Biol. 2010, 190, 197–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leslie, M. Mitosis first, DNA repair later. J. Cell Biol. 2010, 190, 160. [Google Scholar] [CrossRef] [Green Version]
- Van Hooser, A.; Goodrich, D.; Allis, C.; Brinkley, B.; Mancini, M. Histone H3 phosphorylation is required for the initiation, but not maintenance, of mammalian chromosome condensation. J. Cell Sci. 1998, 111, 3497–3506. [Google Scholar] [CrossRef]
- Weyemi, U.; Paul, B.D.; Bhattacharya, D.; Malla, A.P.; Boufraqech, M.; Harraz, M.M.; Bonner, W.M.; Snyder, S.H. Histone H2AX promotes neuronal health by controlling mitochondrial homeostasis. Proc. Natl. Acad. Sci. USA 2019, 116, 7471–7476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, D.; Shaham, S.; Horvitz, H.R. The Caenorhabditis elegans cell-death protein CED-3 is a cysteine protease with substrate specificities similar to those of the human CPP32 protease. Genes Dev. 1996, 10, 1073–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, M.; Hakem, R.; Soengas, M.; Duncan, G.S.; Shahinian, A.; Kägi, D.; Hakem, A.; McCurrach, M.; Khoo, W.; Kaufman, S.A.; et al. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998, 12, 806–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikka, T.; Usenius, T.; Tenhunen, M.; Keinänen, R.; Koistinaho, J. Tetracycline derivatives and ceftriaxone, a cephalosporin antibiotic, protect neurons against apoptosis induced by ionizing radiation. J. Neurochem. 2001, 78, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Guida, P.; Vazquez, M.E.; Otto, S. Cytotoxic Effects of Low- and High-LET Radiation on Human Neuronal Progenitor Cells: Induction of Apoptosis andTP53Gene Expression. Radiat. Res. 2005, 164, 545–551. [Google Scholar] [CrossRef]
- Ivanov, V.N.; Hei, T.K. A role for TRAIL/TRAIL-R2 in radiation-induced apoptosis and radiation-induced bystander response of human neural stem cells. Apoptosis 2013, 19, 399–413. [Google Scholar] [CrossRef] [Green Version]
- Duque, A.R.; Rakic, P. Identification of proliferating and migrating cells by BrdU and other thymidine analogs: Benefits and limitations. In Immunocytochemistry and Related Techniques; Merighi, A., Lossi, L., Eds.; Humana Press: New York, NY, USA, 2015; Volume 101, pp. 123–139. [Google Scholar]
- Morin, X.; Jaouen, F.; Durbec, P. Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat. Neurosci. 2007, 10, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Hendzel, M.; Wei, Y.; Mancini, M.A.; Van Hooser, A.; Ranalli, T.; Brinkley, B.R.; Bazett-Jones, D.P.; Allis, C.D. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 1997, 106, 348–360. [Google Scholar] [CrossRef]
- Le Hegarat, L.; Roudot, A.-C.; Fessard, V. Benchmark dose analyses of γH2AX and pH3 endpoints for quantitative comparison of in vitro genotoxicity potential of lipophilic phycotoxins. Mutat. Res. Toxicol. Environ. Mutagen. 2020, 852, 503169. [Google Scholar] [CrossRef] [PubMed]
- Khoury, L.; Zalko, D.; Audebert, M. Complementarity of phosphorylated histones H2AX and H3 quantification in different cell lines for genotoxicity screening. Arch. Toxicol. 2015, 90, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, O.; Zhu, X.; Lee, H.-G.; Raina, A.; Obrenovich, M.E.; Bowser, R.; Ghanbari, H.A.; Castellani, R.J.; Perry, G.; Smith, M.A. Ectopic localization of phosphorylated histone H3 in Alzheimer’s disease: A mitotic catastrophe? Acta Neuropathol. 2003, 105, 524–528. [Google Scholar] [CrossRef]
- Yu, J.; Fleming, S.L.; Williams, B.; Williams, E.V.; Li, Z.; Somma, P.; Rieder, C.L.; Goldberg, M.L. Greatwall kinase. J. Cell Biol. 2004, 164, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, R.S.; Zupanc, M.M.; Lösche, A.; Westra, J.; Chun, J.; Zupanc, G.K. Numerical chromosome variation and mitotic segregation defects in the adult brain of teleost fish. Dev. Neurobiol. 2007, 67, 1334–1347. [Google Scholar] [CrossRef]
- Wang, H.; Tian, C.; Xu, Y.; Xie, W.-L.; Zhang, J.; Zhang, B.-Y.; Ren, K.; Wang, K.; Chen, C.; Wang, S.-B.; et al. Abortive Cell Cycle Events in the Brains of Scrapie-Infected Hamsters with Remarkable Decreases of PLK3/Cdc25C and Increases of PLK1/Cyclin B1. Mol. Neurobiol. 2013, 48, 655–668. [Google Scholar] [CrossRef]
- Roman, D.D.; Sperduto, P.W. Neuropsychological effects of cranial radiation: Current knowledge and future directions. Int. J. Radiat. Oncol. 1995, 31, 983–998. [Google Scholar] [CrossRef]
- Pinkham, M.; Sanghera, P.; Wall, G.; Dawson, B.; Whitfield, G. Neurocognitive Effects Following Cranial Irradiation for Brain Metastases. Clin. Oncol. 2015, 27, 630–639. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Moore, E.; Robbins, M.E. Molecular Pathways: Radiation-Induced Cognitive Impairment. Clin. Cancer Res. 2013, 19, 2294–2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.R.; Liu, L.; Wang, H.; Ni Ho, K.J.; Sethi, G. Spatiotemporal dynamics of γH2AX in the mouse brain after acute irradiation at different postnatal days with special reference to the dentate gyrus of the hippocampus. Aging 2021, 13, 15815–15832. [Google Scholar] [CrossRef] [PubMed]
- Wallin, A.; Kapaki, E.; Boban, M.; Engelborghs, S.; Hermann, D.M.; Huisa, B.; Jonsson, M.; Kramberger, M.G.; Lossi, L.; Malojcic, B.; et al. Biochemical markers in vascular cognitive impairment associated with subcortical small vessel disease—A consensus report. BMC Neurol. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Chilukuri, S.; Burela, N. Memantine for Prevention of Brain Irradiation–Induced Cognitive Toxicity: A Tale of an Underappreciated and Underused Intervention. JCO Glob. Oncol. 2020, 6, 1384–1388. [Google Scholar] [CrossRef]
- Galbiati, A.; Beauséjour, C.; Di Fagagna, F.D. A novel single-cell method provides direct evidence of persistent DNA damage in senescent cells and aged mammalian tissues. Aging Cell 2017, 16, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgoulis, A.; Vorgias, C.E.; Chrousos, G.P.; Rogakou, E.P. Genome Instability and γH2AX. Int. J. Mol. Sci. 2017, 18, 1979. [Google Scholar] [CrossRef] [PubMed]
- Erwin, J.; Marchetto, M.C.; Gage, F.H. Mobile DNA elements in the generation of diversity and complexity in the brain. Nat. Rev. Neurosci. 2014, 15, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Gasior, S.; Wakeman, T.P.; Xu, B.; Deininger, P.L. The Human LINE-1 Retrotransposon Creates DNA Double-strand Breaks. J. Mol. Biol. 2006, 357, 1383–1393. [Google Scholar] [CrossRef] [Green Version]
- Jurkowski, M.P.; Bettio, L.; Woo, E.K.; Patten, A.; Yau, S.-Y.; Gil-Mohapel, J. Beyond the Hippocampus and the SVZ: Adult Neurogenesis Throughout the Brain. Front. Cell. Neurosci. 2020, 14, 576444. [Google Scholar] [CrossRef]
- Riches, L.C.; Lynch, A.M.; Gooderham, N.J. Early events in the mammalian response to DNA double-strand breaks. Mutagenesis 2008, 23, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Pospelova, T.V.; Demidenko, Z.N.; Bukreeva, E.I.; Pospelov, V.A.; Gudkov, A.; Blagosklonny, M.V. Pseudo-DNA damage response in senescent cells. Cell Cycle 2009, 8, 4112–4118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, P. DNA repair in neural cells: Basic science and clinical implications. Mutat. Res. Mol. Mech. Mutagen. 2002, 509, 93–108. [Google Scholar] [CrossRef]
- Kruman, I.I. Why do Neurons Enter the Cell Cycle? Cell Cycle 2004, 3, 767–771. [Google Scholar] [CrossRef]
- Fishel, M.; Vasko, M.R.; Kelley, M.R. DNA repair in neurons: So if they don’t divide what’s to repair? Mutat. Res. Mol. Mech. Mutagen. 2007, 614, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, A. DNA damage, neuronal and glial cell death and neurodegeneration. Apoptosis 2010, 15, 1371–1381. [Google Scholar] [CrossRef]
- Hou, Y.; Song, H.; Croteau, D.L.; Akbari, M.; Bohr, V.A. Genome instability in Alzheimer disease. Mech. Ageing Dev. 2017, 161, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Saez-Atienzar, S.; Masliah, E. Cellular senescence and Alzheimer disease: The egg and the chicken scenario. Nat. Rev. Neurosci. 2020, 21, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Suberbielle, E.; E Sanchez, P.; Kravitz, A.; Wang, X.; Ho, K.; Eilertson, K.; Devidze, N.; Kreitzer, A.C.; Mucke, L. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat. Neurosci. 2013, 16, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.; Sun, Y.; Xie, L.; Batteur, S.; Mao, X.O.; Smelick, C.; Logvinova, A.; Greenberg, D.A. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell 2003, 2, 175–183. [Google Scholar] [CrossRef]
- Maslov, A.Y.; Barone, T.A.; Plunkett, R.J.; Pruitt, S.C. Neural Stem Cell Detection, Characterization, and Age-Related Changes in the Subventricular Zone of Mice. J. Neurosci. 2004, 24, 1726–1733. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Daniels, S.B.; Lennington, J.B.; Notti, R.Q.; Conover, J.C. The aging neurogenic subventricular zone. Aging Cell 2006, 5, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Cooper-Kuhn, C.M.; Aigner, R.; Winkler, J.; Kuhn, H.G. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur. J. Neurosci. 2002, 16, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Dayer, A.G.; Ford, A.A.; Cleaver, K.M.; Yassaee, M.; Cameron, H. Short-term and long-term survival of new neurons in the rat dentate gyrus. J. Comp. Neurol. 2003, 460, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, H.G. Control of Cell Survival in Adult Mammalian Neurogenesis. Cold Spring Harb. Perspect. Biol. 2015, 7, a018895. [Google Scholar] [CrossRef] [Green Version]
- Keller, D.; Erö, C.; Markram, H. Cell Densities in the Mouse Brain: A Systematic Review. Front. Neuroanat. 2018, 12, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attili, S.M.; Silva, M.F.M.; Nguyen, T.-V.; Ascoli, G.A. Cell numbers, distribution, shape, and regional variation throughout the murine hippocampal formation from the adult brain Allen Reference Atlas. Brain Struct. Funct. 2019, 224, 2883–2897. [Google Scholar] [CrossRef]





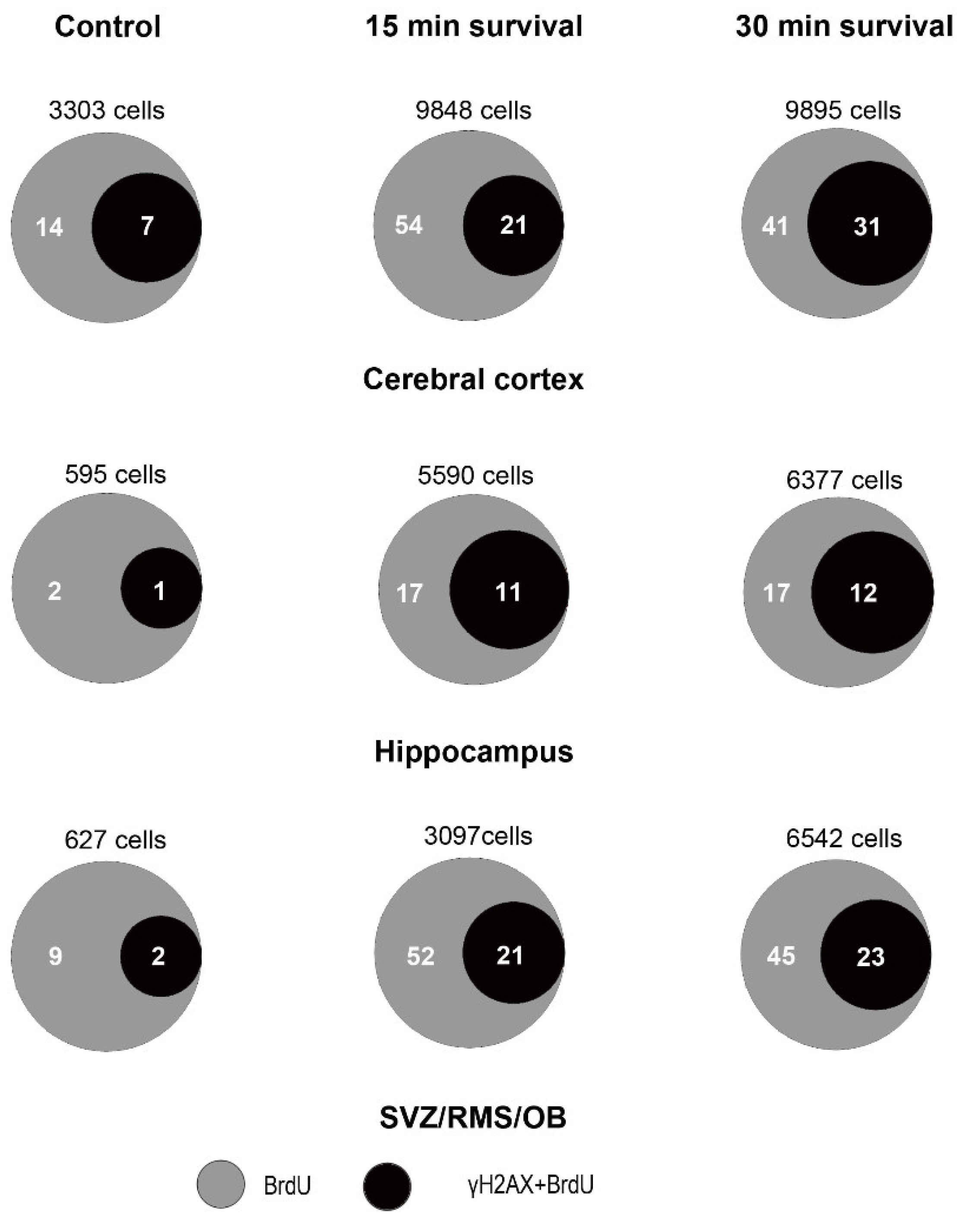

| Forebrain Areas—Animal Groups | % γH2AX | ‰ cCASP3 | PCP (%) | OCP (%) |
|---|---|---|---|---|
| Cerebral cortex-Control | 8.055 | 6.296 | 5.0714 | 68.75 |
| Cerebral cortex-15 min survival | 39.65 | 10.66 | 42.267 | 85.9 |
| Cerebral cortex-30 min survival | 35.97 | 14.55 | 52.336 | 89.17 |
| Hippocampus-Control | 4.106 | 4.394 | 1.804 | 68.75 |
| Hippocampus-15 min survival | 55.78 | 6.517 | 36.352 | 89.06 |
| Hippocampus-30 min survival | 56.22 | 7.079 | 39.798 | 95.31 |
| SVZ/RMS/OB—Control | 7.036 | 10.48 | 7.374 | 43.21 |
| SVZ/RMS/OB-15 min survival | 30.72 | 10.29 | 31.611 | 87.81 |
| SVZ/RMS/OB-30 min survival | 37.39 | 23,6 | 88.240 | 93.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gionchiglia, N.; Granato, A.; Merighi, A.; Lossi, L. Association of Caspase 3 Activation and H2AX γ Phosphorylation in the Aging Brain: Studies on Untreated and Irradiated Mice. Biomedicines 2021, 9, 1166. https://doi.org/10.3390/biomedicines9091166
Gionchiglia N, Granato A, Merighi A, Lossi L. Association of Caspase 3 Activation and H2AX γ Phosphorylation in the Aging Brain: Studies on Untreated and Irradiated Mice. Biomedicines. 2021; 9(9):1166. https://doi.org/10.3390/biomedicines9091166
Chicago/Turabian StyleGionchiglia, Nadia, Alberto Granato, Adalberto Merighi, and Laura Lossi. 2021. "Association of Caspase 3 Activation and H2AX γ Phosphorylation in the Aging Brain: Studies on Untreated and Irradiated Mice" Biomedicines 9, no. 9: 1166. https://doi.org/10.3390/biomedicines9091166
APA StyleGionchiglia, N., Granato, A., Merighi, A., & Lossi, L. (2021). Association of Caspase 3 Activation and H2AX γ Phosphorylation in the Aging Brain: Studies on Untreated and Irradiated Mice. Biomedicines, 9(9), 1166. https://doi.org/10.3390/biomedicines9091166






