Comparison of Outcomes between 3 Monthly Brolucizumab and Aflibercept Injections for Polypoidal Choroidal Vasculopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Follow-Up
2.3. Statistical Analyses
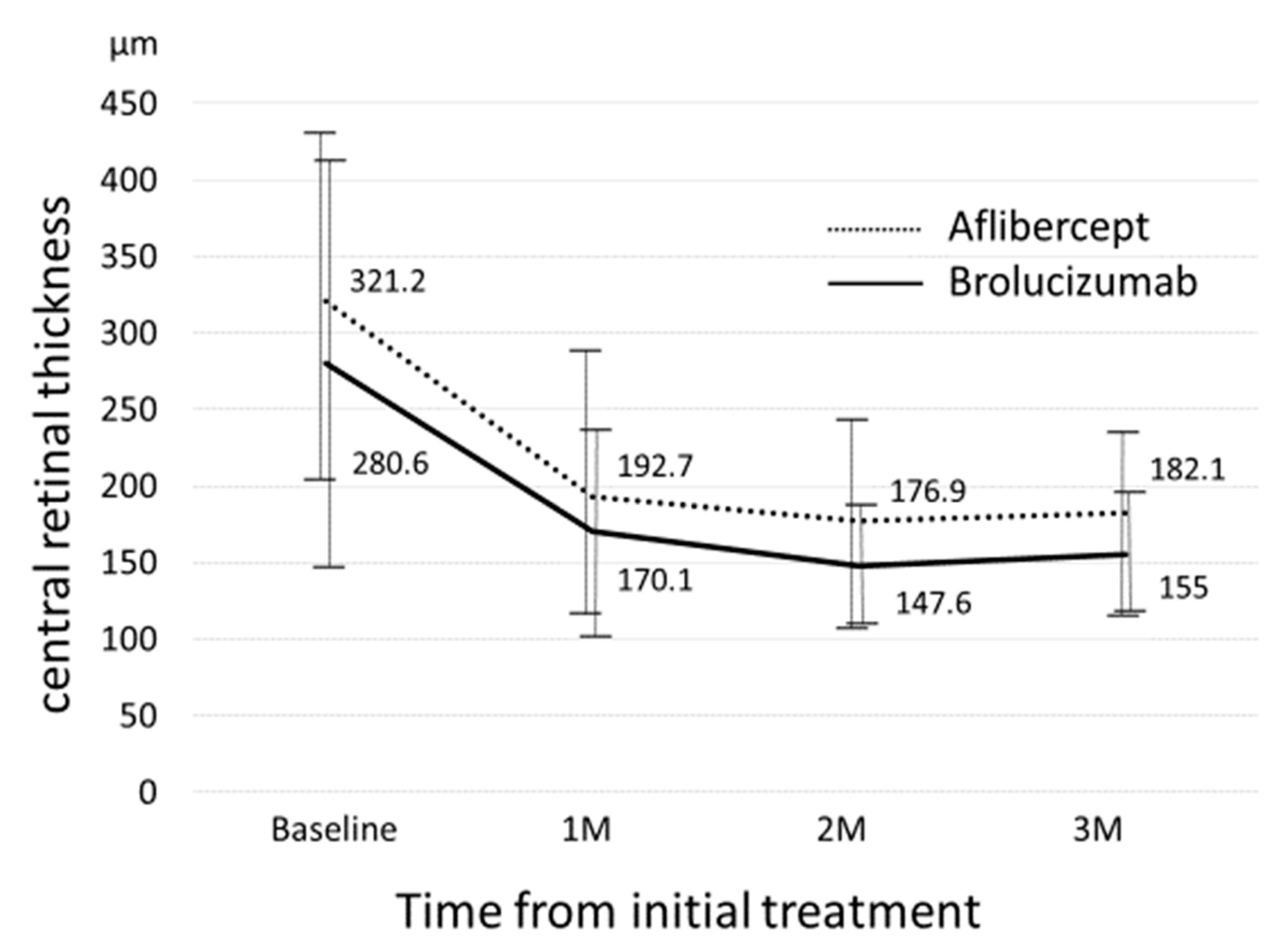
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yannuzzi, L.A.; Sorenson, J.; Spaide, R.F.; Lipson, B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990, 10, 1–8. [Google Scholar] [CrossRef]
- Spaide, R.F.; Yannuzzi, L.A.; Slakter, J.S.; Sorenson, J.; Orlach, D.A. Indocyanine Green Videoangiography of Idiopathic Polypoidal Choroidal Vasculopathy. Retina 1995, 15, 100–110. [Google Scholar] [CrossRef]
- Sakurada, Y.; Yoneyama, S.; Sugiyama, A.; Tanabe, N.; Kikushima, W.; Mabuchi, F.; Kume, A.; Kubota, T.; Iijima, H. Prevalence and Genetic Characteristics of Geographic Atrophy among Elderly Japanese with Age-Related Macular Degeneration. PLoS ONE 2016, 11, e0149978. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Kaiser, P.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S. Ranibizumab versus Verteporfin for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.; Chung, C.Y.; Kim, R.Y. Ranibizumab for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; Lee, W.K.; Chen, L.-J.; Chen, S.-J.; Hashad, Y.; Kim, H.; Lai, T.; Pilz, S.; Ruamviboonsuk, P.; Tokaji, E.; et al. Everest Study. Retina 2012, 32, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Kikushima, W.; Sakurada, Y.; Sugiyama, A.; Yoneyama, S.; Tanabe, N.; Matsubara, M.; Mabuchi, F.; Iijima, H. Comparison of two-year outcomes after photodynamic therapy with ranibizumab or aflibercept for polypoidal choroidal vasculopathy. Sci. Rep. 2017, 7, 16461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, M.; Sakurada, Y.; Sugiyama, A.; Fukuda, Y.; Parikh, R.; Kashiwagi, K. Response to photodynamic therapy combined with intravitreal aflibercept for polypoidal choroidal vasculopathy depending on fellow-eye condition:2-year results. PLoS ONE 2020, 15, e0237330. [Google Scholar] [CrossRef]
- Lee, W.K.; Iida, T.; Ogura, Y.; Chen, S.-J.; Wong, T.Y.; Mitchell, P.; Cheung, C.M.G.; Zhang, Z.; Leal, S.; Ishibashi, T.; et al. Efficacy and Safety of Intravitreal Aflibercept for Polypoidal Choroidal Vasculopathy in the PLANET Study. JAMA Ophthalmol. 2018, 136, 786–793. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Das, A.; Do, D.V.; Dugel, P.U.; Gomes, A.; Holz, F.G.; Koh, A.; Pan, C.K.; Sepah, Y.J.; Patel, N.; et al. Brolucizumab: Evolution through Preclinical and Clinical Studies and the Implications for the Management of Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hoshino, J.; Mukai, R.; Nakamura, K.; Akiyama, H. Short-term outcomes of intravitreal brolucizumab for treatment-naïve neovascular age-related macular degeneration with type 1 choroidal neovascularization including polypoidal choroidal vasculopathy. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, A.; Kodjikian, L.; Vasavada, S.; Jha, S.; Srivastava, S.; Sudhalkar, A.; Mathis, T. Brolucizumab for Choroidal Neovascular Membrane with Pigment Epithelial Tear and Subretinal Fluid. J. Clin. Med. 2021, 10, 2425. [Google Scholar] [CrossRef]
- Bilgic, A.; Kodjikian, L.; de Ribot, F.M.; Vasavada, V.; Gonzalez-Cortes, J.; Abukashabah, A.; Sudhalkar, A.; Mathis, T. Real-World Experience with Brolucizumab in Wet Age-Related Macular Degeneration: The REBA Study. J. Clin. Med. 2021, 10, 2758. [Google Scholar] [CrossRef]
- Kikushima, W.; Sakurada, Y.; Sugiyama, A.; Tanabe, N.; Kume, A.; Iijima, H. Comparison of initial treatment between 3-monthly intravitreal aflibercept monotherapy and combined photodynamic therapy with single intravitreal aflibercept for polypoidal choroidal vasculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 255, 311–316. [Google Scholar] [CrossRef]
- Morimoto, M.; Matsumoto, H.; Mimura, K.; Akiyama, H. Two-year results of a treat-and-extend regimen with aflibercept for polypoidal choroidal vasculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Kano, M.; Yamamoto, A.; Saito, M.; Maruko, I.; Sekiryu, T.; Okada, A.A.; Iida, T. Subfoveal Choroidal Thickness during Aflibercept Therapy for Neovascular Age-Related Macular Degeneration. Ophthalmology 2016, 123, 617–624. [Google Scholar] [CrossRef]
- Fukuda, Y.; Sakurada, Y.; Sugiyama, A.; Yoneyama, S.; Matsubara, M.; Kikushima, W.; Tanabe, N.; Parikh, R.; Kashiwagi, K. Title: Pachydrusen in Fellow Eyes Predict Response to Aflibercept Monotherapy in Patients with Polypoidal Choroidal Vasculopathy. J. Clin. Med. 2020, 9, 2459. [Google Scholar] [CrossRef]
- Sakurada, Y.; Sugiyama, A.; Tanabe, N.; Kikushima, W.; Kume, A.; Iijima, H. Choroidal Thickness as a Prognostic Factor of Photodynamic Therapy with Aflibercept or Ranibizumab for Polypoidal Choroidal Vasculopathy. Retina 2017, 37, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Baumal, C.R.; Bodaghi, B.; Singer, M.; Tanzer, D.J.; Seres, A.; Joshi, M.R.; Feltgen, N.; Gale, R. Expert Opinion on Management of Intraocular Inflammation, Retinal Vasculitis, and Vascular Occlusion after Brolucizumab Treatment. Ophthalmol. Retina 2021, 5, 519–527. [Google Scholar] [CrossRef]
- Hikichi, T. Three Japanese cases of intraocular inflammation after intravitreal brolucizumab injections in one clinic. Jpn. J. Ophthalmol. 2021, 65, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Horiguchi, E.; Kawano, K.; Ushida, H.; Nakano, Y.; Ito, Y.; Terasaki, H. Three cases of brolucizumab-associated retinal vasculitis treated with systemic and local steroid therapy. Jpn. J. Ophthalmol. 2021, 65, 199–207. [Google Scholar] [CrossRef] [PubMed]




| Aflibercept | Brolucizumab | p-Value | |
|---|---|---|---|
| (n = 38) | (n = 14) | ||
| Age(years) 95%CI (confidence interval) | 71.3 ± 10.0 69.0–73.7 | 74.7 ± 7.3 69.0–80.5 | 0.25 |
| Male 95%CI | 27 (71.1%) 56–86.2% | 10 (71.4%) 44.4–98.5% | 1.0 |
| Presence of subretinal fluid | 38 (100%) | 14 (100%) | 1.0 |
| Mean baseline | 0.30 ± 0.30 0.20–0.39 | 0.27 ± 0.34 0.07–0.47 | 0.57 |
| log MAR BCVA 95%CI | |||
| Mean baseline CRT(μm) 95%CI | 321.2 ± 112.8 284–358 | 280.5 ± 131.7 205–357 | 0.16 |
| Mean baseline SCT(μm) 95%CI | 226.0 ± 100.2 193–259 | 190.5 ± 73.2 148–233 | 0.33 |
| Mean number of polyps 95%CI | 2.79 ± 3.2 1.72–3.85 | 2.29 ± 1.5 1.43–3.15 | 0.16 |
| Mean maximum diameter of polyp(μm) | 393.52 ± 234.6 | 280.2 ± 127.0 | 0.15 |
| Aflibercept | Brolucizumab | p-Value | |
|---|---|---|---|
| (n = 38) | (n = 14) | ||
| Baseline SRF (%) | 38 (100%) | 14 (100%) | 1.0 |
| SRF at 1-month visit (%) 95%CI | 21 (55.3%) 38.7–71.8% | 5 (35.7%) 7.0–64.4% | 0.35 |
| SRF at 2-month visit (%) 95%CI | 9 (23.7%) 9.5–37.9% | 2 (14.3%) 0–35.3% | 0.72 |
| SRF at 3-month visit (%) 95%CI | 6 (15.8%) 3.6–27.9% | 0 (0%) 0% | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, Y.; Sakurada, Y.; Matsubara, M.; Hasebe, Y.; Sugiyama, A.; Kikushima, W.; Kashiwagi, K. Comparison of Outcomes between 3 Monthly Brolucizumab and Aflibercept Injections for Polypoidal Choroidal Vasculopathy. Biomedicines 2021, 9, 1164. https://doi.org/10.3390/biomedicines9091164
Fukuda Y, Sakurada Y, Matsubara M, Hasebe Y, Sugiyama A, Kikushima W, Kashiwagi K. Comparison of Outcomes between 3 Monthly Brolucizumab and Aflibercept Injections for Polypoidal Choroidal Vasculopathy. Biomedicines. 2021; 9(9):1164. https://doi.org/10.3390/biomedicines9091164
Chicago/Turabian StyleFukuda, Yoshiko, Yoichi Sakurada, Mio Matsubara, Yuka Hasebe, Atsushi Sugiyama, Wataru Kikushima, and Kenji Kashiwagi. 2021. "Comparison of Outcomes between 3 Monthly Brolucizumab and Aflibercept Injections for Polypoidal Choroidal Vasculopathy" Biomedicines 9, no. 9: 1164. https://doi.org/10.3390/biomedicines9091164
APA StyleFukuda, Y., Sakurada, Y., Matsubara, M., Hasebe, Y., Sugiyama, A., Kikushima, W., & Kashiwagi, K. (2021). Comparison of Outcomes between 3 Monthly Brolucizumab and Aflibercept Injections for Polypoidal Choroidal Vasculopathy. Biomedicines, 9(9), 1164. https://doi.org/10.3390/biomedicines9091164







