Modified Vaccinia Virus Ankara as a Viral Vector for Vaccine Candidates against Chikungunya Virus
Abstract
:1. Vaccinia Virus and the Success against Smallpox
2. Second-Generation Smallpox Vaccines
3. Limitations of First- and Second-Generation Smallpox Vaccines
4. Third-Generation Smallpox Vaccines: MVA
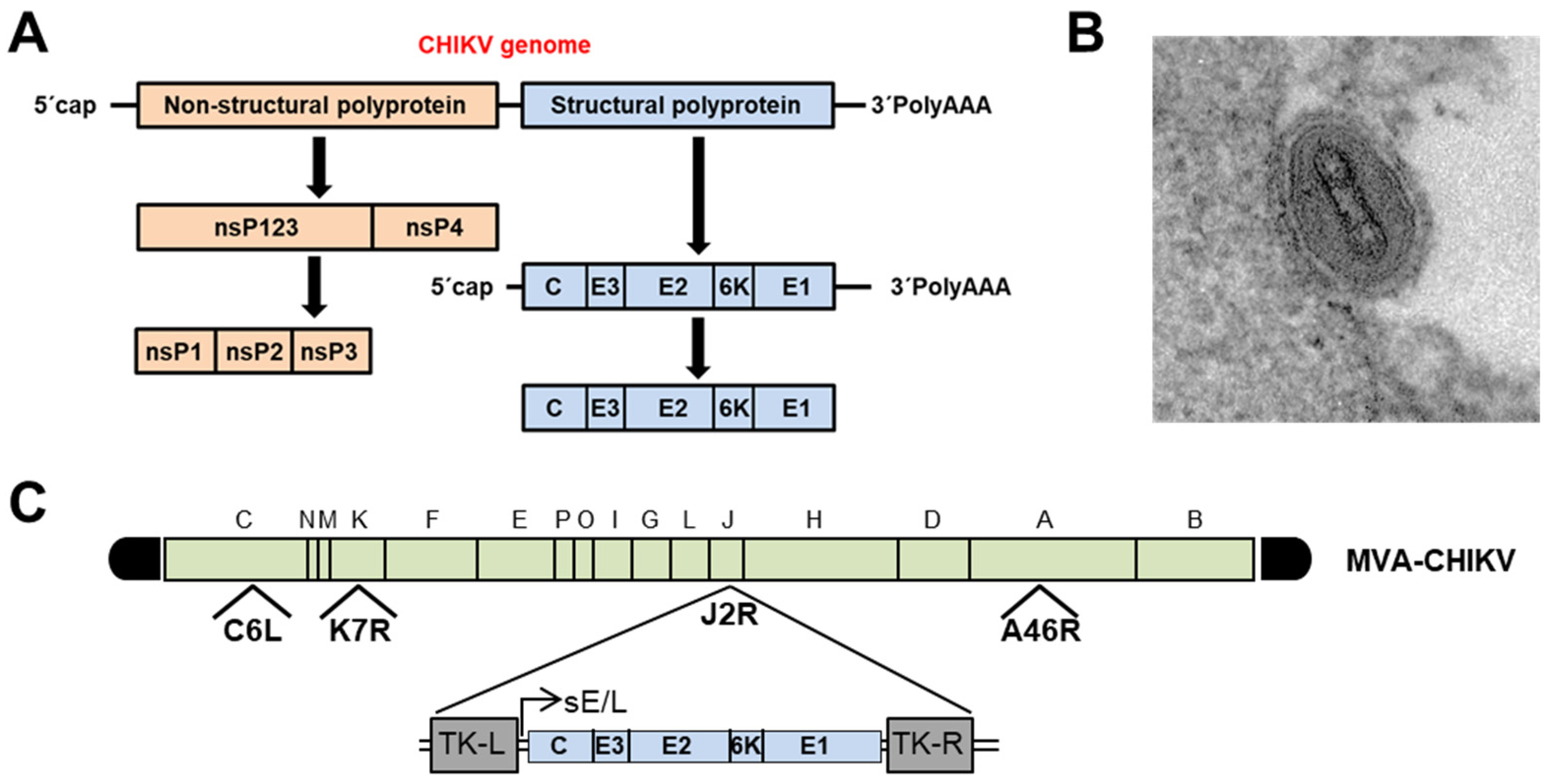
5. History, Pathology and Structure of Chikungunya Virus
6. Recombinant MVAs as Potential Vaccine Candidates against Chikungunya Virus
7. Other Vaccination Strategies against CHIKV
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henderson, D.A. The eradication of smallpox—An overview of the past, present, and future. Vaccine 2011, 29 (Suppl. 4), D7–D9. [Google Scholar] [CrossRef] [PubMed]
- Barquet, N.; Domingo, P. Smallpox: The triumph over the most terrible of the ministers of death. Ann. Intern. Med. 1997, 127, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I. Smallpox and Its Eradication; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Esparza, J.; Schrick, L.; Damaso, C.R.; Nitsche, A. Equination (inoculation of horsepox): An early alternative to vaccination (inoculation of cowpox) and the potential role of horsepox virus in the origin of the smallpox vaccine. Vaccine 2017, 35, 7222–7230. [Google Scholar] [CrossRef] [PubMed]
- Mark, C.; Rigau-Perez, J.G. The world’s first immunization campaign: The Spanish Smallpox Vaccine Expedition, 1803–1813. Bull. Hist. Med. 2009, 83, 63–94. [Google Scholar] [CrossRef]
- Esparza, J. Three different paths to introduce the smallpox vaccine in early 19th century United States. Vaccine 2020, 38, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Upton, C.; Hazes, B.; Evans, D.H. Genomic analysis of the vaccinia virus strain variants found in Dryvax vaccine. J. Virol. 2011, 85, 13049–13060. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Sampedro, L.; Perdiguero, B.; Mejias-Perez, E.; Garcia-Arriaza, J.; Di, P.M.; Esteban, M. The Evolution of Poxvirus Vaccines. Viruses 2015, 7, 1726–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, J.M.; Ruben, F.L.; Neff, J.M.; Millar, J.D. Complications of smallpox vaccination, 1968. N. Engl. J. Med. 1969, 281, 1201–1208. [Google Scholar] [CrossRef]
- Rosenthal, S.R.; Merchlinsky, M.; Kleppinger, C.; Goldenthal, K.L. Developing new smallpox vaccines. Emerg. Infect. Dis. 2001, 7, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Halsell, J.S.; Riddle, J.R.; Atwood, J.E.; Gardner, P.; Shope, R.; Poland, G.A.; Gray, G.C.; Ostroff, S.; Eckart, R.E.; Hospenthal, D.R.; et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA 2003, 289, 3283–3289. [Google Scholar] [CrossRef]
- Mayr, A.; Munz, E. Changes in the vaccinia virus through continuing passages in chick embryo fibroblast cultures. Zentralbl. Bakteriol. Orig. 1964, 195, 24–35. [Google Scholar]
- Antoine, G.; Scheiflinger, F.; Dorner, F.; Falkner, F.G. The complete genomic sequence of the modified vaccinia Ankara strain: Comparison with other orthopoxviruses. Virology 1998, 244, 365–396. [Google Scholar] [CrossRef]
- Meisinger-Henschel, C.; Schmidt, M.; Lukassen, S.; Linke, B.; Krause, L.; Konietzny, S.; Goesmann, A.; Howley, P.; Chaplin, P.; Suter, M.; et al. Genomic sequence of chorioallantois vaccinia virus Ankara, the ancestor of modified vaccinia virus Ankara. J. Gen. Virol. 2007, 88 Pt 5, 3249–3259. [Google Scholar] [CrossRef]
- Blanchard, T.J.; Alcami, A.; Andrea, P.; Smith, G.L. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: Implications for use as a human vaccine. J. Gen. Virol. 1998, 79, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Sutter GMoss, B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 1992, 89, 10847–10851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutter, G.; Wyatt, L.S.; Foley, P.L.; Bennink, J.R.; Moss, B. A recombinant vector derived from the host range- restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine 1994, 12, 1032–1040. [Google Scholar] [CrossRef]
- Gomez, C.E.; Najera, J.L.; Krupa, M.; Esteban, M. The poxvirus vectors MVA and NYVAC as gene delivery systems for vaccination against infectious diseases and cancer. Curr. Gene Ther. 2008, 8, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.S.; Greenberg, R.N. IMVAMUNE: Modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert. Rev. Vaccines 2009, 8, 13–24. [Google Scholar] [CrossRef]
- Pittman, P.R.; Hahn, M.; Lee, H.S.; Koca, C.; Samy, N.; Schmidt, D.; Hornung, J.; Weidenthaler, H.; Heery, C.R.; Meyer, T.P.H.; et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N. Engl. J. Med. 2019, 381, 1897–1908. [Google Scholar] [CrossRef]
- Stickl, H.; Hochstein-Mintzel, V.; Mayr, A.; Huber, H.C.; Schafer, H.; Holzner, A. MVA vaccination against smallpox: Clinical tests with an attenuated live vaccinia virus strain (MVA) (author’s transl). Dtsch. Med. Wochenschr. 1974, 99, 2386–2392. [Google Scholar] [CrossRef]
- Gomez, C.E.; Perdiguero, B.; Garcia-Arriaza, J.; Esteban, M. Poxvirus vectors as HIV/AIDS vaccines in humans. Hum. Vaccin Immunother. 2012, 8, 1192–1207. [Google Scholar] [CrossRef] [Green Version]
- Tomori, O.; Kolawole, M.O. Ebola virus disease: Current vaccine solutions. Curr. Opin. Immunol. 2021, 71, 27–33. [Google Scholar] [CrossRef]
- Her, Z.; Kam, Y.W.; Lin, R.T.; Ng, L.F. Chikungunya: A bending reality. Microbes. Infect. 2009, 11, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Dupuis-Maguiraga, L.; Noret, M.; Brun, S.; Le, G.R.; Gras, G.; Roques, P. Chikungunya disease: Infection-associated markers from the acute to the chronic phase of arbovirus-induced arthralgia. PLoS Negl. Trop. Dis. 2012, 6, e1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, O.; Albert, M.L. Biology and pathogenesis of chikungunya virus. Nat. Rev. Microbiol. 2010, 8, 491–500. [Google Scholar] [CrossRef]
- Robinson, M.C. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–1953. I. Clinical features. Trans. R Soc. Trop. Med. Hyg. 1955, 49, 28–32. [Google Scholar] [CrossRef]
- Halstead, S.B.; Scanlon, J.E.; Umpaivit, P.; Udomsakdi, S. Dengue and chikungunya virus infection in man in Thailand, 1962-1964, IV. Epidemiologic studies in the Bangkok metropolitan area. Am. J. Trop. Med. Hyg. 1969, 18, 997–1021. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.M.; Logue, C.H. Changing patterns of chikungunya virus: Re-emergence of a zoonotic arbovirus. J. Gen. Virol. 2007, 88, 2363–2377. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Singh, M.; Mishra, N.; Lakshmi, V. Resurgence of chikungunya virus in India: An emerging threat. Eurosurveillance 2006, 11, E060810. [Google Scholar] [CrossRef]
- Patterson, J.; Sammon, M.; Garg, M. Dengue, Zika and Chikungunya: Emerging Arboviruses in the New World. West. J. Emerg. Med. 2016, 17, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, B.; Muyembe-Tamfum, J.J.; Bessaud, M.; Tock, F.; Tolou, H.; Durand, J.P.; Peyrefitte, C.N. Epidemic resurgence of Chikungunya virus in democratic Republic of the Congo: Identification of a new central African strain. J. Med. Virol. 2004, 74, 277–282. [Google Scholar] [CrossRef]
- Barzon, L. Ongoing and emerging arbovirus threats in Europe. J. Clin. Virol. 2018, 107, 38–47. [Google Scholar] [CrossRef]
- Weaver, S.C.; Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Solignat, M.; Gay, B.; Higgs, S.; Briant, L.; Devaux, C. Replication cycle of chikungunya: A re-emerging arbovirus. Virology 2009, 393, 183–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallego-Gomez, J.C.; Risco, C.; Rodriguez, D.; Cabezas, P.; Guerra, S.; Carrascosa, J.L.; Esteban, M. Differences in virus-induced cell morphology and in virus maturation between MVA and other strains (WR, Ankara, and NYCBH) of vaccinia virus in infected human cells. J. Virol. 2003, 77, 10606–10622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goebel, S.J.; Johnson, G.P.; Perkus, M.E.; Davis, S.W.; Winslow, J.P.; Paoletti, E. The complete DNA sequence of vaccinia virus. Virology 1990, 179, 247–263. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Sisler, J.R.; Moss, B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques 1997, 23, 1094–1097. [Google Scholar] [CrossRef]
- Garcia-Arriaza, J.; Cepeda, V.; Hallengard, D.; Sorzano, C.O.; Kummerer, B.M.; Liljestrom, P.; Esteban, M. A novel poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and protects mice against chikungunya infection. J. Virol. 2014, 88, 3527–3547. [Google Scholar] [CrossRef] [Green Version]
- Lorente, E.; Barriga, A.; Barnea, E.; Palomo, C.; Garcia-Arriaza, J.; Mir, C.; Esteban, M.; Admon, A.; Lopez, D. Immunoproteomic analysis of a Chikungunya poxvirus-based vaccine reveals high HLA class II immunoprevalence. PLoS Negl. Trop. Dis. 2019, 13, e0007547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorente, E.; Barriga, A.; Garcia-Arriaza, J.; Lemonnier, F.A.; Esteban, M.; Lopez, D. Complex antigen presentation pathway for an HLA-A*0201-restricted epitope from Chikungunya 6K protein. PLoS Negl. Trop. Dis. 2017, 11, e0006036. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.A. Immunologic basis of vaccine vectors. Immunity 2010, 33, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, M.L.; Ljungberg, K.; Kakoulidou, M.; Kostic, L.; Hallengard, D.; Garcia-Arriaza, J.; Merits, A.; Esteban, M.; Liljestrom, P. Kinetic and phenotypic analysis of CD8+ T cell responses after priming with alphavirus replicons and homologous or heterologous booster immunizations. J. Virol. 2014, 88, 12438–12451. [Google Scholar] [CrossRef] [Green Version]
- Roques, P.; Ljungberg, K.; Kummerer, B.M.; Gosse, L.; Dereuddre-Bosquet, N.; Tchitchek, N.; Hallengard, D.; Garcia-Arriaza, J.; Meinke, A.; Esteban, M.; et al. Attenuated and vectored vaccines protect nonhuman primates against Chikungunya virus. JCI Insight 2017, 2, e83527. [Google Scholar] [CrossRef] [PubMed]
- Hallengard, D.; Kakoulidou, M.; Lulla, A.; Kummerer, B.M.; Johansson, D.X.; Mutso, M.; Lulla, V.; Fazakerley, J.K.; Roques, P.; Le, G.R.; et al. Novel attenuated Chikungunya vaccine candidates elicit protective immunity in C57BL/6 mice. J. Virol. 2014, 88, 2858–2866. [Google Scholar] [CrossRef] [Green Version]
- Hallengard, D.; Lum, F.M.; Kummerer, B.M.; Lulla, A.; Lulla, V.; Garcia-Arriaza, J.; Fazakerley, J.K.; Roques, P.; Le, G.R.; Merits, A.; et al. Prime-boost immunization strategies against Chikungunya virus. J. Virol. 2014, 88, 13333–13343. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Arriaza, J.; Esteban, M. Enhancing poxvirus vectors vaccine immunogenicity. Hum. Vaccin. Immunother. 2014, 10, 2235–2244. [Google Scholar] [CrossRef] [Green Version]
- Weger-Lucarelli, J.; Chu, H.; Aliota, M.T.; Partidos, C.D.; Osorio, J.E. A novel MVA vectored Chikungunya virus vaccine elicits protective immunity in mice. PLoS Negl. Trop. Dis. 2014, 8, e2970. [Google Scholar] [CrossRef]
- van den Doel, P.; Volz, A.; Roose, J.M.; Sewbalaksing, V.D.; Pijlman, G.P.; van Middelkoop, I.; Duiverman, V.; van de Wetering, E.; Sutter, G.; Osterhaus, A.D.; et al. Recombinant modified vaccinia virus Ankara expressing glycoprotein E2 of Chikungunya virus protects AG129 mice against lethal challenge. PLoS Negl. Trop. Dis. 2014, 8, e3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, C.; Buchner, S.M.; Schnierle, B.S. A small antigenic determinant of the Chikungunya virus E2 protein is sufficient to induce neutralizing antibodies which are partially protective in mice. PLoS Negl. Trop. Dis. 2015, 9, e0003684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Song, S.; Zhang, L. Recent Progress in Vaccine Development Against Chikungunya Virus. Front. Microbiol. 2019, 10, 2881. [Google Scholar] [CrossRef] [Green Version]
- DeFilippis, V.R. Chikungunya Virus Vaccines: Platforms, Progress, and Challenges. Curr. Top. Microbiol. Immunol. 2019, 1–26. [Google Scholar]
- Weaver, S.C.; Osorio, J.E.; Livengood, J.A.; Chen, R.; Stinchcomb, D.T. Chikungunya virus and prospects for a vaccine. Expert. Rev. Vaccines 2012, 11, 1087–1101. [Google Scholar] [CrossRef]
- Harder, T.; Koch, J.; Vygen-Bonnet, S.; Kulper-Schiek, W.; Pilic, A.; Reda, S.; Scholz, S.; Wichmann, O. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: Interim results of a living systematic review, 1 January to 14 May 2021. Eurosurveillance 2021, 26, 2100563. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arriaza, J.; Garaigorta, U.; Perez, P.; Lazaro-Frias, A.; Zamora, C.; Gastaminza, P.; Del, F.C.; Casasnovas, J.M.; Sorzano, C.O.S.; Sancho, D.; et al. COVID-19 vaccine candidates based on modified vaccinia virus Ankara expressing the SARS-CoV-2 spike induce robust T- and B-cell immune responses and full efficacy in mice. J. Virol. 2021, 95, e02260-20. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Americo, J.L.; Cotter, C.A.; Earl, P.L.; Erez, N.; Peng, C.; Moss, B. One or two injections of MVA-vectored vaccine shields hACE2 transgenic mice from SARS-CoV-2 upper and lower respiratory tract infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2026785118. [Google Scholar] [CrossRef] [PubMed]
- Tscherne, A.; Schwarz, J.H.; Rohde, C.; Kupke, A.; Kalodimou, G.; Limpinsel, L.; Okba, N.M.A.; Bosnjak, B.; Sandrock, I.; Odak, I.; et al. Immunogenicity and efficacy of the COVID-19 candidate vector vaccine MVA-SARS-2-S in preclinical vaccination. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Chiuppesi, F.; Salazar, M.D.; Contreras, H.; Nguyen, V.H.; Martinez, J.; Park, S.; Nguyen, J.; Kha, M.; Iniguez, A.; Zhou, Q.; et al. Development of a Synthetic Poxvirus-Based SARS-CoV-2 Vaccine. bioRxiv 2020. [Google Scholar] [CrossRef]
- Chiuppesi, F.; Salazar, M.D.; Contreras, H.; Nguyen, V.H.; Martinez, J.; Park, Y.; Nguyen, J.; Kha, M.; Iniguez, A.; Zhou, Q.; et al. Development of a multi-antigenic SARS-CoV-2 vaccine candidate using a synthetic poxvirus platform. Nat. Commun. 2020, 11, 6121. [Google Scholar] [CrossRef]
| Name | CHIKV Viral Proteins Expressed | Vaccination Strategy a | In Vitro Effects | In Vivo Effects (Mice or Nonhuman Primates) | Limitations | Reference |
|---|---|---|---|---|---|---|
| MVA-CHIKV (C/E3/E2/6K/E1) | C, E3, E2, 6K and E1 structural proteins | Single and/or double dose | Multiprotein expression. Activation of human dendritic cells and macrophages. |
| [39,40,41,43,44] | |
| MVA-CHIK | E3 and E2 structural proteins | Two doses | E3/E2 expression |
| Low or undetectable levels of neutralizing Abs. | [45] |
| MVA-E3E2 (E3/E2) | E3 and E2, structural proteins | Single dose | E3/E2 expression |
| Low levels of neutralizing antibodies. | [46] |
| MVA-6KE1 (6K/E1) | 6K and E1 structural proteins | Single dose | E1 expression |
| Low levels of infectious CHIKV were isolated from the spleen of mice immunized. | [46] |
| MVA-E3E26KE1 (E3/E2/6K/E1) | E3, E2, 6K and E1 structural proteins | Single dose | E3/E2/E1 Expression |
| [46] | |
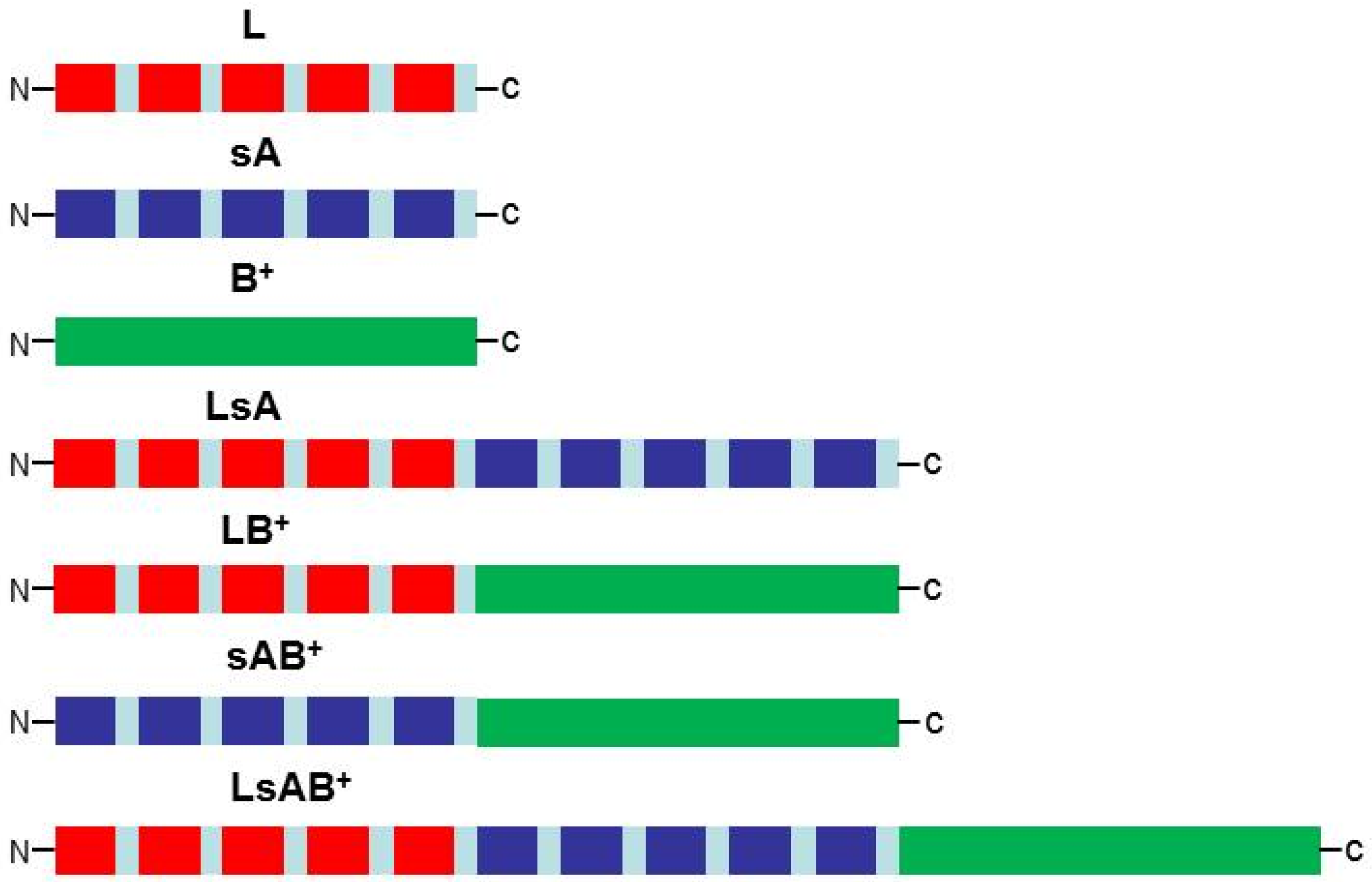
| MVA-CHIKV-sAB+ | 5 putative linear antigens of domain A from CHIKV E2 protein assembled with glycine-serine plus the whole domain B from E2 | Four doses | Multiepitope expression |
|
| [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Arriaza, J.; Esteban, M.; López, D. Modified Vaccinia Virus Ankara as a Viral Vector for Vaccine Candidates against Chikungunya Virus. Biomedicines 2021, 9, 1122. https://doi.org/10.3390/biomedicines9091122
García-Arriaza J, Esteban M, López D. Modified Vaccinia Virus Ankara as a Viral Vector for Vaccine Candidates against Chikungunya Virus. Biomedicines. 2021; 9(9):1122. https://doi.org/10.3390/biomedicines9091122
Chicago/Turabian StyleGarcía-Arriaza, Juan, Mariano Esteban, and Daniel López. 2021. "Modified Vaccinia Virus Ankara as a Viral Vector for Vaccine Candidates against Chikungunya Virus" Biomedicines 9, no. 9: 1122. https://doi.org/10.3390/biomedicines9091122
APA StyleGarcía-Arriaza, J., Esteban, M., & López, D. (2021). Modified Vaccinia Virus Ankara as a Viral Vector for Vaccine Candidates against Chikungunya Virus. Biomedicines, 9(9), 1122. https://doi.org/10.3390/biomedicines9091122






