Cytokine (IL-10, IL-6, TNF-α and TGF-β1) Gene Polymorphisms in Chronic Hepatitis C Virus Infection among Malay Male Drug Abusers
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Ethical Clearance
2.2. DNA Extraction
2.3. Genetic Study
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blach, S.; Zeuzem, S.; Manns, M.; Altraif, I.; Duberg, A.S.; Muljono, D.H.; Waked, I.; Alavian, S.M.; Lee, M.H.; Negro, F.; et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef]
- Dore, G.J.; Law, M.; MacDonald, M.; Kaldor, J.M. Epidemiology of hepatitis C virus infection in Australia. J. Clin. Virol 2003, 26, 171–184. [Google Scholar] [CrossRef]
- Diaz, T.; Des Jarlais, D.C.; Vlahov, D.; Perlis, T.E.; Edwards, V.; Friedman, S.R.; Rockwell, R.; Hoover, D.; Williams, I.T.; Monterroso, E.R. Factors associated with prevalent hepatitis C: Differences among young adult injection drug users in lower and upper Manhattan, New York City. Am. J. Public Health 2001, 91, 23. [Google Scholar] [PubMed]
- Yeung, C.Y.; Lee, H.C.; Chan, W.T.; Jiang, C.B.; Chang, S.W.; Chuang, C.K. Vertical transmission of hepatitis C virus: Current knowledge and perspectives. World J. Hepatol. 2014, 6, 643. [Google Scholar] [CrossRef] [PubMed]
- Who.int. Hepatitis C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 14 June 2021).
- Hallager, S.; Ladelund, S.; Christensen, P.B.; Kjær, M.; Roege, B.T.; Grønbæk, K.E.; Belard, E.; Barfod, T.S.; Madsen, L.G.; Gerstoft, J.; et al. Liver-related morbidity and mortality in patients with chronic hepatitis C and cirrhosis with and without sustained virologic response. Clin. Epidemiol. 2017, 9, 501. [Google Scholar] [CrossRef]
- Napoli, J.; Bishop, G.A.; McGuinness, P.H.; Painter, D.M.; McCaughan, G.W. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology 1996, 24, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Stanley, A.C.; Lacy, P. Pathways for cytokine secretion. Physiology 2010, 25, 218–229. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferri, C.; Ferrari, S.M.; Corrado, A.; Sansonno, D.; Antonelli, A. Cytokines and HCV-related disorders. Clin. Dev. Immunol. 2012, 2012, 468107. [Google Scholar] [CrossRef] [PubMed]
- Lacy, P.; Stow, J.L. Cytokine release from innate immune cells: Association with diverse membrane trafficking pathways. Blood 2011, 118, 9–18. [Google Scholar] [CrossRef]
- Bidwell, J.; Keen, L.; Gallagher, G.; Kimberly, R.; Huizinga, T.; McDermott, M.F.; Oksenberg, J.; McNicholl, J.; Pociot, F.; Hardt, C.; et al. Cytokine gene polymorphism in human disease: On-line databases. Genes Immun. 1999, 1, 3–19. [Google Scholar] [CrossRef]
- Corwin, E.J. Understanding cytokines part I: Physiology and mechanism of action. Biol. Res. Nurs. 2000, 2, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.R.; Zheng, B.X.; Liu, Z.F. Association between TGFB1 915G/C polymorphism and susceptibility to chronic hepatitis C virus infection: A meta-analysis. Biomed. Rep. 2014, 2, 239–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vidigal, P.G.; Germer, J.J.; Zein, N.N. Polymorphisms in the interleukin-10, tumor necrosis factor-α, and transforming growth factor-β1 genes in chronic hepatitis C patients treated with interferon and ribavirin. J. Hepatol. 2002, 36, 271–277. [Google Scholar] [CrossRef]
- Naeemi, H.; Aslam, R.; Raza, S.M.; Shahzad, M.A.; Naz, S.; Manzoor, S.; Khaliq, S. Distribution of IL28B and IL10 polymorphisms as genetic predictors of treatment response in Pakistani HCV genotype 3 patients. Arch. Virol. 2018, 163, 997–1008. [Google Scholar] [CrossRef]
- Barrett, S.; Collins, M.; Kenny, C.; Ryan, E.; Keane, C.O.; Crowe, J. Polymorphisms in tumour necrosis factor-α, transforming growth factor-β, interleukin-10, interleukin-6, interferon-γ, and outcome of hepatitis C virus infection. J. Med. Virol. 2003, 71, 212–218. [Google Scholar] [CrossRef]
- Pasha, H.F.; Radwan, M.I.; Hagrass, H.A.; Tantawy, E.A.; Emara, M.H. Cytokines genes polymorphisms in chronic hepatitis C: Impact on susceptibility to infection and response to therapy. Cytokine 2013, 61, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.S.; Wang, Z.Q.; Jiang, Y.K.; Zhang, X.Y.; Jiang, W.M. Association of cytokine gene polymorphisms with chronic hepatitis C virus genotype 1b infection in Chinese Han population: An observational study. Medicine 2020, 18, 99. [Google Scholar] [CrossRef]
- da Silva, N.M.; Germano, F.N.; Vidales-Braz, B.M.; do Carmo Zanella, R.; dos Santos, D.M.; Lobato, R.; de Martinez, A.M. Polymorphisms of IL-10 gene in patients infected with HCV under antiviral treatment in southern Brazil. Cytokine 2015, 73, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.S.; Tahir, S.; Salman, A.; Baig, T.A.; Shafi, T.; Zaidi, N.U.; Qadri, I. Analysis of interleukin-10 gene polymorphisms and hepatitis C susceptibility in Pakistan. J. Infect. Dev. Ctries 2011, 5, 473–479. [Google Scholar] [CrossRef][Green Version]
- Eskdale, J.; Kube, D.; Tesch, H.; Gallagher, G. Mapping of the human IL10 gene and further characterization of the 5’ flanking sequence. Immunogenet 1997, 46, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Opdal, S.H. IL-10 gene polymorphisms in infectious disease and SIDS. FEMS Immunol. Med. Microbiol. 2004, 42, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Gonsalkorale, W.M.; Perrey, C.; Pravica, V.; Whorwell, P.J.; Hutchinson, I.V. Interleukin 10 genotypes in irritable bowel syndrome: Evidence for an inflammatory component? Gut 2003, 52, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Z.; Ye, Q. Interleukin-6 gene polymorphisms and susceptibility to liver diseases: A meta-analysis. Medicine 2019, 98, e18408. [Google Scholar] [CrossRef]
- Fabrício-Silva, G.M.; Poschetzky, B.S.; de Mello Perez, R.; Dos Santos, R.C.; Cavalini, L.T.; Porto, L.C. Association of cytokine gene polymorphisms with hepatitis C virus infection in a population from Rio de Janeiro, Brazil. Hepatic Med. Evid. Res. 2015, 7, 71. [Google Scholar]
- Deeb, A.S.; Nasr, M.Y.; Badra, G.; El-Sayed, I.H. The Relationship between Interleukin-6 Polymorphism and Susceptibility to Hepatitis C-virus Infected Patients. Jordan Med. J. 2019, 53, 1–2. [Google Scholar]
- Asifa, G.Z.; Liaquat, A.; Murtaza, I.; Kazmi, S.A.; Javed, Q. Tumor necrosis factor-alpha gene promoter region polymorphism and the risk of coronary heart disease. Sci. World J. 2013, 2013, 203492. [Google Scholar] [CrossRef]
- Farid, S.; Rashid, L.; Swelam, S. Tumour Necrosis Factor-Alpha Gene Expression in Chronic Hepatitis C Virus Infection. Egypt J. Hosp. Med. 2013, 51, 395–404. [Google Scholar] [CrossRef]
- Ghoneim, A.M. Lack of association between 4 key TNF-alpha promoter polymorphisms and hepatitis C virus infection in a population of Egyptian patients. Biomed. Res. Ther. 2019, 6, 3156–3165. [Google Scholar] [CrossRef]
- Dogra, G.; Chakravarti, A.; Kar, P.; Chawla, Y.K. Polymorphism of tumor necrosis factor-α and interleukin-10 gene promoter region in chronic hepatitis C virus patients and their effect on pegylated interferon-α therapy response. Hum. Immunol. 2011, 72, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.R.; Lentz, J.J.; Rose, S.L.; Rabkin, J.; Corless, C.L.; Taylor, K. Donor polymorphism of tumor necrosis factor gene: Relationship with variable severity of hepatitis C recurrence after liver transplantation. Transplantation 1999, 68, 1898–1902. [Google Scholar] [CrossRef]
- Yee, L.J.; Tang, J.; Herrera, J.; Kaslow, R.A.; van Leeuwen, D.J. Tumor necrosis factor gene polymorphisms in patients with cirrhosis from chronic hepatitis C virus infection. Genes Immun. 2000, 1, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Rebbani, K.; Ezzikouri, S.; Marchio, A.; Ababou, M.; Kitab, B.; Dejean, A.; Kandil, M.; Pineau, P.; Benjelloun, S. Common polymorphic effectors of immunity against hepatitis B and C modulate susceptibility to infection and spontaneous clearance in a Moroccan population. Infect. Genet. Evol. 2014, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Liu, S.; Sun, X.; Xu, L. Association of TGF-β1 polymorphisms and chronic hepatitis C infection: A Meta-analysis. BMC Infect. Dis. 2019, 19, 758. [Google Scholar] [CrossRef]
- Fujii, D.; Brissenden, J.E.; Derynck, R.; Francke, U. Transforming growth factor β gene maps to human chromosome 19 long arm and to mouse chromosome 7. Somatic Cell Mol. Genet. 1986, 12, 281–288. [Google Scholar] [CrossRef]
- Lawrence, D.A. Transforming growth factor-beta: A general review. Eur. Cytokine Netw. 1996, 7, 363–374. [Google Scholar] [PubMed]
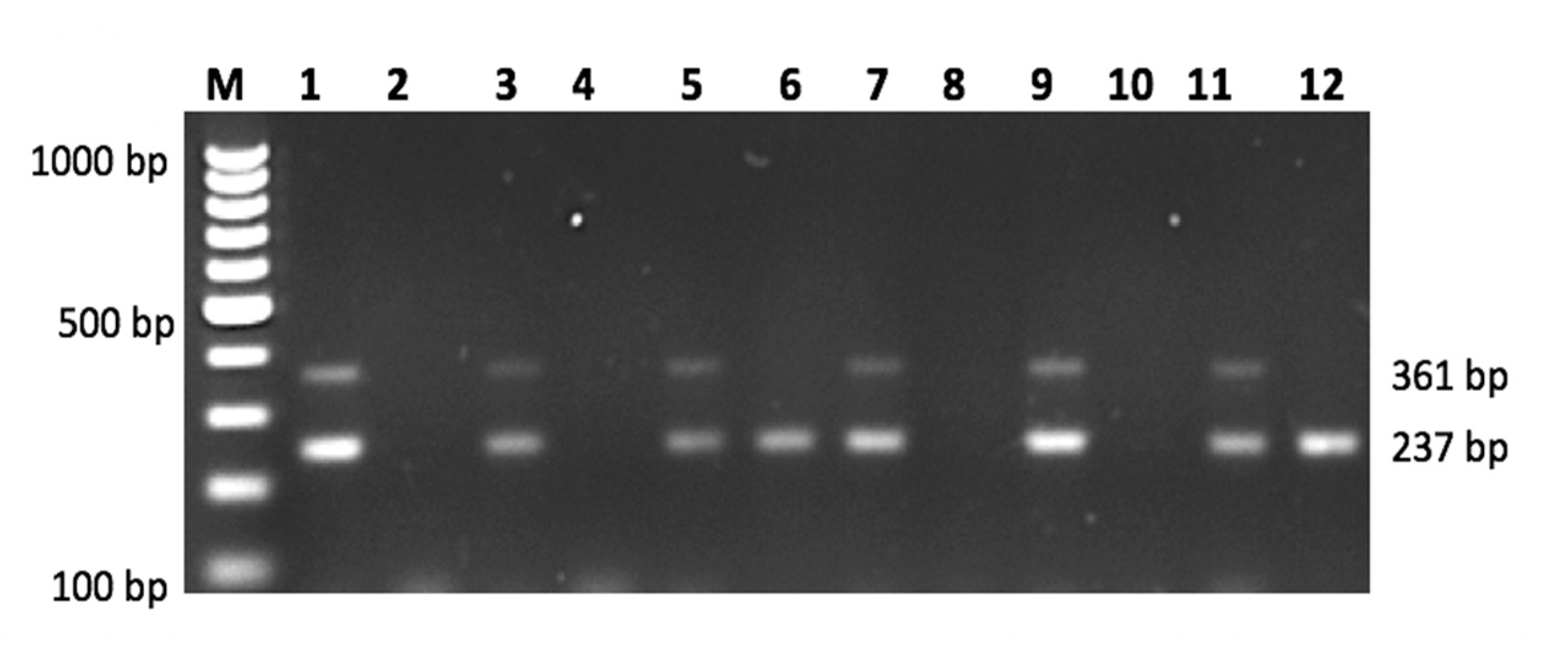
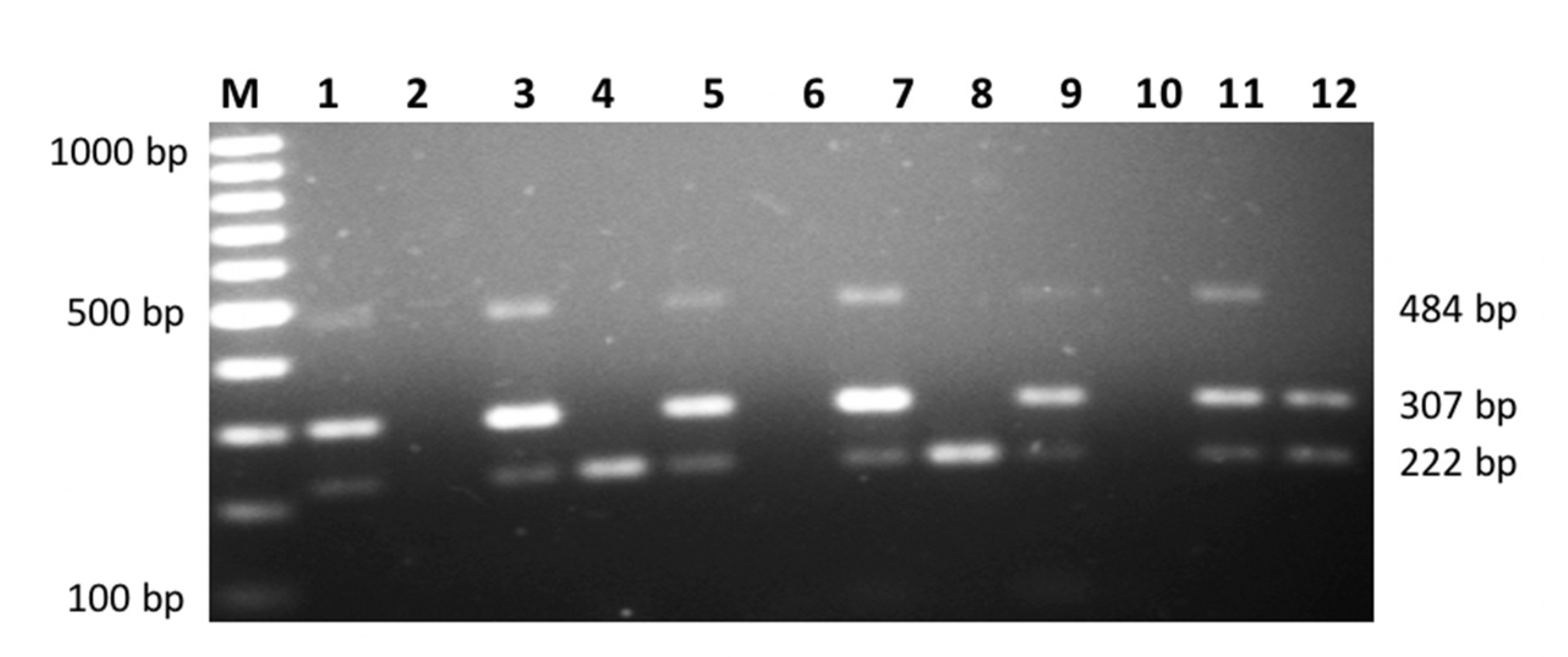
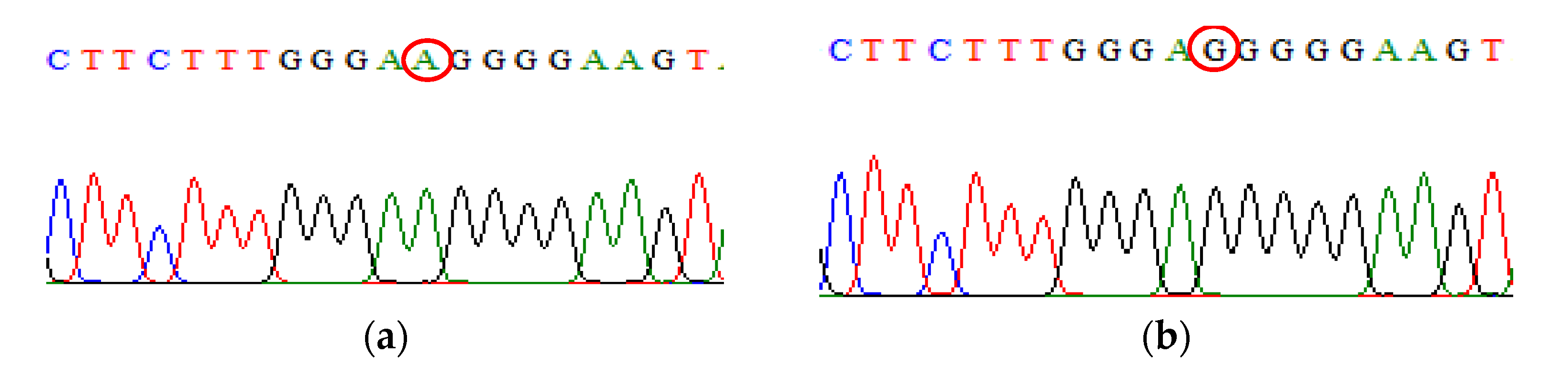
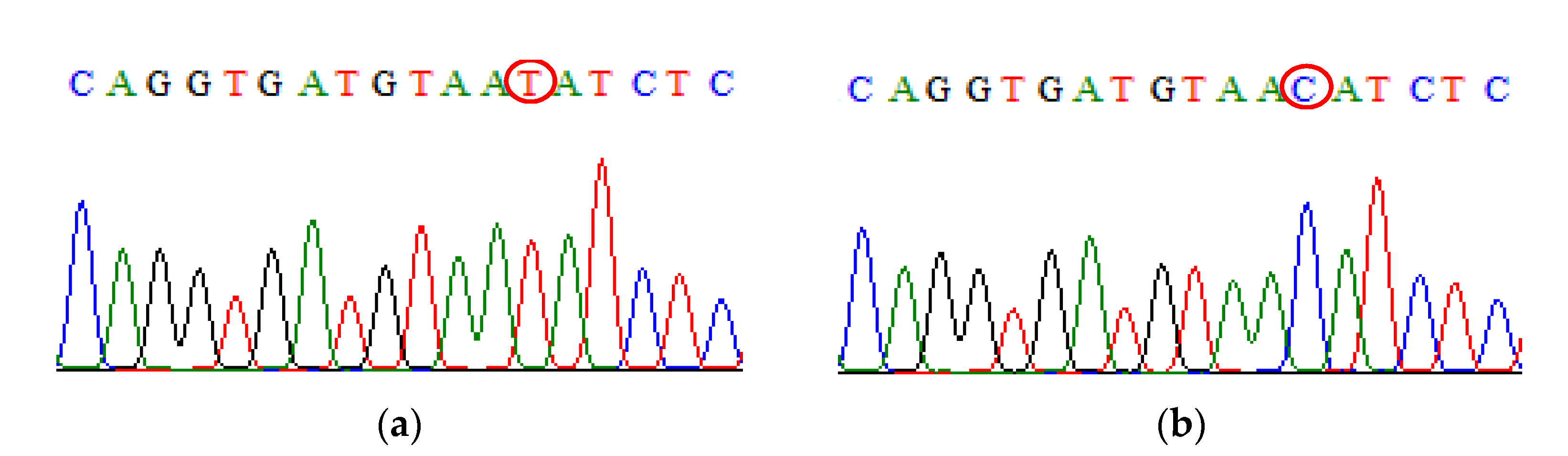
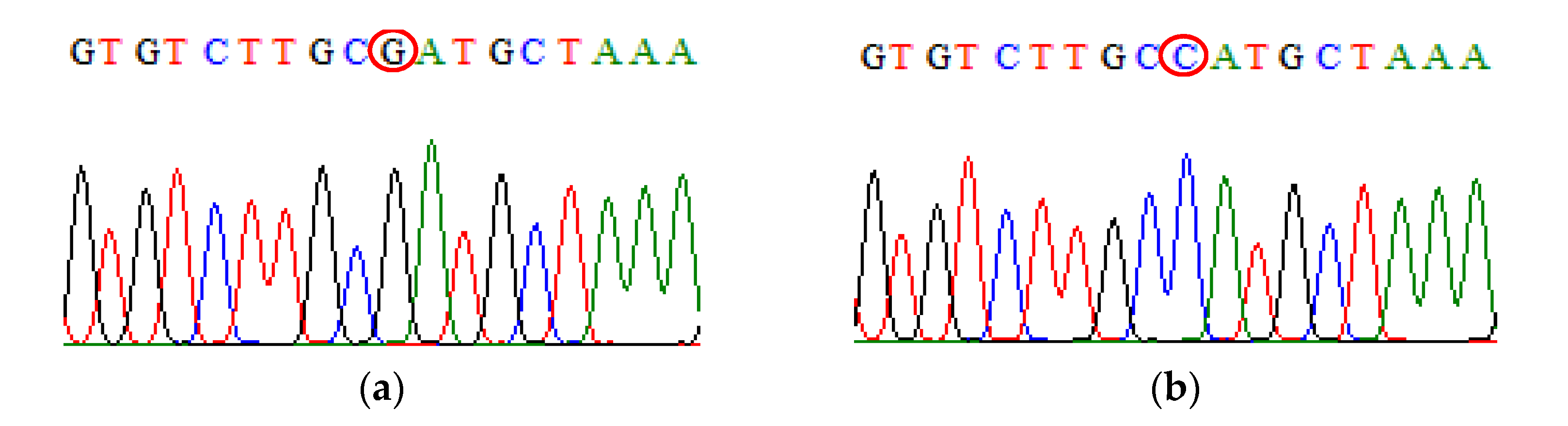
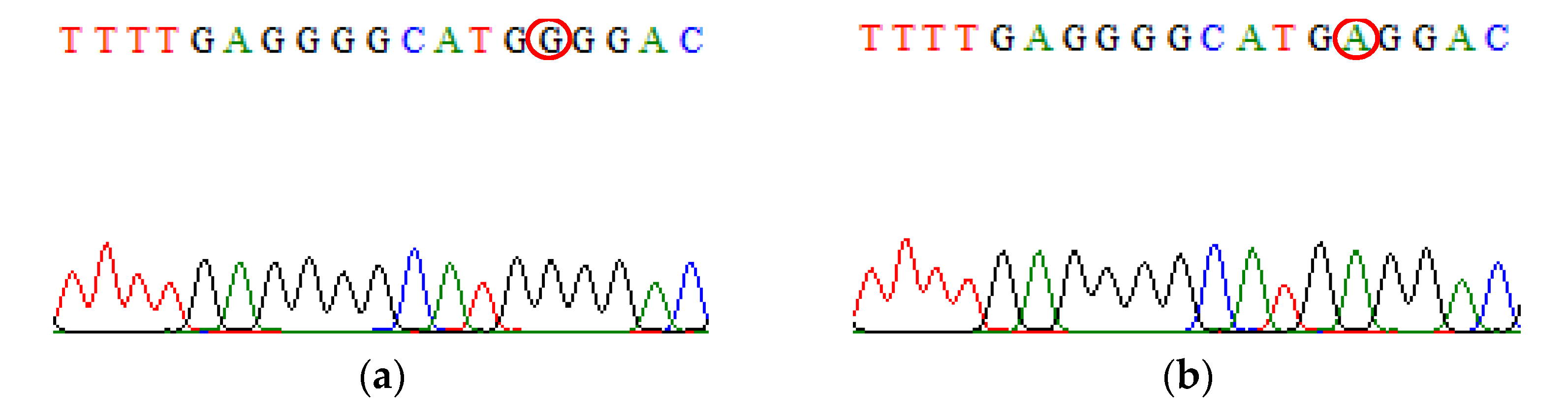
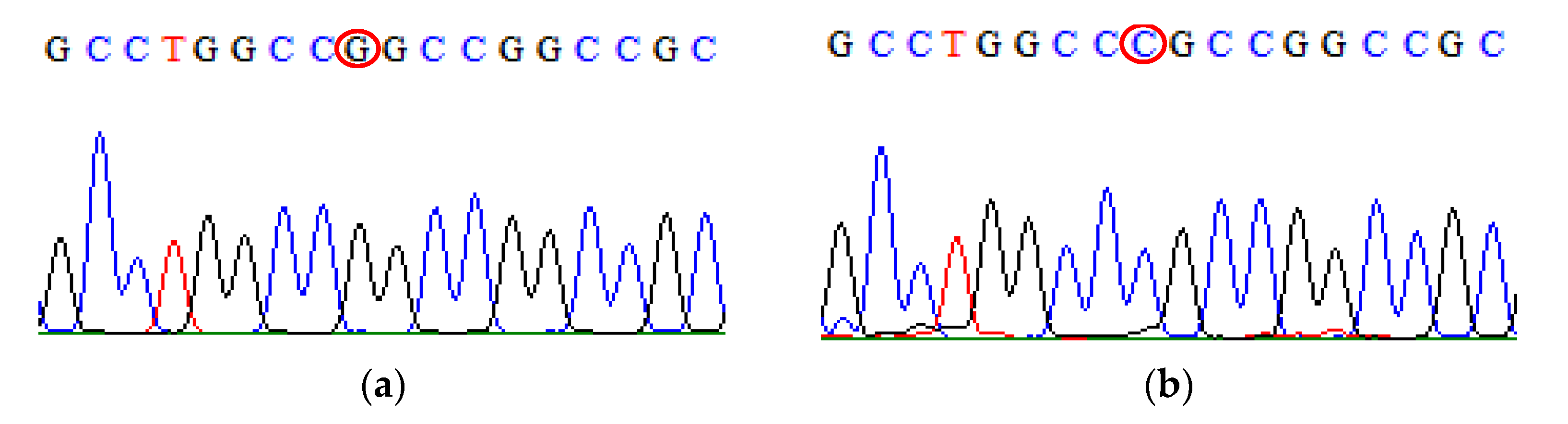
| SNP | Primer | Primer Sequences (5′ → 3′) | Size (bp) |
|---|---|---|---|
|
IL-10 rs1800896 | Primer A Primer G | Forward: ACTACTAAGGCTTCTTTGGGAA Forward: CTACTAAGGCTTCTTTGGGAG Reverse: TACCCTTGTACAGGTGATGTAAT | 484 484 |
|
IL-10 rs1800871 | Primer T Primer C | Forward: TACCCTTGTACAGGTGATGTAAT Forward: TACCCTTGTACAGGTGATGTAAC Reverse: TACCCTTGTACAGGTGATGTAAT | 222 222 |
|
IL-6 rs1800795 | Primer G Primer C | Forward: CCCCCCTAGTTGTGTCTTGCG Forward: CCCCCTAGTTGTGTCTTGCC Reverse: CAGTTCCAGGGCTAAGGATTTC | 307 307 |
|
TNF-α rs1800629 | Primer G Primer A | Forward: GCAATAGGTTTTGAGGGGCATGG Forward: GCAATAGGTTTTGAGGGCATGA Reverse: TGCTGTCCTTGCTGAGGGAGC | 361 361 |
|
TGF-β1 rs1800471 | Primer G Primer C | Forward: TACTGGTGCTGACGCCTGGCCG Forward: TACTGGTGCTGACGCCTGGCCC Reverse: GCTCCGGTTCTGCACTCTCCC | 237 237 |
| Characteristics | Drug Abusers with Positive Chronic HCV (HP Group) (n = 76) Mean (SD) | Drug Abusers without HCV (Control Group) (n = 40) Mean (SD) | Statistics p-Value |
|---|---|---|---|
| Age (years) a | 42.50 (5.24) c | 43.00 (6.90) c | 0.2809 |
| Height (m) b | 163.8 (5.37) | 164.5 (7.58) | 0.5789 |
| Weight (kg) b | 60.03 (8.70) | 60.88 (10.12) | 0.6396 |
| BMI (kg/m2) b | 22.40 (3.31) | 24.03 (3.00) | 0.5578 |
| Brachial systolic BP (mm/Hg) | 121.6 (16.04) | 121.7 (14.05) | 0.9676 |
| Brachial diastolic BP (mm/Hg) | 79.83 (9.74) | 78.75 (8.69) | 0.5576 |
| Total protein (g/L) | 80.51 (8.53) | 79.78 (8.95) | 0.6959 |
| Albumin (g/L) | 39.40 (3.90) | 40.92 (3.30) | 0.0576 |
| Total bilirubin (umol/L) | 9.48 (8.18) | 5.984 (2.50) | * 0.0148 |
| Alanine aminotransferase, ALT (U/L) | 35.89 (29.74) | 42.33 (42.29) | 0.3957 |
| Aspartate transaminase, AST (U/L) | 51.62 (32.25) | 49.00 (35.88) | 0.7181 |
| Alkaline phosphatase, ALP (U/L) | 110.2 (46.6) | 95.53 (39.21) | 0.1224 |
| Gamma-glutamyl transpeptidase, GGT (U/L) | 97.38 (159.5) | 62.36 (61.29) | 0.2123 |
| SNP | Genotype and Alleles | Drug Abusers with Chronic HCV (HP Group) (n = 76) | Drug Abusers without HCV (Control Group) (n = 40) | Statistics a p-Value |
|---|---|---|---|---|
| IL-10 rs1800896 | AA | 62 (81.6%) | 38 (95%) | 0.16 |
| AG | 9 (11.2%) | 2 (5%) | ||
| GG | 5 (6.6%) | 0 (%) | ||
| A | 133 (87.5%) | 78 (97.5%) | * 0.0142 | |
| G | 19 (12.5%) | 2 (2.5%) | ||
| IL-10 rs1800871 | TT | 30 (39.5%) | 11 (27.5%) | * 0.0386 |
| TC | 31 (40.8%) | 26 (65%) | ||
| CC | 15 (19.7%) | 3 (7.5%) | ||
| T | 91 (59.9%) | 48 (60%) | >0.9999 | |
| C | 61 (40.1%) | 32 (40%) | ||
| IL-6 rs1800795 | GG | 75 (98.7%) | 38 (95%) | 0.4228 |
| GC | 1 (1.3%) | 1 (2.5%) | ||
| CC | 0 | 1 (2.5%) | ||
| G | 151 (99.3%) | 77 (96.25%) | 0.1196 | |
| C | 1 (0.7%) | 3 (3.75%) | ||
| TNF-α rs1800629 | GG | 73 (96.1%) | 38 (95%) | 0.2544 |
| GA | 1 (1.3%) | 2 (5%) | ||
| AA | 2 (2.6%) | 0 | ||
| G | 147 (96.7%) | 78 (97.5%) | >0.9999 | |
| A | 5 (3.3%) | 2 (2.5%) | ||
| TGF-β 1rs1800471 | GG | 67 (88.2%) | 39 (97.5%) | 0.1291 |
| GC | 7 (9.2%) | 0 | ||
| CC | 2 (2.6%) | 1 (2.5%) | ||
| G | 141 (92.8%) | 78 (97.5%) | 0.2282 | |
| C | 11 (7.2%) | 2 (2.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, I.C.; Ahmad, I.; Suraiya, S.; Musa, N.F.; Nurul, A.A.; Ruzilawati, A.B. Cytokine (IL-10, IL-6, TNF-α and TGF-β1) Gene Polymorphisms in Chronic Hepatitis C Virus Infection among Malay Male Drug Abusers. Biomedicines 2021, 9, 1115. https://doi.org/10.3390/biomedicines9091115
Noh IC, Ahmad I, Suraiya S, Musa NF, Nurul AA, Ruzilawati AB. Cytokine (IL-10, IL-6, TNF-α and TGF-β1) Gene Polymorphisms in Chronic Hepatitis C Virus Infection among Malay Male Drug Abusers. Biomedicines. 2021; 9(9):1115. https://doi.org/10.3390/biomedicines9091115
Chicago/Turabian StyleNoh, Ismail Che, Imran Ahmad, Siti Suraiya, Nur Fadhlina Musa, Asma Abdullah Nurul, and Abu Bakar Ruzilawati. 2021. "Cytokine (IL-10, IL-6, TNF-α and TGF-β1) Gene Polymorphisms in Chronic Hepatitis C Virus Infection among Malay Male Drug Abusers" Biomedicines 9, no. 9: 1115. https://doi.org/10.3390/biomedicines9091115
APA StyleNoh, I. C., Ahmad, I., Suraiya, S., Musa, N. F., Nurul, A. A., & Ruzilawati, A. B. (2021). Cytokine (IL-10, IL-6, TNF-α and TGF-β1) Gene Polymorphisms in Chronic Hepatitis C Virus Infection among Malay Male Drug Abusers. Biomedicines, 9(9), 1115. https://doi.org/10.3390/biomedicines9091115







