Evaluation of β-Catenin Inhibition of Axitinib and Nitazoxanide in Human Monocyte-Derived Dendritic Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. MTS Assay (CellTiter 96 Aqueous One Solution Cell Proliferation Assay)
2.3. Western Blots
2.4. Indirect Immunofluorescence Microscopy
2.5. Flow Cytometry Analyses
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Mixed Leukocyte Reaction (MLR)
2.8. Endocytosis Assay
2.9. Chemotaxis Assay
2.10. RNA-Sequencing and Analyses
2.11. Software Tools Used for RNA-Seq Analysis
2.12. Statistical Analysis
3. Results
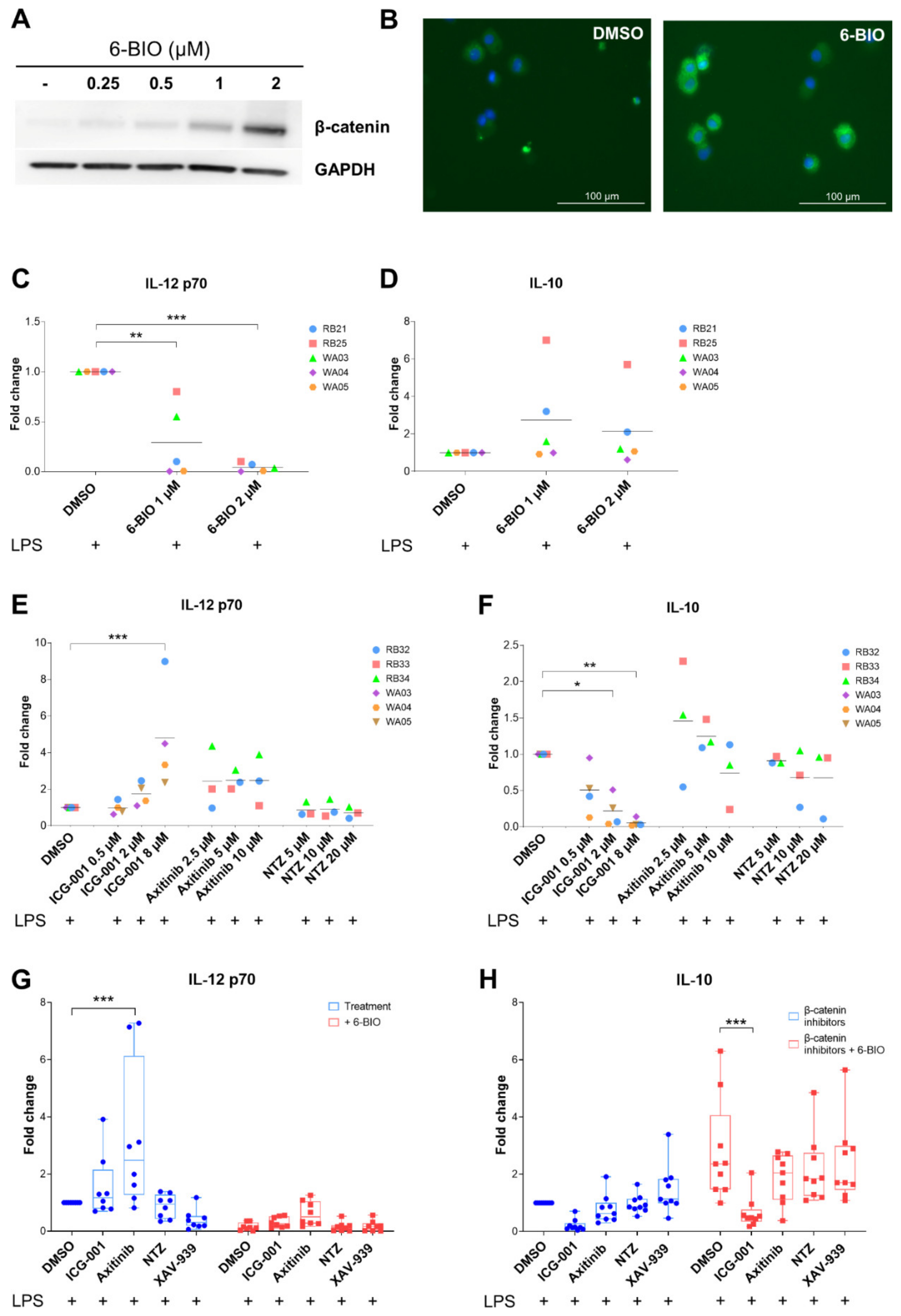
3.1. Compounds Affect the Metabolic Activity in Human moDCs
3.2. Dose-Dependent Effects of β-Catenin Modulators in LPS-Matured moDCs
3.3. Effect of Axitinib and Nitazoxanide in Competition with 6-BIO on Cytokine Secretion
3.4. Effects of Axitinib and Nitazoxanide on moDC Surface Markers
3.5. Effects of 6-BIO versus Potential β-Catenin Inhibitors on Migration of moDCs
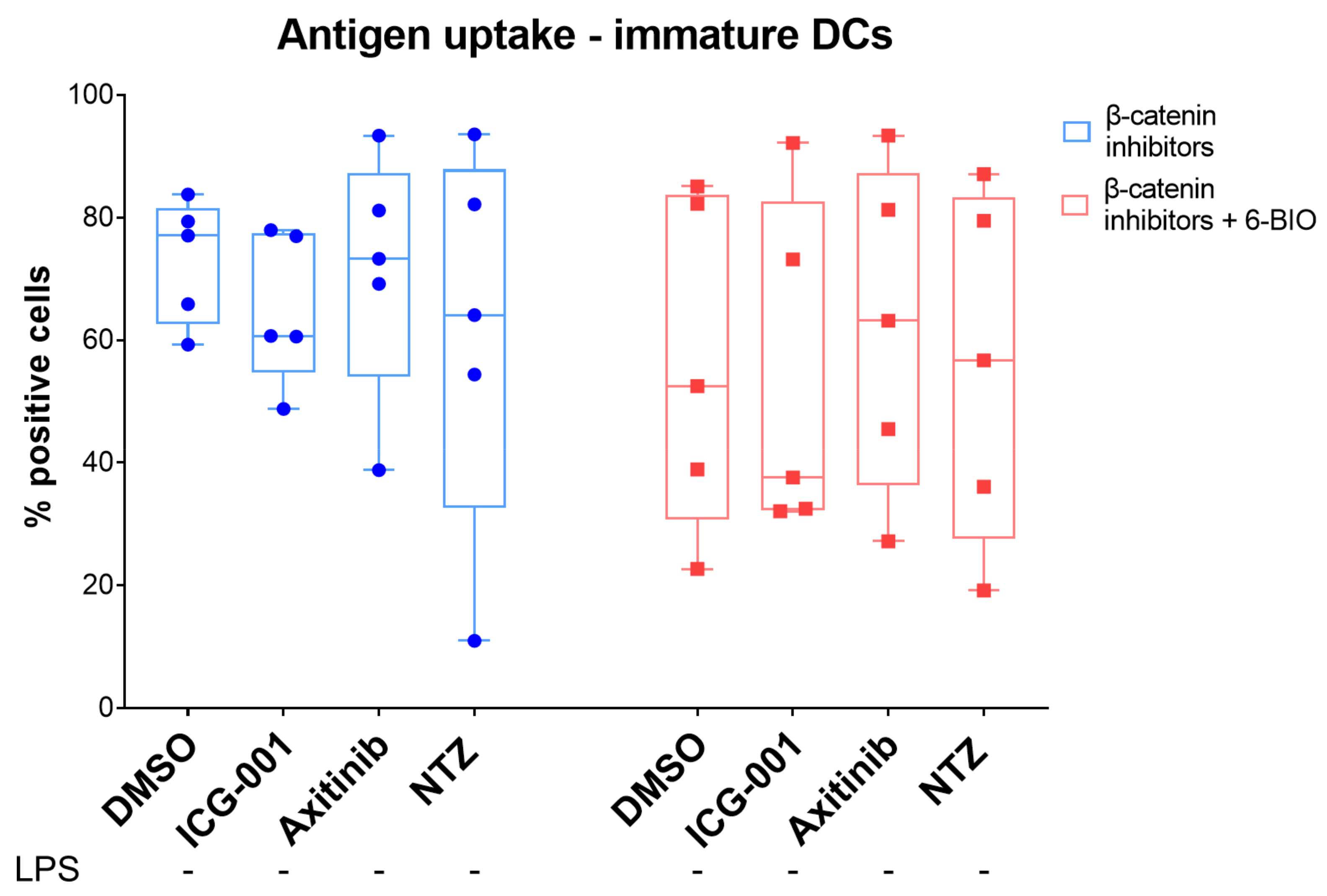
3.6. 6-BIO Decreased Antigen Uptake in Immature moDCs
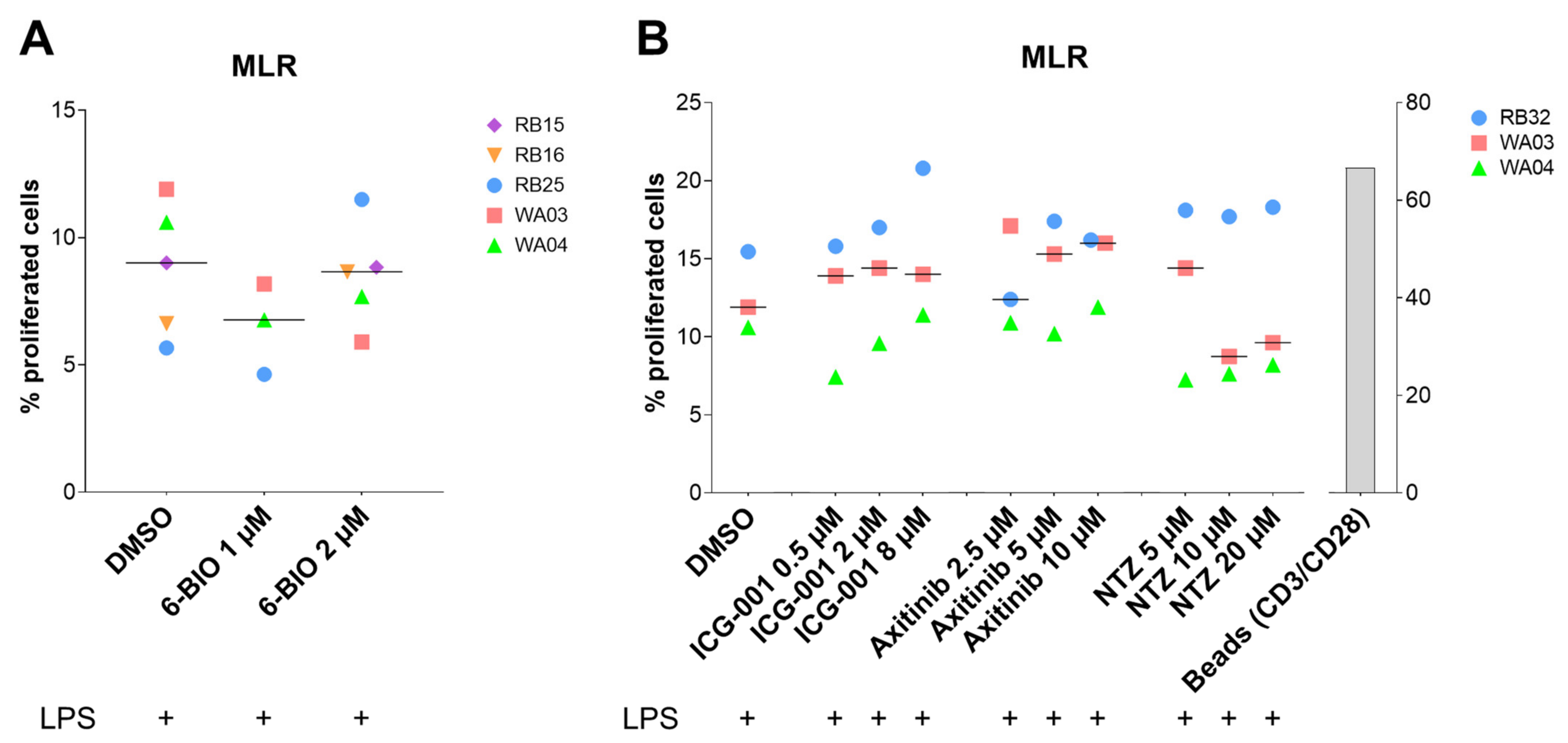
3.7. Compounds Tested in the Allogeneic Mixed Leukocyte Reaction (MLR)
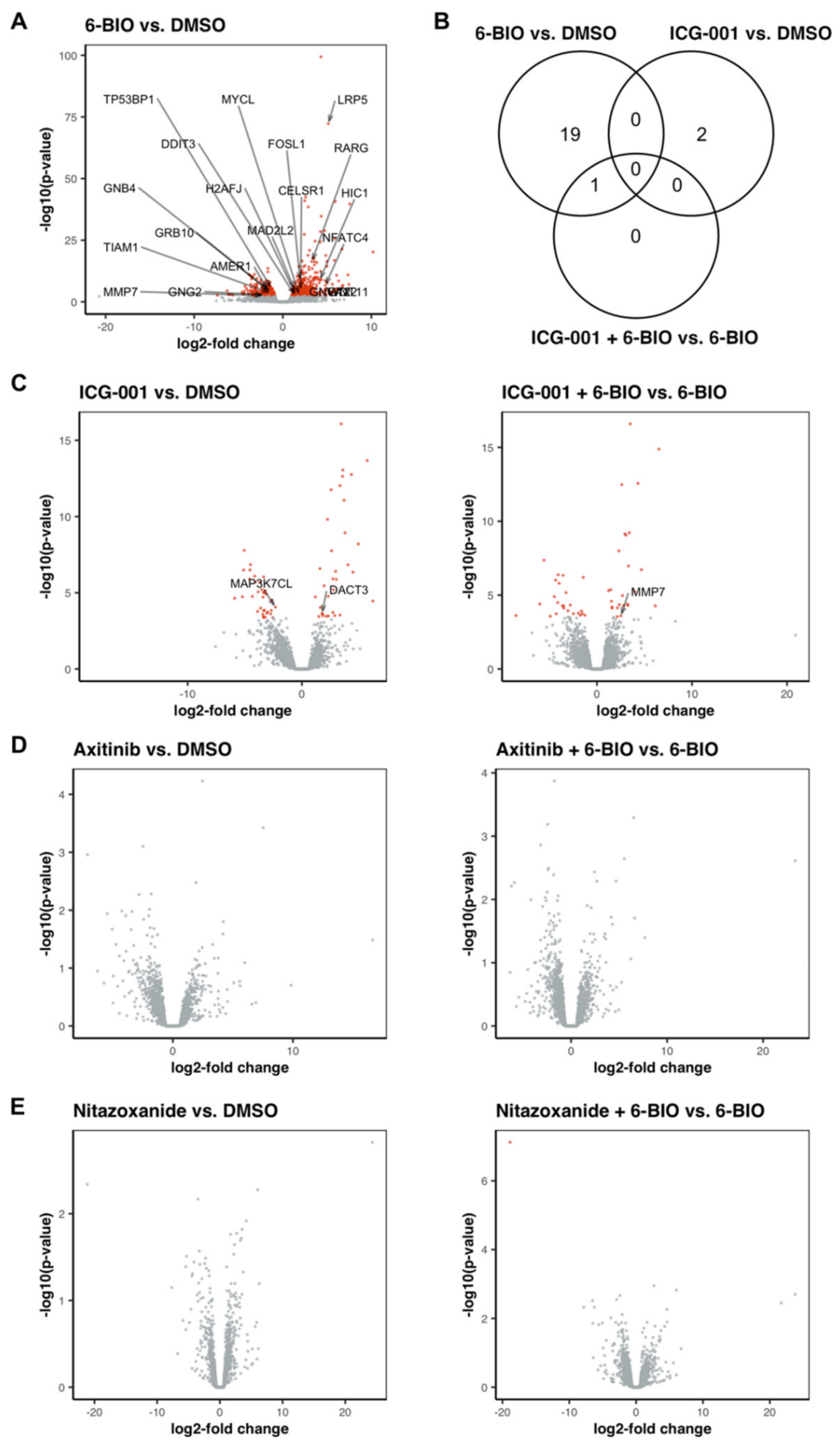
3.8. Treatment of iDC with LPS Induced Extensive Changes in Gene Expression Pattern
3.9. 6-BIO Induced the Expression of Genes Involved in the Wnt Signaling Pathway in LPS-Matured DC
3.10. Treatment with ICG-001 Alone or in Combination with 6-BIO Resulted in Changes in the Gene Expression Pattern of LPS-Matured DC, While Axitinib and Nitazoxanide Did Not Show Strong Effects on the Transcriptome of LPS-Matured DC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murillo-Garzón, V.; Kypta, R. WNT signalling in prostate cancer. Nat. Rev. Urol. 2017, 14, 683–696. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- El-Sahli, S.; Xie, Y.; Wang, L.; Liu, S. Wnt signaling in cancer metabolism and immunity. Cancers 2019, 11, 904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Spranger, S.; Fuchs, E.; López-Soto, A. WNT signaling in cancer immunosurveillance. Trends Cell Biol. 2019, 29, 44–65. [Google Scholar] [CrossRef]
- Luke, J.J.; Bao, R.; Sweis, R.F.; Spranger, S.; Gajewski, T.F. WNT/beta-catenin pathway activation correlates with immune exclusion across human cancers. Clin. Cancer Res. 2019, 25, 3074–3083. [Google Scholar] [CrossRef] [Green Version]
- Martin-Orozco, E.; Sanchez-Fernandez, A.; Ortiz-Parra, I.; Nicolas, M.A.-S. WNT signaling in tumors: The way to evade drugs and immunity. Front. Immunol. 2019, 10, 2854. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Qu, Y. Targeting the β-catenin signaling for cancer therapy. Pharmacol. Res. 2020, 160, 104794. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, A.; Hussein, M.S.; Prasad, P.D.; Manicassamy, S. Wnt signaling cascade in dendritic cells and regulation of anti-tumor immunity. Front. Immunol. 2020, 11, 122. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Tian, T.; Kalland, K.H.; Ke, X.; Qu, Y. Targeting Wnt/beta-Catenin Signaling for Cancer Immunotherapy. Trends Pharm. Sci. 2018, 39, 648–658. [Google Scholar] [CrossRef]
- Oderup, C.; LaJevic, M.; Butcher, E.C. Canonical and noncanonical Wnt proteins program dendritic cell responses for tolerance. J. Immunol. 2013, 190, 6126–6134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manicassamy, S.; Reizis, B.; Ravindran, R.; Nakaya, H.; Salazar-Gonzalez, R.M.; Wang, Y.-C.; Pulendran, B. Activation of -catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 2010, 329, 849–853. [Google Scholar] [CrossRef] [Green Version]
- Azeem, W.; Bakke, R.M.; Appel, S.; Øyan, A.M.; Kalland, K.-H. Dual Pro- and Anti-Inflammatory Features of Monocyte-Derived Dendritic Cells. Front. Immunol. 2020, 11, 438. [Google Scholar] [CrossRef]
- Blagodatski, A.; Klimenko, A.; Jia, L.; Katanaev, V.L. Small molecule wnt pathway modulators from natural sources: History, state of the art and perspectives. Cells 2020, 9, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, C.; Zhou, X.; Zhang, W.; Qu, Y.; Ke, X. Is β-Catenin a druggable target for cancer therapy? Trends Biochem. Sci. 2018, 43, 623–634. [Google Scholar] [CrossRef]
- Qu, Y.; Gharbi, N.; Yuan, X.; Olsen, J.R.; Blicher, P.; Dalhus, B.; Brokstad, K.A.; Lin, B.; Øyan, A.M.; Zhang, W.; et al. Axitinib blocks Wnt/β-catenin signaling and directs asymmetric cell division in cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 9339–9344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Kalland, K.-H.; Ke, X. Small molecule induces Wnt asymmetry in cancer. Cell Cycle 2016, 16, 141–142. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Olsen, J.R.; Yuan, X.; Cheng, P.F.; Levesque, M.P.; Brokstad, K.A.; Hoffman, P.S.; Oyan, A.M.; Zhang, W.; Kalland, K.-H.; et al. Small molecule promotes β-catenin citrullination and inhibits Wnt signaling in cancer. Nat. Chem. Biol. 2017, 14, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Herbst, R.S.; Liu, G.; Park, J.W.; Kies, M.S.; Steinfeldt, H.M.; Pithavala, Y.K.; Reich, S.D.; Freddo, J.L.; Wilding, G. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: Pharmacokinetic and clinical results. J. Clin. Oncol. 2005, 23, 5474–5483. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, A.S.; Devarajan, P.V. A review on possible mechanistic insights of Nitazoxanide for repurposing in COVID-19. Eur. J. Pharmacol. 2021, 891, 173748. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Vara-Perez, M.; Schaaf, M.; Agostinis, P.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: Dendritic cell-based anticancer immunotherapy. Oncoimmunology 2017, 6, e1328341. [Google Scholar] [CrossRef]
- Sprater, F.; Azeem, W.; Appel, S. Activation of peroxisome proliferator-activated receptor gamma leads to upregulation of ESE-3 expression in human monocyte-derived dendritic cells. Scand. J. Immunol. 2013, 79, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Alexa, A.; Rahnenfuhrer, J. Enrichment Analysis for Gene Ontology, R package version 2.42.0. [CrossRef]
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2017, 27, 74–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, M.; Balan, S.; Roudko, V.; Bhardwaj, N. Towards superior dendritic-cell vaccines for cancer therapy. Nat. Biomed. Eng. 2018, 2, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Bhardwaj, N. Re-emergence of dendritic cell vaccines for cancer treatment. Trends Cancer 2018, 4, 119–137. [Google Scholar] [CrossRef]
- Heine, A.; Held, S.A.E.; Daecke, S.N.; Riethausen, K.; Kotthoff, P.; Flores, C.; Kurts, C.; Brossart, P. The VEGF-receptor inhibitor axitinib impairs dendritic cell phenotype and function. PLoS ONE 2015, 10, e0128897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.-M.A.; Mishina, Y.M.; Liu, S.; Cheung, A.; Stegmeier, F.; Michaud, G.A.; Charlat, O.; Wiellette, E.; Zhang, Y.; Wiessner, S.; et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009, 461, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Lövgren, T.; Sarhan, D.; Truxova, I.; Choudhary, B.; Maas, R.; Melief, J.; Nyström, M.; Edbäck, U.; Vermeij, R.; Scurti, G.; et al. Enhanced stimulation of human tumor-specific T cells by dendritic cells matured in the presence of interferon-γ and multiple toll-like receptor agonists. Cancer Immunol. Immunother. 2017, 66, 1333–1344. [Google Scholar] [CrossRef] [Green Version]
- Stehle, F.; Schulz, K.; Fahldieck, C.; Kalich, J.; Lichtenfels, R.; Riemann, D.; Seliger, B. Reduced immunosuppressive properties of Axitinib in comparison with other tyrosine kinase inhibitors. J. Biol. Chem. 2013, 288, 16334–16347. [Google Scholar] [CrossRef] [Green Version]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Donninelli, G.; Sanseverino, I.; Purificato, C.; Gessani, S.; Gauzzi, M.C. Dual requirement for STAT signaling in dendritic cell immunobiology. Immunobiology 2018, 223, 342–347. [Google Scholar] [CrossRef]
- Du Four, S.; Maenhout, S.K.; Niclou, S.; Thielemans, K.; Neyns, B.; Aerts, J.L. Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. Am. J. Cancer Res. 2016, 6, 2514–2531. [Google Scholar] [PubMed]
- Bose, A.; Lowe, D.B.; Rao, A.; Storkus, W.J. Combined vaccine+axitinib therapy yields superior antitumor efficacy in a murine melanoma model. Melanoma Res. 2012, 22, 236–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Four, S.; Maenhout, S.K.; Benteyn, D.; De Keersmaecker, B.; Duerinck, J.; Thielemans, K.; Neyns, B.; Aerts, J.L. Disease progression in recurrent glioblastoma patients treated with the VEGFR inhibitor axitinib is associated with increased regulatory T cell numbers and T cell exhaustion. Cancer Immunol. Immunother. 2016, 65, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Hipp, M.M.; Hilf, N.; Walter, S.; Werth, D.; Brauer, K.M.; Radsak, M.P.; Weinschenk, T.; Singh-Jasuja, H.; Brossart, P. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood 2008, 111, 5610–5620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, L.; Flippot, R.; Escudier, B.; Albiges, L. Immunomodulatory Roles of VEGF Pathway Inhibitors in Renal Cell Carcinoma. Drugs 2020, 80, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Tanaka, T.; Akasaki, Y.; Murayama, Y.; Yoshida, K.; Sasaki, H. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: Perspectives for therapeutic implications. Med. Oncol. 2019, 37, 2. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yan, J.; Liu, B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018, 9, 978. [Google Scholar] [CrossRef] [Green Version]
- Zizzari, I.G.; Napoletano, C.; Botticelli, A.; Caponnetto, S.; Calabrò, F.; Gelibter, A.; Rughetti, A.; Ruscito, I.; Rahimi, H.; Rossi, E.; et al. TK inhibitor pazopanib primes DCs by downregulation of the β-catenin pathway. Cancer Immunol. Res. 2018, 6, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Miner, K.; Labitzke, K.; Liu, B.; Wang, P.; Henckels, K.; Gaida, K.; Elliott, R.; Chen, J.J.; Liu, L.; Leith, A.; et al. Drug repurposing: The anthelmintics Niclosamide and Nitazoxanide are potent TMEM16A antagonists that fully bronchodilate airways. Front. Pharmacol. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, J.; Naguleswaran, A.; Müller, N.; Hemphill, A. Neospora caninum: Functional inhibition of protein disulfide isomerase by the broad-spectrum anti-parasitic drug nitazoxanide and other thiazolides. Exp. Parasitol. 2008, 118, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.; Bhat, H.R.; Ghosh, S.K. Update on Nitazoxanide: A multifunctional chemotherapeutic agent. Curr. Drug Discov. Technol. 2018, 15, 201–213. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azeem, W.; Bakke, R.M.; Gabriel, B.; Appel, S.; Øyan, A.M.; Kalland, K.-H. Evaluation of β-Catenin Inhibition of Axitinib and Nitazoxanide in Human Monocyte-Derived Dendritic Cells. Biomedicines 2021, 9, 949. https://doi.org/10.3390/biomedicines9080949
Azeem W, Bakke RM, Gabriel B, Appel S, Øyan AM, Kalland K-H. Evaluation of β-Catenin Inhibition of Axitinib and Nitazoxanide in Human Monocyte-Derived Dendritic Cells. Biomedicines. 2021; 9(8):949. https://doi.org/10.3390/biomedicines9080949
Chicago/Turabian StyleAzeem, Waqas, Ragnhild Maukon Bakke, Benjamin Gabriel, Silke Appel, Anne Margrete Øyan, and Karl-Henning Kalland. 2021. "Evaluation of β-Catenin Inhibition of Axitinib and Nitazoxanide in Human Monocyte-Derived Dendritic Cells" Biomedicines 9, no. 8: 949. https://doi.org/10.3390/biomedicines9080949
APA StyleAzeem, W., Bakke, R. M., Gabriel, B., Appel, S., Øyan, A. M., & Kalland, K.-H. (2021). Evaluation of β-Catenin Inhibition of Axitinib and Nitazoxanide in Human Monocyte-Derived Dendritic Cells. Biomedicines, 9(8), 949. https://doi.org/10.3390/biomedicines9080949





