The Anticancer Efficacy of Plasma-Oxidized Saline (POS) in the Ehrlich Ascites Carcinoma Model In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Generation of Cold Physical Plasma-Oxidized Saline (POS)
2.2. Analysis of Plasma-Oxidized Saline (POS)
2.3. Ehrlich Ascites Carcinoma (EAC) Mouse Model
2.4. Metabolic Activity
2.5. Systemic Oxidative Profile
2.6. Quantitative Immuno-Fluorescence Imaging
2.7. Multiplex Cytokine Analysis
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition of POS and Its Effects on the Metabolic Activity of EAC Cells Ex Vivo
3.2. POS Reduced EAC Burden and Altered Antioxidant Capacity In Vivo
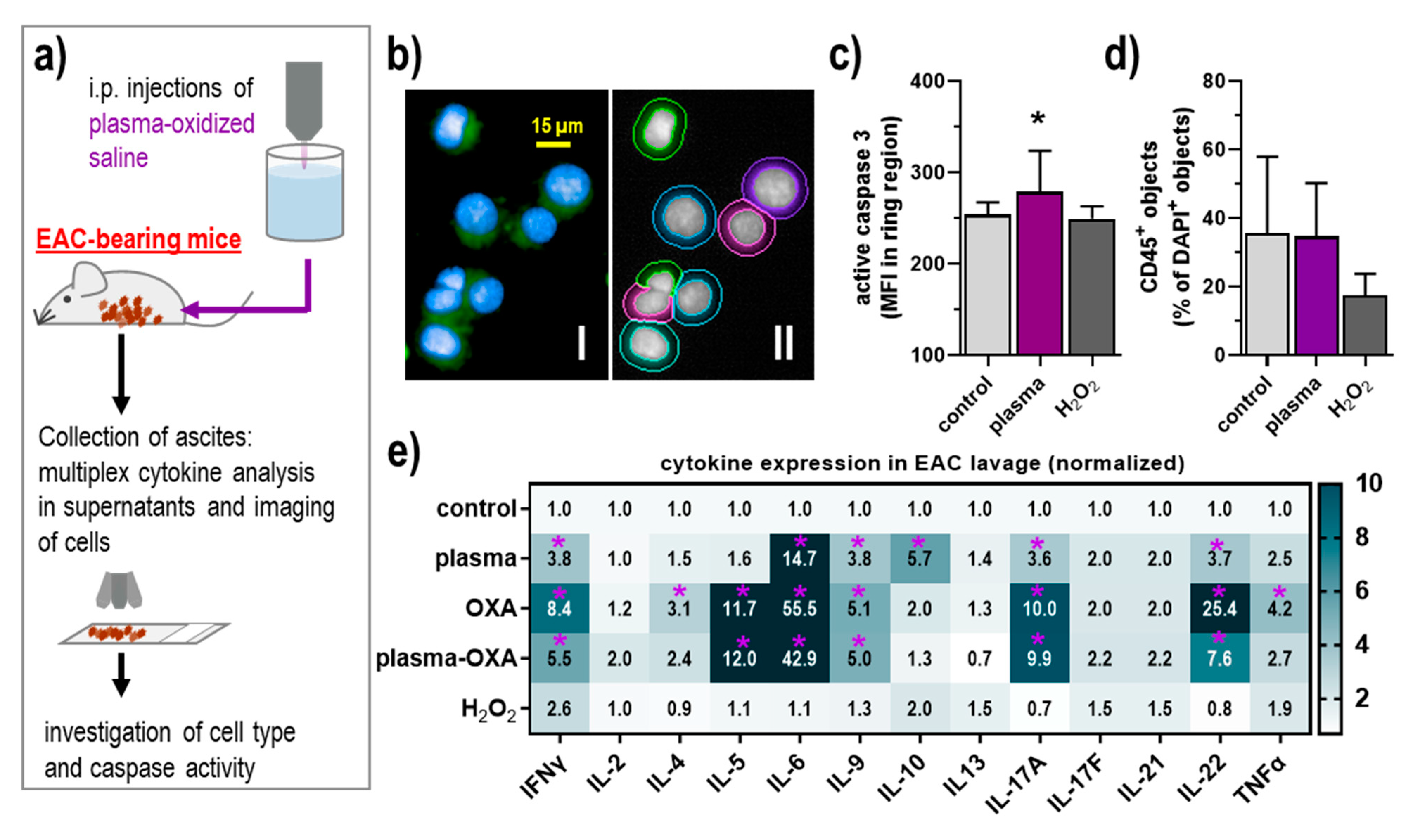
3.3. POS Elicited Apoptosis and Modest Inflammatory Changes in the Tumor Microenvironment of the Peritoneal Cavity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [CrossRef]
- Verwaal, V.J.; Bruin, S.; Boot, H.; van Slooten, G.; van Tinteren, H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.S.; Al-Adra, D.P.; Nagendran, J.; Campbell, S.; Shi, X.; Haase, E.; Schiller, D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and hipec: A systematic review of survival, mortality, and morbidity. J. Surg. Oncol. 2011, 104, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ros-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kinpen-a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; Schmidt, A.; Niessner, F.; Gerling, T.; Weltmann, K.D.; Wende, K. Basic research in plasma medicine—A throughput approach from liquids to cells. J. Vis. Exp. 2017, e56331. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; Clemen, R.; Niessner, F.; Sagwal, S.K.; Freund, E.; Schmidt, A. Medical gas plasma jet technology targets murine melanoma in an immunogenic fashion. Adv. Sci. 2020, 7, 1903438. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; Moritz, J.; Helfrich, I.; Boeckmann, L.; Weltmann, K.D.; Emmert, S.; Metelmann, H.R.; Stoffels, I.; von Woedtke, T. Ex vivo exposure of human melanoma tissue to cold physical plasma elicits apoptosis and modulates inflammation. Appl. Sci. 2020, 10, 1971. [Google Scholar] [CrossRef] [Green Version]
- Freund, E.; Liedtke, K.R.; Gebbe, R.; Heidecke, A.K.; Partecke, L.-I.; Bekeschus, S. In vitro anticancer efficacy of six different clinically approved types of liquids exposed to physical plasma. IEEE Trans. Rad. Plasma Med. Sci. 2019, 3, 588–596. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; van der Linde, J.; Metelmann, H.R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Physical plasma-treated saline promotes an immunogenic phenotype in ct26 colon cancer cells in vitro and in vivo. Sci. Rep. 2019, 9, 634. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The plasma jet kinpen—A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Bekeschus, S.; Lin, A.; Fridman, A.; Wende, K.; Weltmann, K.D.; Miller, V. A comparison of floating-electrode dbd and kinpen jet: Plasma parameters to achieve similar growth reduction in colon cancer cells under standardized conditions. Plasma Chem. Plasma Process. 2018, 38, 1–12. [Google Scholar] [CrossRef]
- Setsukinai, K.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2003, 278, 3170–3175. [Google Scholar] [CrossRef] [Green Version]
- Charbouillot, T.; Brigante, M.; Mailhot, G.; Maddigapu, P.R.; Minero, C.; Vione, D. Performance and selectivity of the terephthalic acid probe for oh as a function of temperature, ph and composition of atmospherically relevant aqueous media. J. Photochem. Photobiol. A Chem. 2011, 222, 70–76. [Google Scholar] [CrossRef]
- Flecha, B.G.; Llesuy, S.; Boveris, A. Hydroperoxide-initiated chemiluminescence—An assay for oxidative stress in biopsies of heart, liver, and muscle. Free Radic. Biol. Med. 1991, 10, 93–100. [Google Scholar] [CrossRef]
- Repetto, M.; Reides, C.; Gomez Carretero, M.L.; Costa, M.; Griemberg, G.; Llesuy, S. Oxidative stress in blood of hiv infected patients. Clin. Chim. Acta 1996, 255, 107–117. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Miller, G.L. Protein determination for large numbers of samples. Anal. Chem. 1959, 31, 964. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Takeda, K.; Nakamura, K.; Utsumi, F.; Kajiyama, H.; Kano, H.; Okazaki, Y.; Toyokuni, S.; et al. Plasma medical science for cancer therapy: Toward cancer therapy using nonthermal atmospheric pressure plasma. IEEE Trans. Plasma Sci. 2014, 42, 3760–3764. [Google Scholar] [CrossRef]
- Torii, K.; Yamada, S.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Tanahashi, K.; Iwata, N.; Kanda, M.; Kobayashi, D.; Tanaka, C.; et al. Effectiveness of plasma treatment on gastric cancer cells. Gastric Cancer 2015, 18, 635–643. [Google Scholar] [CrossRef]
- Judee, F.; Fongia, C.; Ducommun, B.; Yousfi, M.; Lobjois, V.; Merbahi, N. Short and long time effects of low temperature plasma activated media on 3d multicellular tumor spheroids. Sci. Rep. 2016, 6, 21421. [Google Scholar] [CrossRef]
- Bauer, G. Targeting protective catalase of tumor cells with cold atmospheric plasma- activated medium (pam). Anticancer Agents Med. Chem. 2018, 18, 784–804. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Park, J.H.; Ali, A.; Choi, E.H. How does plasma activated media treatment differ from direct cold plasma treatment? Anticancer Agents Med. Chem. 2018, 18, 805–814. [Google Scholar] [CrossRef]
- Bauer, G.; Sersenova, D.; Graves, D.B.; Machala, Z. Cold atmospheric plasma and plasma-activated medium trigger rons-based tumor cell apoptosis. Sci. Rep. 2019, 9, 14210. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yu, L.; Zou, F.; Hu, H.; Liu, K.; Lin, Z. Gene expression profiling and functional analysis reveals that p53 pathway-related gene expression is highly activated in cancer cells treated by cold atmospheric plasma-activated medium. PeerJ 2017, 5, e3751. [Google Scholar] [CrossRef] [Green Version]
- Hara, H.; Kobayashi, M.; Shiiba, M.; Kamiya, T.; Adachi, T. Sublethal treatment with plasma-activated medium induces senescence-like growth arrest of a549 cells: Involvement of intracellular mobile zinc. J. Clin. Biochem. Nutr. 2019, 65, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, T.; Tanaka, H.; Nonomura, S.; Hara, H.; Kondo, S.; Hori, M. Plasma-activated medium induces a549 cell injury via a spiral apoptotic cascade involving the mitochondrial-nuclear network. Free Radic. Biol. Med. 2015, 79, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Gandhirajan, R.K.; Rodder, K.; Bodnar, Y.; Pasqual-Melo, G.; Emmert, S.; Griguer, C.E.; Weltmann, K.D.; Bekeschus, S. Cytochrome c oxidase inhibition and cold plasma-derived oxidants synergize in melanoma cell death induction. Sci. Rep. 2018, 8, 12734. [Google Scholar] [CrossRef]
- Adachi, T.; Kano, A.; Nonomura, S.; Kamiya, T.; Hara, H. Histone deacetylase inhibitors stimulate the susceptibility of a549 cells to a plasma-activated medium treatment. Arch. Biochem. Biophys. 2016, 606, 120–127. [Google Scholar] [CrossRef]
- Masur, K.; von Behr, M.; Bekeschus, S.; Weltmann, K.D.; Hackbarth, C.; Heidecke, C.D.; von Bernstorff, W.; von Woedtke, T.; Partecke, L.I. Synergistic inhibition of tumor cell proliferation by cold plasma and gemcitabine. Plasma Process. Polym. 2015, 12, 1377–1382. [Google Scholar] [CrossRef]
- Tentes, A.A.; Kyziridis, D.; Kakolyris, S.; Pallas, N.; Zorbas, G.; Korakianitis, O.; Mavroudis, C.; Courcoutsakis, N.; Prasopoulos, P. Preliminary results of hyperthermic intraperitoneal intraoperative chemotherapy as an adjuvant in resectable pancreatic cancer. Gastroenterol. Res. Pract. 2012, 2012, 506571. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Bekeschus, S.; Kaeding, A.; Hackbarth, C.; Kuehn, J.P.; Heidecke, C.D.; von Bernstorff, W.; von Woedtke, T.; Partecke, L.I. Non-thermal plasma-treated solution demonstrates antitumor activity against pancreatic cancer cells in vitro and in vivo. Sci. Rep. 2017, 7, 8319. [Google Scholar] [CrossRef]
- Duan, J.; Lu, X.; He, G. The selective effect of plasma activated medium in an in vitro co-culture of liver cancer and normal cells. J. Appl. Phys. 2017, 121, 013302. [Google Scholar] [CrossRef]
- Tanaka, H.; Bekeschus, S.; Yan, D.; Hori, M.; Keidar, M.; Laroussi, M. Plasma-treated solutions (pts) in cancer therapy. Cancers 2021, 13, 1737. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Kano, A.; Kamiya, T.; Hara, H.; Adachi, T. Enhanced ability of plasma-activated lactated ringer’s solution to induce a549cell injury. Arch. Biochem. Biophys. 2018, 656, 19–30. [Google Scholar] [CrossRef]
- Bisag, A.; Bucci, C.; Coluccelli, S.; Girolimetti, G.; Laurita, R.; De Iaco, P.; Perrone, A.M.; Gherardi, M.; Marchio, L.; Porcelli, A.M.; et al. Plasma-activated ringer’s lactate solution displays a selective cytotoxic effect on ovarian cancer cells. Cancers 2020, 12, 476. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, K.; Hosoi, Y.; Tanaka, H.; Jiang, L.; Toyokuni, S.; Nakamura, K.; Kajiyama, H.; Kikkawa, F.; Mizuno, M.; Hori, M. Non-thermal plasma-activated lactate solution kills u251sp glioblastoma cells in an innate reductive manner with altered metabolism. Arch. Biochem. Biophys. 2020, 688, 108414. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizuno, M.; Katsumata, Y.; Ishikawa, K.; Kondo, H.; Hashizume, H.; Okazaki, Y.; Toyokuni, S.; Nakamura, K.; Yoshikawa, N.; et al. Oxidative stress-dependent and -independent death of glioblastoma cells induced by non-thermal plasma-exposed solutions. Sci. Rep. 2019, 9, 13657. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ishikawa, K.; Miron, C.; Hashizume, H.; Tanaka, H.; Hori, M. Hydrogen peroxide in lactate solutions irradiated by non-equilibrium atmospheric pressure plasma. Plasma Sources Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakamura, K.; Mizuno, M.; Ishikawa, K.; Takeda, K.; Kajiyama, H.; Utsumi, F.; Kikkawa, F.; Hori, M. Non-thermal atmospheric pressure plasma activates lactate in ringer’s solution for anti-tumor effects. Sci. Rep. 2016, 6, 36282. [Google Scholar] [CrossRef] [Green Version]
- Van Boxem, W.; Van der Paal, J.; Gorbanev, Y.; Vanuytsel, S.; Smits, E.; Dewilde, S.; Bogaerts, A. Anti-cancer capacity of plasma-treated pbs: Effect of chemical composition on cancer cell cytotoxicity. Sci. Rep. 2017, 7, 16478. [Google Scholar] [CrossRef] [Green Version]
- Privat-Maldonado, A.; Gorbanev, Y.; Dewilde, S.; Smits, E.; Bogaerts, A. Reduction of human glioblastoma spheroids using cold atmospheric plasma: The combined effect of short- and long-lived reactive species. Cancers 2018, 10, 394. [Google Scholar] [CrossRef] [Green Version]
- Van Loenhout, J.; Flieswasser, T.; Freire Boullosa, L.; De Waele, J.; Van Audenaerde, J.; Marcq, E.; Jacobs, J.; Lin, A.; Lion, E.; Dewitte, H.; et al. Cold atmospheric plasma-treated pbs eliminates immunosuppressive pancreatic stellate cells and induces immunogenic cell death of pancreatic cancer cells. Cancers 2019, 11, 1597. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; Clemen, R.; Metelmann, H.-R. Potentiating anti-tumor immunity with physical plasma. Clin. Plasma Med. 2018, 12, 17–22. [Google Scholar] [CrossRef]
- Iuchi, K.; Morisada, Y.; Yoshino, Y.; Himuro, T.; Saito, Y.; Murakami, T.; Hisatomi, H. Cold atmospheric-pressure nitrogen plasma induces the production of reactive nitrogen species and cell death by increasing intracellular calcium in hek293t cells. Arch. Biochem. Biophys. 2018, 654, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G. Intercellular singlet oxygen-mediated bystander signaling triggered by long-lived species of cold atmospheric plasma and plasma-activated medium. Redox Biol. 2019, 26, 101301. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Wende, K.; Hefny, M.M.; Rodder, K.; Jablonowski, H.; Schmidt, A.; Woedtke, T.V.; Weltmann, K.D.; Benedikt, J. Oxygen atoms are critical in rendering thp-1 leukaemia cells susceptible to cold physical plasma-induced apoptosis. Sci. Rep. 2017, 7, 2791. [Google Scholar] [CrossRef] [Green Version]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.S.; Burgeiro, A.; Garcia, R.; Moreno, A.J.; Carvalho, R.A.; Oliveira, P.J. Doxorubicin-induced cardiotoxicity: From bioenergetic failure and cell death to cardiomyopathy. Med. Res. Rev. 2014, 34, 106–135. [Google Scholar] [CrossRef]
- Ma, Y.; Ha, C.S.; Hwang, S.W.; Lee, H.J.; Kim, G.C.; Lee, K.W.; Song, K. Non-thermal atmospheric pressure plasma preferentially induces apoptosis in p53-mutated cancer cells by activating ros stress-response pathways. PLoS ONE 2014, 9, e91947. [Google Scholar] [CrossRef]
- Sagwal, S.K.; Pasqual-Melo, G.; Bodnar, Y.; Gandhirajan, R.K.; Bekeschus, S. Combination of chemotherapy and physical plasma elicits melanoma cell death via upregulation of slc22a16. Cell Death Dis. 2018, 9, 1179. [Google Scholar] [CrossRef] [PubMed]
- Vijayarangan, V.; Delalande, A.; Dozias, S.; Pouvesle, J.M.; Robert, E.; Pichon, C. New insights on molecular internalization and drug delivery following plasma jet exposures. Int. J. Pharm. 2020, 589, 119874. [Google Scholar] [CrossRef]
- Liao, Y.C.; Liang, W.G.; Chen, F.W.; Hsu, J.H.; Yang, J.J.; Chang, M.S. Il-19 induces production of il-6 and tnf-alpha and results in cell apoptosis through tnf-alpha. J. Immunol. 2002, 169, 4288–4297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, A.; Fabri, M. Vitamin d regulates cytokine patterns secreted by dendritic cells to promote differentiation of il-22-producing t cells. PLoS ONE 2015, 10, e0130395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawrylowicz, C.M. Regulatory t cells and il-10 in allergic inflammation. J. Exp. Med. 2005, 202, 1459–1463. [Google Scholar] [CrossRef]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the treatment of cancer. J. Interferon Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef]
- Lin, C.F.; Lin, C.M.; Lee, K.Y.; Wu, S.Y.; Feng, P.H.; Chen, K.Y.; Chuang, H.C.; Chen, C.L.; Wang, Y.C.; Tseng, P.C.; et al. Escape from ifn-gamma-dependent immunosurveillance in tumorigenesis. J. Biomed. Sci. 2017, 24, 10. [Google Scholar] [CrossRef] [Green Version]
- Vegran, F.; Berger, H.; Boidot, R.; Mignot, G.; Bruchard, M.; Dosset, M.; Chalmin, F.; Rebe, C.; Derangere, V.; Ryffel, B.; et al. The transcription factor irf1 dictates the il-21-dependent anticancer functions of th9 cells. Nat. Immunol. 2014, 15, 758–766. [Google Scholar] [CrossRef]
- Senovilla, L.; Vacchelli, E.; Galon, J.; Adjemian, S.; Eggermont, A.; Fridman, W.H.; Sautes-Fridman, C.; Ma, Y.; Tartour, E.; Zitvogel, L.; et al. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology 2012, 1, 1323–1343. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, W.A.S.; Freund, E.; Nascimento, T.D.H.d.; Pasqual-Melo, G.; Sanches, L.J.; Dionísio, J.H.R.; Fumegali, W.C.; Miebach, L.; Cecchini, A.L.; Bekeschus, S. The Anticancer Efficacy of Plasma-Oxidized Saline (POS) in the Ehrlich Ascites Carcinoma Model In Vitro and In Vivo. Biomedicines 2021, 9, 932. https://doi.org/10.3390/biomedicines9080932
Brito WAS, Freund E, Nascimento TDHd, Pasqual-Melo G, Sanches LJ, Dionísio JHR, Fumegali WC, Miebach L, Cecchini AL, Bekeschus S. The Anticancer Efficacy of Plasma-Oxidized Saline (POS) in the Ehrlich Ascites Carcinoma Model In Vitro and In Vivo. Biomedicines. 2021; 9(8):932. https://doi.org/10.3390/biomedicines9080932
Chicago/Turabian StyleBrito, Walison Augusto Silva, Eric Freund, Thiago Daniel Henrique do Nascimento, Gabriella Pasqual-Melo, Larissa Juliani Sanches, Joyce Hellen Ribeiro Dionísio, William Capellari Fumegali, Lea Miebach, Alessandra Lourenço Cecchini, and Sander Bekeschus. 2021. "The Anticancer Efficacy of Plasma-Oxidized Saline (POS) in the Ehrlich Ascites Carcinoma Model In Vitro and In Vivo" Biomedicines 9, no. 8: 932. https://doi.org/10.3390/biomedicines9080932
APA StyleBrito, W. A. S., Freund, E., Nascimento, T. D. H. d., Pasqual-Melo, G., Sanches, L. J., Dionísio, J. H. R., Fumegali, W. C., Miebach, L., Cecchini, A. L., & Bekeschus, S. (2021). The Anticancer Efficacy of Plasma-Oxidized Saline (POS) in the Ehrlich Ascites Carcinoma Model In Vitro and In Vivo. Biomedicines, 9(8), 932. https://doi.org/10.3390/biomedicines9080932






