Exosomal Components and Modulators in Colorectal Cancer: Novel Diagnosis and Prognosis Biomarkers
Abstract
1. Background of CRC
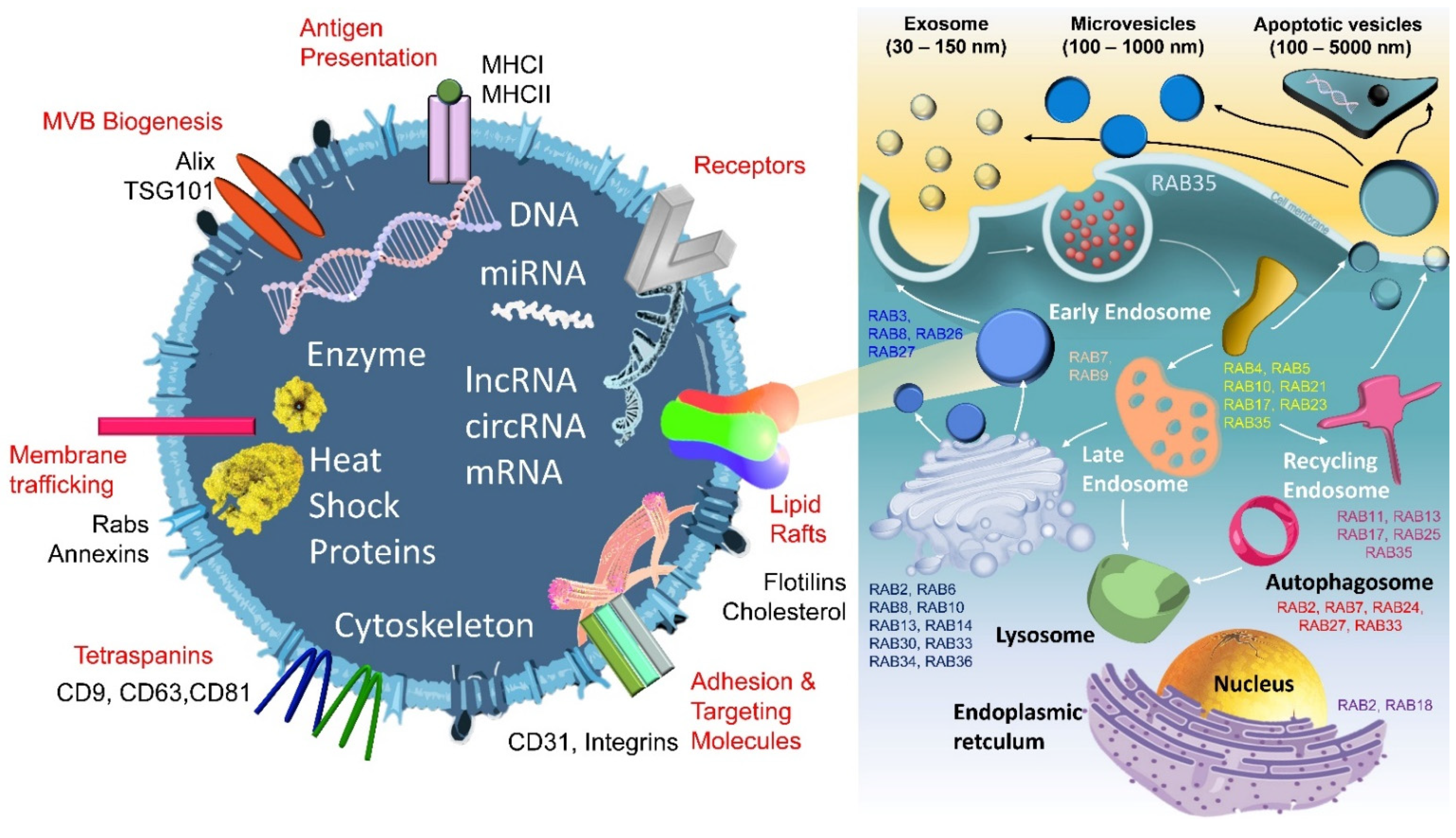
2. General Definition, Classification, and Application of EVs
3. Modulation and Regulation Mechanisms for EVs and Their Components
3.1. ESCRT Complex
3.2. RAB Family
3.3. Genetic Alterations
4. Role of EVs in Colorectal Tumorigenesis
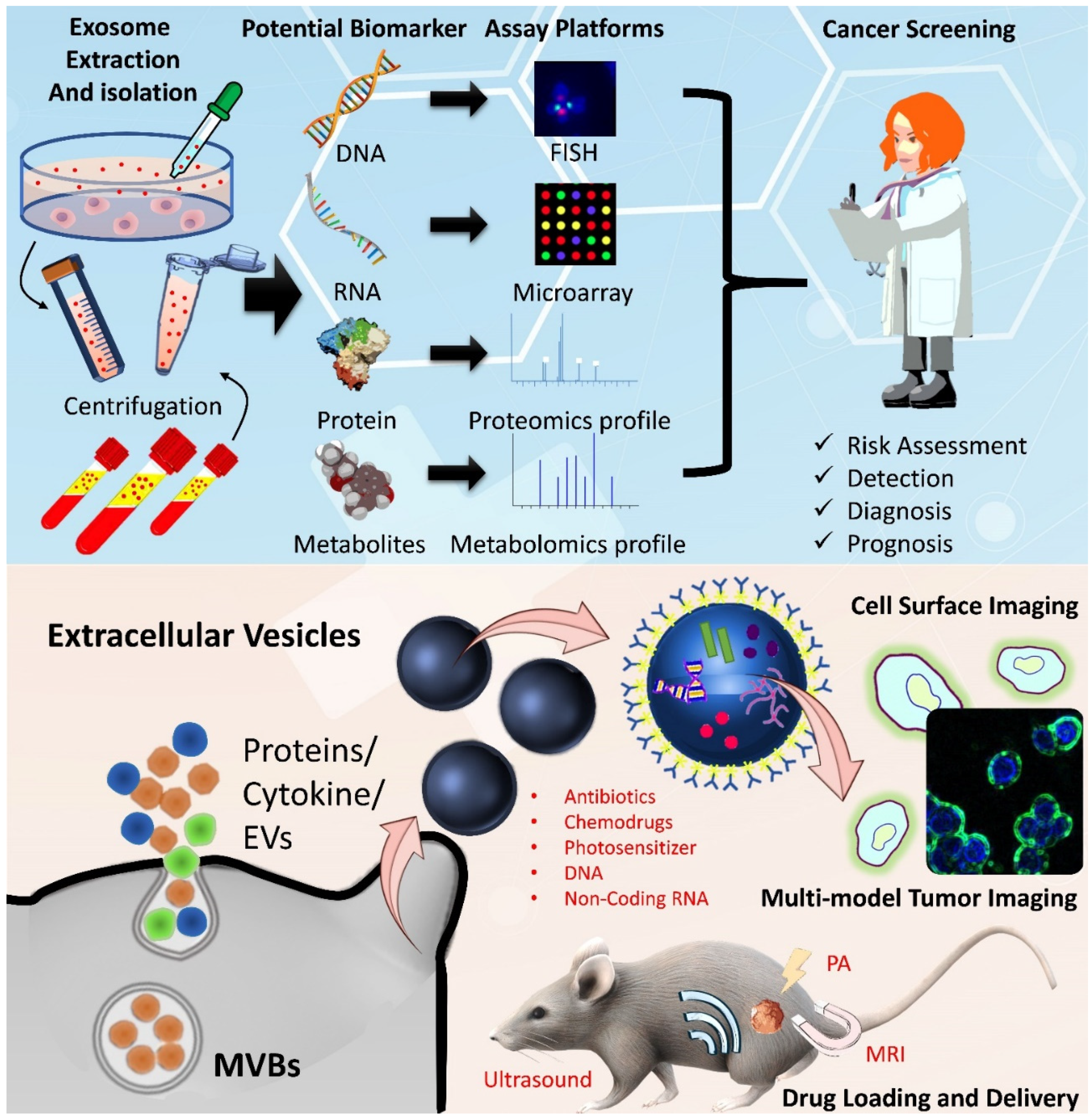
5. Available Omics Datasets of EVs for CRC
6. Current Combinations and Clinical Trials of EV-Based Carriers
7. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Wong, M.C.; Ding, H.; Wang, J.; Chan, P.S.; Huang, J. Prevalence and risk factors of colorectal cancer in Asia. Intest. Res. 2019, 17, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Hopirtean, C.; Nagy, V. Optimizing the use of anti VEGF targeted therapies in patients with metastatic colorectal cancer: Review of literature. Clujul Med. 2018, 91, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lieu, C.H.; Tran, H.; Jiang, Z.Q.; Mao, M.; Overman, M.J.; Lin, E.; Eng, C.; Morris, J.; Ellis, L.; Heymach, J.V.; et al. The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. PLoS ONE 2013, 8, e77117. [Google Scholar] [CrossRef] [PubMed]
- Dercle, L.; Lu, L.; Schwartz, L.H.; Qian, M.; Tejpar, S.; Eggleton, P.; Zhao, B.; Piessevaux, H. Radiomics Response Signature for Identification of Metastatic Colorectal Cancer Sensitive to Therapies Targeting EGFR Pathway. J. Natl. Cancer Inst. 2020, 112, 902–912. [Google Scholar] [CrossRef]
- Martinelli, E.; Ciardiello, D.; Martini, G.; Troiani, T.; Cardone, C.; Vitiello, P.P.; Normanno, N.; Rachiglio, A.M.; Maiello, E.; Latiano, T.; et al. Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: Challenges and future perspectives. Ann. Oncol. 2020, 31, 30–40. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Bender, U.; Rho, Y.S.; Barrera, I.; Aghajanyan, S.; Acoba, J.; Kavan, P. Adjuvant therapy for stages II and III colon cancer: Risk stratification, treatment duration, and future directions. Curr. Oncol. 2019, 26, S43–S52. [Google Scholar] [CrossRef]
- Benson, A.B., 3rd; Schrag, D.; Somerfield, M.R.; Cohen, A.M.; Figueredo, A.T.; Flynn, P.J.; Krzyzanowska, M.K.; Maroun, J.; McAllister, P.; Van Cutsem, E.; et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J. Clin. Oncol. 2004, 22, 3408–3419. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined with Cetuximab or Bevacizumab on Overall Survival in Patients with KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef]
- Kuismanen, S.A.; Holmberg, M.T.; Salovaara, R.; de la Chapelle, A.; Peltomäki, P. Genetic and epigenetic modification of MLH1 accounts for a major share of microsatellite-unstable colorectal cancers. Am. J. Pathol. 2000, 156, 1773–1779. [Google Scholar] [CrossRef]
- Xiao, J.; Li, W.; Huang, Y.; Huang, M.; Li, S.; Zhai, X.; Zhao, J.; Gao, C.; Xie, W.; Qin, H.; et al. A next-generation sequencing-based strategy combining microsatellite instability and tumor mutation burden for comprehensive molecular diagnosis of advanced colorectal cancer. BMC Cancer 2021, 21, 282. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Sokol, E.S.; Frampton, G.M.; Lippman, S.M.; Kurzrock, R. Microsatellite-Stable Tumors with High Mutational Burden Benefit from Immunotherapy. Cancer Immunol. Res. 2019, 7, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Bibeau, F.; Ligtenberg, M.J.L.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.Y.; Andre, F.; et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019, 30, 1232–1243. [Google Scholar] [CrossRef]
- Schrock, A.B.; Ouyang, C.; Sandhu, J.; Sokol, E.; Jin, D.; Ross, J.S.; Miller, V.A.; Lim, D.; Amanam, I.; Chao, J.; et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann. Oncol. 2019, 30, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Hewson, C.; Capraro, D.; Burdach, J.; Whitaker, N.; Morris, K.V. Extracellular vesicle associated long non-coding RNAs functionally enhance cell viability. Noncoding RNA Res. 2016, 1, 3–11. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M.; et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom. 2013, 14, 319. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef]
- Erozenci, L.A.; Böttger, F.; Bijnsdorp, I.V.; Jimenez, C.R. Urinary exosomal proteins as (pan-)cancer biomarkers: Insights from the proteome. FEBS Lett. 2019, 593, 1580–1597. [Google Scholar] [CrossRef]
- Guha, D.; Lorenz, D.R.; Misra, V.; Chettimada, S.; Morgello, S.; Gabuzda, D. Proteomic analysis of cerebrospinal fluid extracellular vesicles reveals synaptic injury, inflammation, and stress response markers in HIV patients with cognitive impairment. J. Neuroinflamm. 2019, 16, 254. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhong, J.; Zhong, B.; Huang, J.; Jiang, L.; Jiang, Y.; Yuan, J.; Sun, J.; Dai, L.; Yang, C.; et al. Exosomes as potential sources of biomarkers in colorectal cancer. Cancer Lett. 2020, 476, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zlotogorski-Hurvitz, A.; Dayan, D.; Chaushu, G.; Korvala, J.; Salo, T.; Sormunen, R.; Vered, M. Human saliva-derived exosomes: Comparing methods of isolation. J. Histochem. Cytochem. 2015, 63, 181–189. [Google Scholar] [CrossRef]
- Yan, S.; Dang, G.; Zhang, X.; Jin, C.; Qin, L.; Wang, Y.; Shi, M.; Huang, H.; Duan, Q. Downregulation of circulating exosomal miR-638 predicts poor prognosis in colon cancer patients. Oncotarget 2017, 8, 72220–72226. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Suthar, J.; Parsons, E.S.; Hoogenboom, B.W.; Williams, G.R.; Guldin, S. Acoustic Immunosensing of Exosomes Using a Quartz Crystal Microbalance with Dissipation Monitoring. Anal. Chem. 2020, 92, 4082–4093. [Google Scholar] [CrossRef]
- Kubota, S.; Chiba, M.; Watanabe, M.; Sakamoto, M.; Watanabe, N. Secretion of small/microRNAs including miR-638 into extracellular spaces by sphingomyelin phosphodiesterase 3. Oncol. Rep. 2015, 33, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, J.; Xie, J.; Wang, Y.; Sun, J.; Liu, T.; Zhao, Y.; Zhao, X.; Wang, X.; Ma, Y.; et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020, 4, 69–83. [Google Scholar] [CrossRef]
- Ambattu, L.A.; Ramesan, S.; Dekiwadia, C.; Hanssen, E.; Li, H.; Yeo, L.Y. High frequency acoustic cell stimulation promotes exosome generation regulated by a calcium-dependent mechanism. Commun. Biol. 2020, 3, 553. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Migliano, S.M.; Teis, D. ESCRT and Membrane Protein Ubiquitination. Prog. Mol. Subcell. Biol. 2018, 57, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Boura, E.; Hurley, J.H. Structural basis for membrane targeting by the MVB12-associated β-prism domain of the human ESCRT-I MVB12 subunit. Proc. Natl. Acad. Sci. USA 2012, 109, 1901–1906. [Google Scholar] [CrossRef]
- Tang, S.; Buchkovich, N.J.; Henne, W.M.; Banjade, S.; Kim, Y.J.; Emr, S.D. ESCRT-III activation by parallel action of ESCRT-I/II and ESCRT-0/Bro1 during MVB biogenesis. eLife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Piper, R.C.; Lehner, P.J. Endosomal transport via ubiquitination. Trends Cell Biol. 2011, 21, 647–655. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, W.; Guo, Z.; Ju, Q.; Zhu, L.; Gao, J.; Zhou, L.; Liu, F.; Xu, Y.; Zhan, Q.; et al. A novel TP53 pathway influences the HGS-mediated exosome formation in colorectal cancer. Sci. Rep. 2016, 6, 28083. [Google Scholar] [CrossRef]
- Džombeta, T.; Kapuralin, K.; Ulamec, M.; Tomas, D.; Gajović, S.; Krušlin, B. Immunohistochemical expression of STAM2 in gastrointestinal stromal tumors. Anticancer Res. 2014, 34, 2291–2296. [Google Scholar] [PubMed]
- Gheytanchi, E.; Zanjani, L.S.; Ghods, R.; Abolhasani, M.; Shahin, M.; Vafaei, S.; Naseri, M.; Fattahi, F.; Madjd, Z. High expression of tumor susceptibility gene 101 (TSG101) is associated with more aggressive behavior in colorectal carcinoma. J. Cancer Res. Clin. Oncol. 2021, 147, 1631–1646. [Google Scholar] [CrossRef]
- Kolmus, K.; Erdenebat, P.; Szymańska, E.; Stewig, B.; Goryca, K.; Derezińska-Wołek, E.; Szumera-Ciećkiewicz, A.; Brewińska-Olchowik, M.; Piwocka, K.; Prochorec-Sobieszek, M.; et al. Concurrent depletion of Vps37 proteins evokes ESCRT-I destabilization and profound cellular stress responses. J. Cell Sci. 2021, 134. [Google Scholar] [CrossRef]
- Qina, J.; Ke, T.; Li, C.; Wei-fang, L.; Rong, W.; Gui-Yuan, L. In silico expression analysis of human novel gene UBAP1 in multiple cancers. Chin. J. Cancer Res. 2002, 14, 157–160. [Google Scholar] [CrossRef]
- Mastrogamvraki, N.; Zaravinos, A. Signatures of co-deregulated genes and their transcriptional regulators in colorectal cancer. NPJ Syst. Biol. Appl. 2020, 6, 23. [Google Scholar] [CrossRef]
- Al-Temaimi, R.A.; Tan, T.Z.; Marafie, M.J.; Thiery, J.P.; Quirke, P.; Al-Mulla, F. Identification of 42 Genes Linked to Stage II Colorectal Cancer Metastatic Relapse. Int. J. Mol. Sci. 2016, 17, 598. [Google Scholar] [CrossRef]
- Mo, J.S.; Han, S.H.; Yun, K.J.; Chae, S.C. MicroRNA 429 regulates the expression of CHMP5 in the inflammatory colitis and colorectal cancer cells. Inflamm. Res. 2018, 67, 985–996. [Google Scholar] [CrossRef]
- Liu, J.; Song, H.; Yao, L.; Liu, Y.; Zhang, Y.; Zhao, H.; Ji, H.; Wang, Y. Over-expression of the overexpressed in lung cancer-1 is associated with poor prognosis in colorectal cancer. Anticancer Res. 2014, 34, 367–372. [Google Scholar]
- Szymańska, E.; Nowak, P.; Kolmus, K.; Cybulska, M.; Goryca, K.; Derezińska-Wołek, E.; Szumera-Ciećkiewicz, A.; Brewińska-Olchowik, M.; Grochowska, A.; Piwocka, K.; et al. Synthetic lethality between VPS4A and VPS4B triggers an inflammatory response in colorectal cancer. EMBO Mol. Med. 2020, 12, e10812. [Google Scholar] [CrossRef] [PubMed]
- Valcz, G.; Galamb, O.; Krenács, T.; Spisák, S.; Kalmár, A.; Patai, Á.V.; Wichmann, B.; Dede, K.; Tulassay, Z.; Molnár, B. Exosomes in colorectal carcinoma formation: ALIX under the magnifying glass. Mod. Pathol. 2016, 29, 928–938. [Google Scholar] [CrossRef]
- Chang, Y.C.; Su, C.Y.; Chen, M.H.; Chen, W.S.; Chen, C.L.; Hsiao, M. Secretory RAB GTPase 3C modulates IL6-STAT3 pathway to promote colon cancer metastasis and is associated with poor prognosis. Mol. Cancer 2017, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Huang, S.; Pisanic Ii, T.R.; Stark, A.; Tao, Y.; Cheng, B.; Li, Y.; Wei, Y.; Zhao, W.; Wang, T.H.; et al. Rab8 GTPase regulates Klotho-mediated inhibition of cell growth and progression by directly modulating its surface expression in human non-small cell lung cancer. EBioMedicine 2019, 49, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Jiang, Y.; Lu, H.; Lu, X.; Wang, S.; Wang, L.; Wei, M.; Lu, W.; Du, Z.; Ye, Z.; et al. Rab27A mediated by NF-κB promotes the stemness of colon cancer cells via up-regulation of cytokine secretion. Oncotarget 2016, 7, 63342–63351. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Cui, J.; Yang, J.; Li, W.; Wang, S.; Wang, X.; Li, X.; Lu, Y.; Xiao, W. Decreased expression of Rab27A and Rab27B correlates with metastasis and poor prognosis in colorectal cancer. Discov. Med. 2015, 20, 357–367. [Google Scholar] [PubMed]
- Hutagalung, A.H.; Novick, P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011, 91, 119–149. [Google Scholar] [CrossRef]
- Kiral, F.R.; Kohrs, F.E.; Jin, E.J.; Hiesinger, P.R. Rab GTPases and Membrane Trafficking in Neurodegeneration. Curr. Biol. 2018, 28, R471–R486. [Google Scholar] [CrossRef]
- Olson, A.L. Regulation of GLUT4 and Insulin-Dependent Glucose Flux. ISRN Mol. Biol. 2012, 2012, 856987. [Google Scholar] [CrossRef]
- Prashar, A.; Schnettger, L.; Bernard, E.M.; Gutierrez, M.G. Rab GTPases in Immunity and Inflammation. Front. Cell Infect. Microbiol. 2017, 7, 435. [Google Scholar] [CrossRef]
- Subramani, D.; Alahari, S.K. Integrin-mediated function of Rab GTPases in cancer progression. Mol. Cancer 2010, 9, 312. [Google Scholar] [CrossRef]
- Mitra, S.; Federico, L.; Zhao, W.; Dennison, J.; Sarkar, T.R.; Zhang, F.; Takiar, V.; Cheng, K.W.; Mani, S.; Lee, J.S.; et al. Rab25 acts as an oncogene in luminal B breast cancer and is causally associated with Snail driven EMT. Oncotarget 2016, 7, 40252–40265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Díaz, J.; Mendoza, P.; Ortiz, R.; Díaz, N.; Leyton, L.; Stupack, D.; Quest, A.F.; Torres, V.A. Rab5 is required in metastatic cancer cells for Caveolin-1-enhanced Rac1 activation, migration and invasion. J. Cell Sci. 2014, 127, 2401–2406. [Google Scholar] [CrossRef] [PubMed]
- Wandinger-Ness, A.; Zerial, M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect. Biol. 2014, 6, a022616. [Google Scholar] [CrossRef]
- Fox, E.J.; Prindle, M.J.; Loeb, L.A. Do mutator mutations fuel tumorigenesis? Cancer Metastasis Rev. 2013, 32, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.J.; Franklin, J.L.; Dou, Y.; Liu, Q.; Higginbotham, J.N.; Beckler, M.D.; Weaver, A.M.; Vickers, K.; Prasad, N.; Levy, S.; et al. KRAS-dependent sorting of miRNA to exosomes. eLife 2015, 4, e07197. [Google Scholar] [CrossRef]
- Beckler, M.D.; Higginbotham, J.N.; Franklin, J.L.; Ham, A.J.; Halvey, P.J.; Imasuen, I.E.; Whitwell, C.; Li, M.; Liebler, D.C.; Coffey, R.J. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol. Cell Proteom. 2013, 12, 343–355. [Google Scholar] [CrossRef]
- Dou, Y.; Cha, D.J.; Franklin, J.L.; Higginbotham, J.N.; Jeppesen, D.K.; Weaver, A.M.; Prasad, N.; Levy, S.; Coffey, R.J.; Patton, J.G.; et al. Circular RNAs are down-regulated in KRAS mutant colon cancer cells and can be transferred to exosomes. Sci. Rep. 2016, 6, 37982. [Google Scholar] [CrossRef] [PubMed]
- Hinger, S.A.; Cha, D.J.; Franklin, J.L.; Higginbotham, J.N.; Dou, Y.; Ping, J.; Shu, L.; Prasad, N.; Levy, S.; Zhang, B.; et al. Diverse Long RNAs Are Differentially Sorted into Extracellular Vesicles Secreted by Colorectal Cancer Cells. Cell Rep. 2018, 25, 715–725.e4. [Google Scholar] [CrossRef] [PubMed]
- Hinger, S.A.; Abner, J.J.; Franklin, J.L.; Jeppesen, D.K.; Coffey, R.J.; Patton, J.G. Rab13 regulates sEV secretion in mutant KRAS colorectal cancer cells. Sci. Rep. 2020, 10, 15804. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Yu, B.H.; Li, D.L.; Ke, H.L.; Guo, X.Z.; Xiao, X.Y. PI3K expression and PIK3CA mutations are related to colorectal cancer metastases. World J. Gastroenterol. 2012, 18, 3745–3751. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Dong, H.; Wang, X.; Gao, F.; Zhang, S.; Zhang, X. Aspirin has a better effect on PIK3CA mutant colorectal cancer cells by PI3K/Akt/Raptor pathway. Mol. Med. 2020, 26, 14. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal. Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.Y.; Gu, R.H.; Yan, B. Downregulation of exosome-encapsulated miR-548c-5p is associated with poor prognosis in colorectal cancer. J. Cell Biochem. 2018. [Google Scholar] [CrossRef]
- Yan, S.; Liu, G.; Jin, C.; Wang, Z.; Duan, Q.; Xu, J.; Xu, D. MicroRNA-6869-5p acts as a tumor suppressor via targeting TLR4/NF-κB signaling pathway in colorectal cancer. J. Cell Physiol. 2018, 233, 6660–6668. [Google Scholar] [CrossRef]
- Zou, S.L.; Chen, Y.L.; Ge, Z.Z.; Qu, Y.Y.; Cao, Y.; Kang, Z.X. Downregulation of serum exosomal miR-150-5p is associated with poor prognosis in patients with colorectal cancer. Cancer Biomark. 2019, 26, 69–77. [Google Scholar] [CrossRef]
- Lu, Y.; Sha, H.; Sun, X.; Zhang, Y.; Wu, Y.; Zhang, J.; Zhang, H.; Wu, J.; Feng, J. CRNDE: An oncogenic long non-coding RNA in cancers. Cancer Cell Int. 2020, 20, 162. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Yang, Y.M.; Du, L.T.; Wang, C.X. Increased expression of the long noncoding RNA CRNDE-h indicates a poor prognosis in colorectal cancer, and is positively correlated with IRX5 mRNA expression. Onco Targets Ther. 2016, 9, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhao, Z.; Wang, G.; Wang, J.; Zhu, W. lncRNA GAS5 inhibits colorectal cancer cell proliferation via the miR-182-5p/FOXO3a axis. Oncol. Rep. 2018, 40, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Ruan, H.; Zhang, X.; Xu, X.; Zhu, Y.; Peng, H.; Zhang, X.; Kong, F.; Guan, M. Long noncoding RNA CCAL transferred from fibroblasts by exosomes promotes chemoresistance of colorectal cancer cells. Int. J. Cancer 2020, 146, 1700–1716. [Google Scholar] [CrossRef]
- Yu, Y.; Nangia-Makker, P.; Farhana, L.; Majumdar, A.P.N. A novel mechanism of lncRNA and miRNA interaction: CCAT2 regulates miR-145 expression by suppressing its maturation process in colon cancer cells. Mol. Cancer 2017, 16, 155. [Google Scholar] [CrossRef]
- Yan, Y.; Su, M.; Qin, B. CircHIPK3 promotes colorectal cancer cells proliferation and metastasis via modulating of miR-1207-5p/FMNL2 signal. Biochem. Biophys. Res. Commun. 2020, 524, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Chen, X.; Xu, M.; Liu, X.; Hu, X.; Xu, T.; Sun, H.; Pan, Y.; He, B.; Wang, S. CircHIPK3 promotes colorectal cancer growth and metastasis by sponging miR-7. Cell Death Dis. 2018, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, H.; Yang, Y.; Wang, X.; Deng, T.; Liu, R.; Ning, T.; Bai, M.; Li, H.; Zhu, K.; et al. Hypoxia induced exosomal circRNA promotes metastasis of Colorectal Cancer via targeting GEF-H1/RhoA axis. Theranostics 2020, 10, 8211–8226. [Google Scholar] [CrossRef]
- Hon, K.W.; Ab-Mutalib, N.S.; Abdullah, N.M.A.; Jamal, R.; Abu, N. Extracellular Vesicle-derived circular RNAs confers chemoresistance in Colorectal cancer. Sci. Rep. 2019, 9, 16497. [Google Scholar] [CrossRef] [PubMed]
- Campanella, C.; Rappa, F.; Sciumè, C.; Gammazza, A.M.; Barone, R.; Bucchieri, F.; David, S.; Curcurù, G.; Bavisotto, C.C.; Pitruzzella, A.; et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 2015, 121, 3230–3239. [Google Scholar] [CrossRef]
- Dong, L.; Lin, W.; Qi, P.; Xu, M.D.; Wu, X.; Ni, S.; Huang, D.; Weng, W.W.; Tan, C.; Sheng, W.; et al. Circulating Long RNAs in Serum Extracellular Vesicles: Their Characterization and Potential Application as Biomarkers for Diagnosis of Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1158–1166. [Google Scholar] [CrossRef]
- Hu, Y.B.; Yan, C.; Mu, L.; Mi, Y.L.; Zhao, H.; Hu, H.; Li, X.L.; Tao, D.D.; Wu, Y.Q.; Gong, J.P.; et al. Exosomal Wnt-induced dedifferentiation of colorectal cancer cells contributes to chemotherapy resistance. Oncogene 2019, 38, 1951–1965. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Guo, X.; Zhou, L.; Jia, Z.; Peng, Z.; Tang, Y.; Liu, W.; Zhu, B.; Wang, L.; et al. GPC1 exosome and its regulatory miRNAs are specific markers for the detection and target therapy of colorectal cancer. J. Cell Mol. Med. 2017, 21, 838–847. [Google Scholar] [CrossRef]
- Kumara, H.M.S.; Grieco, M.J.; Caballero, O.L.; Su, T.; Ahmed, A.; Ritter, E.; Gnjatic, S.; Cekic, V.; Old, L.J.; Simpson, A.J.; et al. MAGE-A3 is highly expressed in a subset of colorectal cancer patients. Cancer Immun. 2012, 12, 16. [Google Scholar]
- Sun, B.; Li, Y.; Zhou, Y.; Ng, T.K.; Zhao, C.; Gan, Q.; Gu, X.; Xiang, J. Circulating exosomal CPNE3 as a diagnostic and prognostic biomarker for colorectal cancer. J. Cell Physiol. 2019, 234, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhou, Y.; Fang, Y.; Li, Z.; Gu, X.; Xiang, J. Colorectal cancer exosomes induce lymphatic network remodeling in lymph nodes. Int. J. Cancer 2019, 145, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ma, L.; Gong, M.; Su, G.; Zhu, S.; Zhang, W.; Wang, S.; Li, Z.; Chen, C.; Li, L.; et al. Protein Profiling and Sizing of Extracellular Vesicles from Colorectal Cancer Patients via Flow Cytometry. ACS Nano 2018, 12, 671–680. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, K.; Tian, J.; Xia, X.; Ma, J.; Tang, X.; Xu, H.; Wang, S. Granulocytic Myeloid-Derived Suppressor Cells Promote the Stemness of Colorectal Cancer Cells through Exosomal S100A9. Adv. Sci. 2019, 6, 1901278. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Sun, D.; Ma, L.; Deng, Y.; Zhang, S.; Dong, L.; Chen, S. Exosomes isolated from CAPS1-overexpressing colorectal cancer cells promote cell migration. Oncol. Rep. 2019, 42, 2528–2536. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Y.; Yuan, Y.; Liu, B.; Pan, S.; Liu, Q.; Qi, X.; Zhou, H.; Dong, W.; Jia, L. The potential of exosomes derived from colorectal cancer as a biomarker. Clin. Chim. Acta 2019, 490, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Takeuchi, A.; Yamaguchi, S.; Mitani, Y.; Watanabe, T.; Matsuda, K.; Hotta, T.; Shively, J.E.; Yamaue, H. Clinical implications of carcinoembryonic antigen distribution in serum exosomal fraction-Measurement by ELISA. PLoS ONE 2017, 12, e0183337. [Google Scholar] [CrossRef]
- Zhong, M.E.; Chen, Y.; Xiao, Y.; Xu, L.; Zhang, G.; Lu, J.; Qiu, H.; Ge, W.; Wu, B. Serum extracellular vesicles contain SPARC and LRG1 as biomarkers of colon cancer and differ by tumour primary location. EBioMedicine 2019, 50, 211–223. [Google Scholar] [CrossRef]
- Ogata-Kawata, H.; Izumiya, M.; Kurioka, D.; Honma, Y.; Yamada, Y.; Furuta, K.; Gunji, T.; Ohta, H.; Okamoto, H.; Sonoda, H.; et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS ONE 2014, 9, e92921. [Google Scholar] [CrossRef]
- Wang, J.; Yan, F.; Zhao, Q.; Zhan, F.; Wang, R.; Wang, L.; Zhang, Y.; Huang, X. Circulating exosomal miR-125a-3p as a novel biomarker for early-stage colon cancer. Sci. Rep. 2017, 7, 4150. [Google Scholar] [CrossRef]
- Fu, F.; Jiang, W.; Zhou, L.; Chen, Z. Circulating Exosomal miR-17-5p and miR-92a-3p Predict Pathologic Stage and Grade of Colorectal Cancer. Transl. Oncol. 2018, 11, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Bjørnetrø, T.; Redalen, K.R.; Meltzer, S.; Thusyanthan, N.S.; Samiappan, R.; Jegerschöld, C.; Handeland, K.R.; Ree, A.H. An experimental strategy unveiling exosomal microRNAs 486-5p, 181a-5p and 30d-5p from hypoxic tumour cells as circulating indicators of high-risk rectal cancer. J. Extracell. Vesicles 2019, 8, 1567219. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Sugimachi, K.; Iinuma, H.; Takahashi, Y.; Kurashige, J.; Sawada, G.; Ueda, M.; Uchi, R.; Ueo, H.; Takano, Y.; et al. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br. J. Cancer 2015, 113, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ren, Y.; Hu, X.; Mu, J.; Samykutty, A.; Zhuang, X.; Deng, Z.; Kumar, A.; Zhang, L.; Merchant, M.L.; et al. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat. Commun. 2017, 8, 14448. [Google Scholar] [CrossRef]
- Ren, D.; Lin, B.; Zhang, X.; Peng, Y.; Ye, Z.; Ma, Y.; Liang, Y.; Cao, L.; Li, X.; Li, R.; et al. Maintenance of cancer stemness by miR-196b-5p contributes to chemoresistance of colorectal cancer cells via activating STAT3 signaling pathway. Oncotarget 2017, 8, 49807–49823. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Masuda, T.; Iinuma, H.; Yamaguchi, R.; Sato, K.; Tobo, T.; Hirata, H.; Kuroda, Y.; Nambara, S.; Hayashi, N.; et al. Circulating exosomal microRNA-203 is associated with metastasis possibly via inducing tumor-associated macrophages in colorectal cancer. Oncotarget 2017, 8, 78598–78613. [Google Scholar] [CrossRef]
- Bigagli, E.; Luceri, C.; Guasti, D.; Cinci, L. Exosomes secreted from human colon cancer cells influence the adhesion of neighboring metastatic cells: Role of microRNA-210. Cancer Biol. Ther. 2016, 17, 1062–1069. [Google Scholar] [CrossRef]
- Jin, G.; Liu, Y.; Zhang, J.; Bian, Z.; Yao, S.; Fei, B.; Zhou, L.; Yin, Y.; Huang, Z. A panel of serum exosomal microRNAs as predictive markers for chemoresistance in advanced colorectal cancer. Cancer Chemother. Pharmacol. 2019, 84, 315–325. [Google Scholar] [CrossRef]
- Karimi, N.; Ali Feizi, M.H.; Safaralizadeh, R.; Hashemzadeh, S.; Baradaran, B.; Shokouhi, B.; Teimourian, S. Serum overexpression of miR-301a and miR-23a in patients with colorectal cancer. J. Chin. Med. Assoc. 2019, 82, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Li, Y.; Pan, Y.; Lan, X.; Song, F.; Sun, J.; Zhou, K.; Liu, X.; Ren, X.; Wang, F.; et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 2018, 9, 5395. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Zeng, K.; Xu, M.; He, B.; Pan, Y.; Sun, H.; Pan, B.; Xu, X.; Xu, T.; et al. DNA-methylation-mediated silencing of miR-486-5p promotes colorectal cancer proliferation and migration through activation of PLAGL2/IGF2/β-catenin signal pathways. Cell Death Dis. 2018, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Han, B.; Gao, S.; Wang, X.; Wang, Z.; Wang, F.; Zhang, J.; Xu, D.; Sun, B. Exosome-encapsulated microRNAs as circulating biomarkers for colorectal cancer. Oncotarget 2017, 8, 60149–60158. [Google Scholar] [CrossRef]
- Yan, S.; Jiang, Y.; Liang, C.; Cheng, M.; Jin, C.; Duan, Q.; Xu, D.; Yang, L.; Zhang, X.; Ren, B.; et al. Exosomal miR-6803-5p as potential diagnostic and prognostic marker in colorectal cancer. J. Cell Biochem. 2018, 119, 4113–4119. [Google Scholar] [CrossRef]
- Hu, J.L.; Wang, W.; Lan, X.L.; Zeng, Z.C.; Liang, Y.S.; Yan, Y.R.; Song, F.Y.; Wang, F.F.; Zhu, X.H.; Liao, W.J.; et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol. Cancer 2019, 18, 91. [Google Scholar] [CrossRef]
- Min, L.; Zhu, S.; Chen, L.; Liu, X.; Wei, R.; Zhao, L.; Yang, Y.; Zhang, Z.; Kong, G.; Li, P.; et al. Evaluation of circulating small extracellular vesicles derived miRNAs as biomarkers of early colon cancer: A comparison with plasma total miRNAs. J. Extracell. Vesicles 2019, 8, 1643670. [Google Scholar] [CrossRef]
- Barbagallo, C.; Brex, D.; Caponnetto, A.; Cirnigliaro, M.; Scalia, M.; Magnano, A.; Caltabiano, R.; Barbagallo, D.; Biondi, A.; Cappellani, A.; et al. LncRNA UCA1, Upregulated in CRC Biopsies and Downregulated in Serum Exosomes, Controls mRNA Expression by RNA-RNA Interactions. Mol. Ther. Nucleic Acids 2018, 12, 229–241. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Wang, J.; Li, B.; Wang, X. Circular RNA expression profile of lung squamous cell carcinoma: Identification of potential biomarkers and therapeutic targets. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Pan, B.; Qin, J.; Liu, X.; He, B.; Wang, X.; Pan, Y.; Sun, H.; Xu, T.; Xu, M.; Chen, X.; et al. Identification of Serum Exosomal hsa-circ-0004771 as a Novel Diagnostic Biomarker of Colorectal Cancer. Front. Genet. 2019, 10, 1096. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ma, J.; Sun, T.; Zhou, Q.; Wang, W.; Wang, G.; Wu, P.; Wang, H.; Jiang, L.; et al. Exosomal circRNAs: Biogenesis, effect and application in human diseases. Mol. Cancer 2019, 18, 116. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Gao, S.; Jing, F.; Yang, Y.; Du, L.; Zheng, G.; Li, P.; Li, C.; Wang, C. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget 2016, 7, 85551–85563. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Meng, T.; Yang, X.H.; Sayim, P.; Lei, C.; Jin, B.; Ge, L.; Wang, H.J. Prognostic and predictive value of long non-coding RNA GAS5 and mircoRNA-221 in colorectal cancer and their effects on colorectal cancer cell proliferation, migration and invasion. Cancer Biomark. 2018, 22, 283–299. [Google Scholar] [CrossRef]
- Hu, D.; Zhan, Y.; Zhu, K.; Bai, M.; Han, J.; Si, Y.; Zhang, H.; Kong, D. Plasma Exosomal Long Non-Coding RNAs Serve as Biomarkers for Early Detection of Colorectal Cancer. Cell Physiol. Biochem. 2018, 51, 2704–2715. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Liu, X.; He, B.; Nie, Z.; Zhu, C.; Zhang, P.; Wang, S. Exosomal lncRNA 91H is associated with poor development in colorectal cancer by modifying HNRNPK expression. Cancer Cell Int. 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Spizzo, R.; Atlasi, Y.; Nicoloso, M.; Shimizu, M.; Redis, R.S.; Nishida, N.; Gafà, R.; Song, J.; Guo, Z.; et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013, 23, 1446–1461. [Google Scholar] [CrossRef]
- Ren, J.; Ding, L.; Zhang, D.; Shi, G.; Xu, Q.; Shen, S.; Wang, Y.; Wang, T.; Hou, Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics 2018, 8, 3932–3948. [Google Scholar] [CrossRef] [PubMed]
- Ogunwobi, O.O.; Mahmood, F.; Akingboye, A. Biomarkers in Colorectal Cancer: Current Research and Future Prospects. Int. J. Mol. Sci. 2020, 21, 5311. [Google Scholar] [CrossRef]
- Wu, H.; Wei, M.; Jiang, X.; Tan, J.; Xu, W.; Fan, X.; Zhang, R.; Ding, C.; Zhao, F.; Shao, X.; et al. lncRNA PVT1 Promotes Tumorigenesis of Colorectal Cancer by Stabilizing miR-16-5p and Interacting with the VEGFA/VEGFR1/AKT Axis. Mol. Ther. Nucleic Acids 2020, 20, 438–450. [Google Scholar] [CrossRef]
- Del Vecchio, F.; Martinez-Rodriguez, V.; Schukking, M.; Cocks, A.; Broseghini, E.; Fabbri, M. Professional killers: The role of extracellular vesicles in the reciprocal interactions between natural killer, CD8+ cytotoxic T-cells and tumour cells. J. Extracell. Vesicles 2021, 10, e12075. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Jiang, S. Immune Cell-Derived Exosomes in the Cancer-Immunity Cycle. Trends Cancer 2020, 6, 506–517. [Google Scholar] [CrossRef]
- Mittal, S.; Gupta, P.; Chaluvally-Raghavan, P.; Pradeep, S. Emerging Role of Extracellular Vesicles in Immune Regulation and Cancer Progression. Cancers 2020, 12, 3563. [Google Scholar] [CrossRef]
- Lan, J.; Sun, L.; Xu, F.; Liu, L.; Hu, F.; Song, D.; Hou, Z.; Wu, W.; Luo, X.; Wang, J.; et al. M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer Res. 2019, 79, 146–158. [Google Scholar] [CrossRef]
- Mannavola, F.; Salerno, T.; Passarelli, A.; Tucci, M.; Internò, V.; Silvestris, F. Revisiting the Role of Exosomes in Colorectal Cancer: Where Are We Now? Front. Oncol. 2019, 9, 521. [Google Scholar] [CrossRef]
- Milane, L.; Singh, A.; Mattheolabakis, G.; Suresh, M.; Amiji, M.M. Exosome mediated communication within the tumor microenvironment. J. Control. Release 2015, 219, 278–294. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, S.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Xu, J.; Xia, K.; Chang, Y.; et al. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol. Cancer 2018, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, K.; Inoue, K.; Fujiwara, A.; Hatakeyama, K.; Kanto, K.; Watanabe, Y.; Muramatsu, K.; Fukuda, Y.; Ogura, S.; Yamaguchi, K.; et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS ONE 2010, 5, e13247. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Chen, M.; Greening, D.W.; He, W.; Rai, A.; Zhang, W.; Simpson, R.J. Deep sequencing of RNA from three different extracellular vesicle (EV) subtypes released from the human LIM1863 colon cancer cell line uncovers distinct miRNA-enrichment signatures. PLoS ONE 2014, 9, e110314. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Chen, B.; Zhao, J.; Yu, S.; Tang, Y.; Zheng, Q.; Li, Y.; Wang, P.; He, X.; et al. exoRBase: A database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018, 46, D106–D112. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Takayama, A.; Arai, T.; Kawauchi, J.; Sudo, H. MicroRNA-8073: Tumor suppressor and potential therapeutic treatment. PLoS ONE 2018, 13, e0209750. [Google Scholar] [CrossRef]
- Kyuno, D.; Bauer, N.; Schnölzer, M.; Provaznik, J.; Ryschich, E.; Hackert, T.; Zöller, M. Distinct Origin of Claudin7 in Early Tumor Endosomes Affects Exosome Assembly. Int. J. Biol. Sci. 2019, 15, 2224–2239. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Rana, S.; Wang, Z.; Zhao, K.; Schnölzer, M.; Provaznik, J.; Hackert, T.; Lv, Q.; Zöller, M. The Pancreatic Cancer-Initiating Cell Marker CD44v6 Affects Transcription, Translation, and Signaling: Consequences for Exosome Composition and Delivery. J. Oncol. 2019, 2019, 3516973. [Google Scholar] [CrossRef]
- Tubita, V.; Segui-Barber, J.; Lozano, J.J.; Banon-Maneus, E.; Rovira, J.; Cucchiari, D.; Moya-Rull, D.; Oppenheimer, F.; Del Portillo, H.; Campistol, J.M.; et al. Effect of immunosuppression in miRNAs from extracellular vesicles of colorectal cancer and their influence on the pre-metastatic niche. Sci. Rep. 2019, 9, 11177. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; He, L.; Pentok, M.; Yang, H.; Yang, Y.; Li, Z.; He, N.; Deng, Y.; Li, S.; Liu, T.; et al. An aptamer-based new method for competitive fluorescence detection of exosomes. Nanoscale 2019, 11, 15589–15595. [Google Scholar] [CrossRef]
- De Voogt, W.S.; Tanenbaum, M.E.; Vader, P. Illuminating RNA trafficking and functional delivery by extracellular vesicles. Adv. Drug Deliv. Rev. 2021, 174, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Lyu, P.; Yoo, K.; Yadav, M.K.; Singh, R.; Atala, A.; Lu, B. Engineered extracellular vesicles as versatile ribonucleoprotein delivery vehicles for efficient and safe CRISPR genome editing. J. Extracell. Vesicles 2021, 10, e12076. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Tan, J.; Wu, C.; Zhang, J.; Liu, T.; Fan, C.; Li, J.; Zhang, Y. Extracellular vesicles engineered with valency-controlled DNA nanostructures deliver CRISPR/Cas9 system for gene therapy. Nucleic Acids Res. 2020, 48, 8870–8882. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Greening, D.W.; Zhu, H.J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008, 16, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Allelein, S.; Medina-Perez, P.; Lopes, A.L.H.; Rau, S.; Hause, G.; Kölsch, A.; Kuhlmeier, D. Potential and challenges of specifically isolating extracellular vesicles from heterogeneous populations. Sci. Rep. 2021, 11, 11585. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
| Complex | Gene Symbol | Hazard Ratio | Expression | Ref. |
|---|---|---|---|---|
| ESCRT-0 | VPS27 (HRS/HGS) | Uni: 2.27 Multi: 3.34 | Up | [36] |
| STAM1/2 | ---- | Up | [37] | |
| ESCRT-I | VPS23 (TSG101) | ---- | Up | [38] |
| VPS28 | ---- | ---- | ----- | |
| VPS37A/B/C/D | ---- | Down | [39] | |
| MVB12A/B | ---- | ---- | ---- | |
| UBAP1 | ---- | Down | [40] | |
| ESCRT-II | VPS22 (SNF8, EAP30) | ---- | ---- | ----- |
| VPS25 (EAP20) | ---- | ---- | ----- | |
| VPS36 (EAP45) | ---- | ---- | ----- | |
| ESCRT-III | VPS2A/B (CHMP2A/B) | ---- | Up | [41] |
| VPS20 (CHMP6) | ---- | ---- | ----- | |
| VPS24 (CHMP3) | ---- | ---- | ----- | |
| SNF7A/B/C (CHMP4A/B/C) | ---- | Down | [42] | |
| VPS60 (CHMP5) | ---- | Up | [43] | |
| DID2A/B (CHMP7, CHMP1A/B) | ---- | Down | [42] | |
| IST1 (OLC1) | Uni: 10.43 Multi: 7.9 | Up | [44] | |
| Vps4-Vta1 | VPS4A/B (SKD1) | ---- | Down | [45] |
| VTA1 (LIP5) | ---- | ---- | ---- | |
| Bro1/ALIX | ALIX (PDCD6IP) | ---- | Down | [46] |
| RABs | RAB3 | Uni: 2.58 Multi: 2.39 | Up | [47] |
| RAB8 | ---- | Up | [48] | |
| RAB26 | ---- | Up | [47] | |
| RAB27 | Multi: 0.45 | Up Down | [49] [50] |
| Category | Name | Express | Function | Ref. |
|---|---|---|---|---|
| miRNA | miR-1246 | Up | Diagnosis | [94] |
| miR-125a-3p | Up | Diagnosis | [95] | |
| miR-150-5p | Down | Diagnosis | [71] | |
| miR-17-5p/miR-92a-3p | Up | Metastasis | [96] | |
| miR-181a-5p | Down | Metastasis | [97] | |
| miR-19a | Up | Recurrence | [98] | |
| miR-193a | Up | Metastasis | [99] | |
| miR-196b-5p | Up | Chemoresistance | [100] | |
| miR-203 | Up | Metastasis | [101] | |
| miR-210 | Up | Chemoresistance/metastasis | [102] | |
| miR-21 | Up | Recurrence/chemoresistance/metastasis | [94] | |
| miR-21-5p/miR-1246/miR-96-5p/miR-1229-5p | Up | Chemoresistance | [103] | |
| miR-23a | Up | Diagnosis | [94,104] | |
| miR-25-3p | Up | Metastasis | [105] | |
| miR-30d-5p | Up | Metastasis | [97] | |
| miR-301a | Up | Diagnosis | [104] | |
| miR-486-5p | Up | Diagnosis | [106] | |
| miR-548c-5p | Down | Metastasis | [69] | |
| miR-638 | Down | Metastasis | [107] | |
| miR-6803-5p | Up | Diagnosis | [108] | |
| miR-6869-5p | Down | Metastasis | [70] | |
| miR-92a-3p | Up | Chemoresistance | [109] | |
| Let-7b-3p/miR-139-3p/miR-145-3p | Up | Diagnosis | [110] | |
| circRNA | circHIPK3 | Up | Diagnosis/metastasis | [78,111] |
| circ-133 | Up | Metastasis | [79] | |
| ciRS-122 | Up | Chemoresistance | [112] | |
| hsa-circ_0004771 | Up | Diagnosis | [113] | |
| hsa-circ_0000338 | Up | Chemoresistance | [80] | |
| circRTN4 | Up | Chemoresistance | [114] | |
| lncRNA | lncRNA CRNDE-h | Up | Recurrence/chemoresistance/ metastasis/diagnosis | [72,115] |
| lncRNA GAS5 | Up | Diagnosis | [116] | |
| LNCV6_116109 | UP | Diagnosis | [117] | |
| LNCV6_98390 | Up | Diagnosis | [117] | |
| LNCV6_84003 | UP | Diagnosis | [117] | |
| LNCV6_98602 | Up | Diagnosis | [117] | |
| LNCV_108266 | Up | Diagnosis | [117] | |
| LNCV6_38772 | Up | Diagnosis | [117] | |
| lncRNA 91H | Up | Recurrence | [118] | |
| lncRNA CCAT2 | Up | Diagnosis | [119] | |
| lncRNA H19 | Up | Chemoresistance | [120] | |
| lncRNA CCAL | Up | Chemoresistance | [75] | |
| lncRNA PVT1 | Up | Metastasis | [121,122] | |
| Protein | Hsp60 | Up | Diagnosis/proliferation | [81] |
| GPC1 | Up | Diagnosis/metastasis | [84] | |
| CD147 | Up | Diagnosis | [88] | |
| CPNE3 | Up | Diagnosis | [86] | |
| TAG72 | Up | Chemoresistance | [91] | |
| S100A9 | Up | Recurrence | [89] | |
| SPARC | Up | Diagnosis/angiogenesis | [93] | |
| LRG1 | Up | Diagnosis | [93] | |
| CEA | Up | Diagnosis/metastasis | [92] | |
| IRF-2 | Up | Metastasis | [87] | |
| Wnt | Up | Chemoresistance | [83] | |
| CAPS1 | Up | Metastasis | [90] | |
| MAGEA3 | Up | Diagnosis/metastasis | [82,85] |
| Array Chip and RNA-Seq Platform | Species | Objects | Ref. | |
|---|---|---|---|---|
| GSE21350 | Agilent-021827. Human miRNA Microarray G4470C | Human | Cell line (SW480,SW620) | [130] |
| GSE39833 | Agilent-021827. Human miRNA Microarray G4470C | Human | CRC patients’ serum | [94] |
| GSE40246 | Agilent-021827. Human miRNA Microarray G4470C | Human | CRC patients’ serum | [94] |
| GSE67004 | Illumina HiSeq 2000 | Human | Cell line (DKO-1,DLD-1,DKs-8) | [60] |
| GSE68979 | Agilent-019052 Homo sapiens 45K | Human | Cell line(SW480) | N/A |
| GSE72577 | Illumina HiSeq 2000 | Human | Cell line (DKO-1,DLD-1,DKs-8) | [62] |
| GSE87839 | Applied Biosystems Taqman Array Human Micro A+B Cards Set v3.0 | Human | Cell line (LIM1863) | [131] |
| GSE100063 | Illumina HiSeq 2000 | Human | CRC patients’ blood | [132] |
| GSE101950 GSE101951 | Illumina HiSeq 2500 | Mus | Cell line (CT26) | N/A |
| GSE114316 GSE114317 GSE114318 | 3D-Gene Human Oligo Chip 25K V2.13D-Gene Human miRNA V21_1.0.0 | Human | Colorectal xenografts | [133] |
| GSE115114 | Illumina NextSeq 500 | Human | CRC patients | N/A |
| GSE116589 | Illumina HiSeq 3000 | Human | CRC patients | N/A |
| GSE119031 | Agilent-040150 EMBL-rel18_30rep 031181 | Human | Cell line (HT-29,SW-948) | [134,135] |
| GSE119032 GSE119033 | Agilent-070156 Human_miRNA_ V21.0_Microarray 046064 | Human | Cell line (HT-29,SW-948) | [134,135] |
| GSE120013 | 3D-Gene Human miRNA V21_1.0.0 | Human | Cell line (HCT116) | N/A |
| GSE121964 | Illumina HiSeq 2000 | Human | Cell line (DKO-1,DLD-1,DKs-8) | [63] |
| GSE123708 GSE123709 GSE123710 | Affymetrix Multispecies miRNA-4 ArrayAffymetrix Clariom S Assay HT, Human | Human | Cell line (HCT116,SW480) | [136] |
| GSE125905 | Illumina HiSeq 2000 | Human | Cell line (DKO-1,Gli36) | [137] |
| GSE173202 | Illumina HiSeq 2000 | Mus | Cell line (CT26,MC38) | N/A |
| Number | Participants | Phase | Status | Application |
|---|---|---|---|---|
| NCT01294072 | 35 | Phase I | Recruiting | Treatment |
| NCT03432806 | 80 | ---- | Recruiting | Diagnostic |
| NCT04394572 | 75 | ---- | Recruiting | Diagnostic |
| NCT04523389 | 172 | ---- | Recruiting | Diagnostic |
| NCT04298398 | 108 | ---- | Not yet recruiting | Diagnostic |
| NCT04394572 | 75 | ---- | Recruiting | Diagnostic |
| NCT04523389 | 172 | ---- | Recruiting | Diagnostic |
| NCT03927898 | 40 | Phase II | Recruiting | Treatment |
| NCT02439008 | 28 | ---- | Terminated | Diagnostic |
| NCT03260179 | 60 | Phase I | Unknown | Treatment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Chan, M.-H.; Li, C.-H.; Fang, C.-Y.; Hsiao, M.; Chen, C.-L. Exosomal Components and Modulators in Colorectal Cancer: Novel Diagnosis and Prognosis Biomarkers. Biomedicines 2021, 9, 931. https://doi.org/10.3390/biomedicines9080931
Chang Y-C, Chan M-H, Li C-H, Fang C-Y, Hsiao M, Chen C-L. Exosomal Components and Modulators in Colorectal Cancer: Novel Diagnosis and Prognosis Biomarkers. Biomedicines. 2021; 9(8):931. https://doi.org/10.3390/biomedicines9080931
Chicago/Turabian StyleChang, Yu-Chan, Ming-Hsien Chan, Chien-Hsiu Li, Chih-Yeu Fang, Michael Hsiao, and Chi-Long Chen. 2021. "Exosomal Components and Modulators in Colorectal Cancer: Novel Diagnosis and Prognosis Biomarkers" Biomedicines 9, no. 8: 931. https://doi.org/10.3390/biomedicines9080931
APA StyleChang, Y.-C., Chan, M.-H., Li, C.-H., Fang, C.-Y., Hsiao, M., & Chen, C.-L. (2021). Exosomal Components and Modulators in Colorectal Cancer: Novel Diagnosis and Prognosis Biomarkers. Biomedicines, 9(8), 931. https://doi.org/10.3390/biomedicines9080931









