Systemic Corticosteroids and the Risk of Venous Thromboembolism in Patients with Severe COPD: A Nationwide Study of 30,473 Outpatients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Source
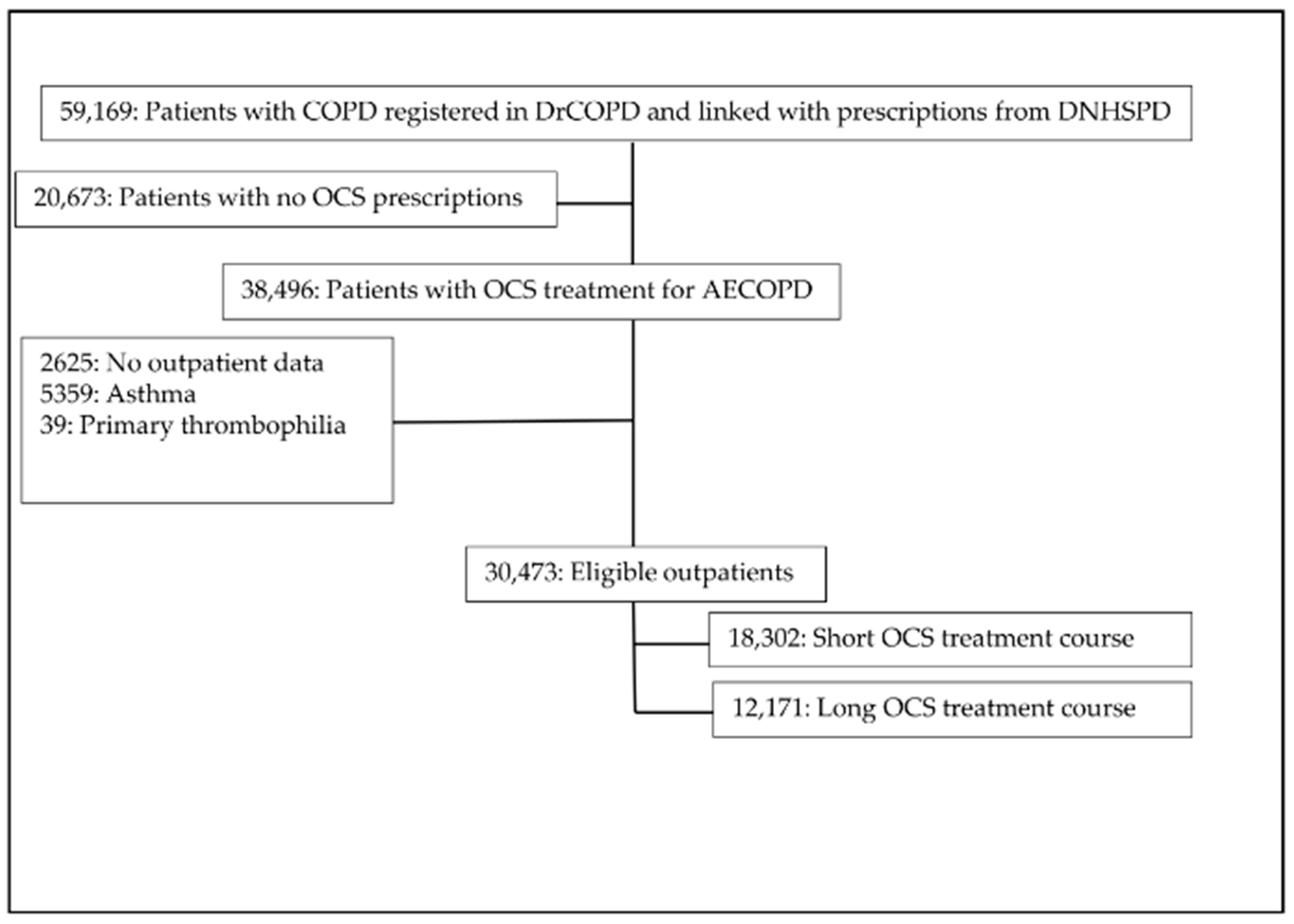
2.3. Study Participants
2.4. Variables
2.5. Statistical Analysis
3. Results
3.1. Descriptive Analyses
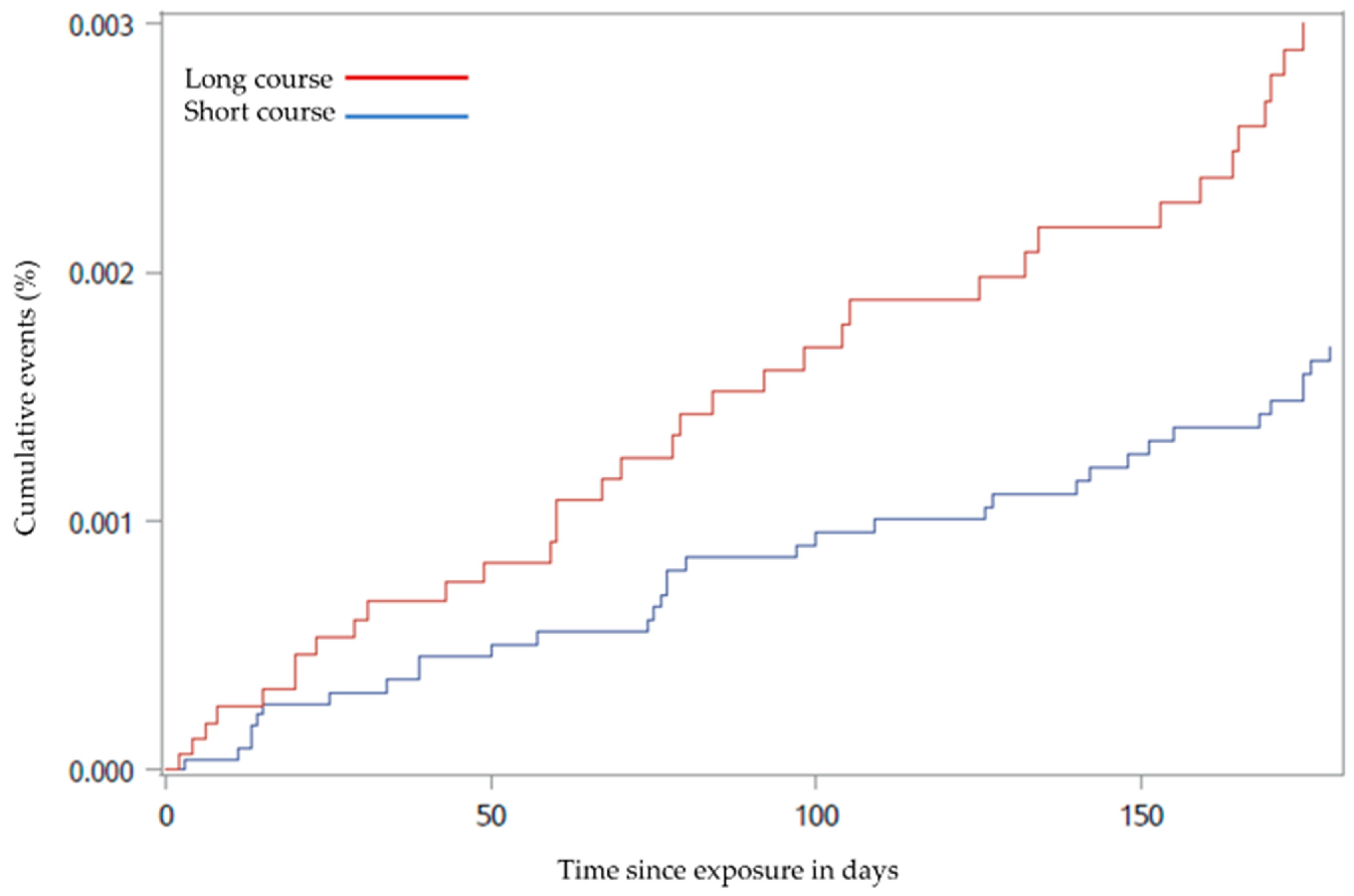
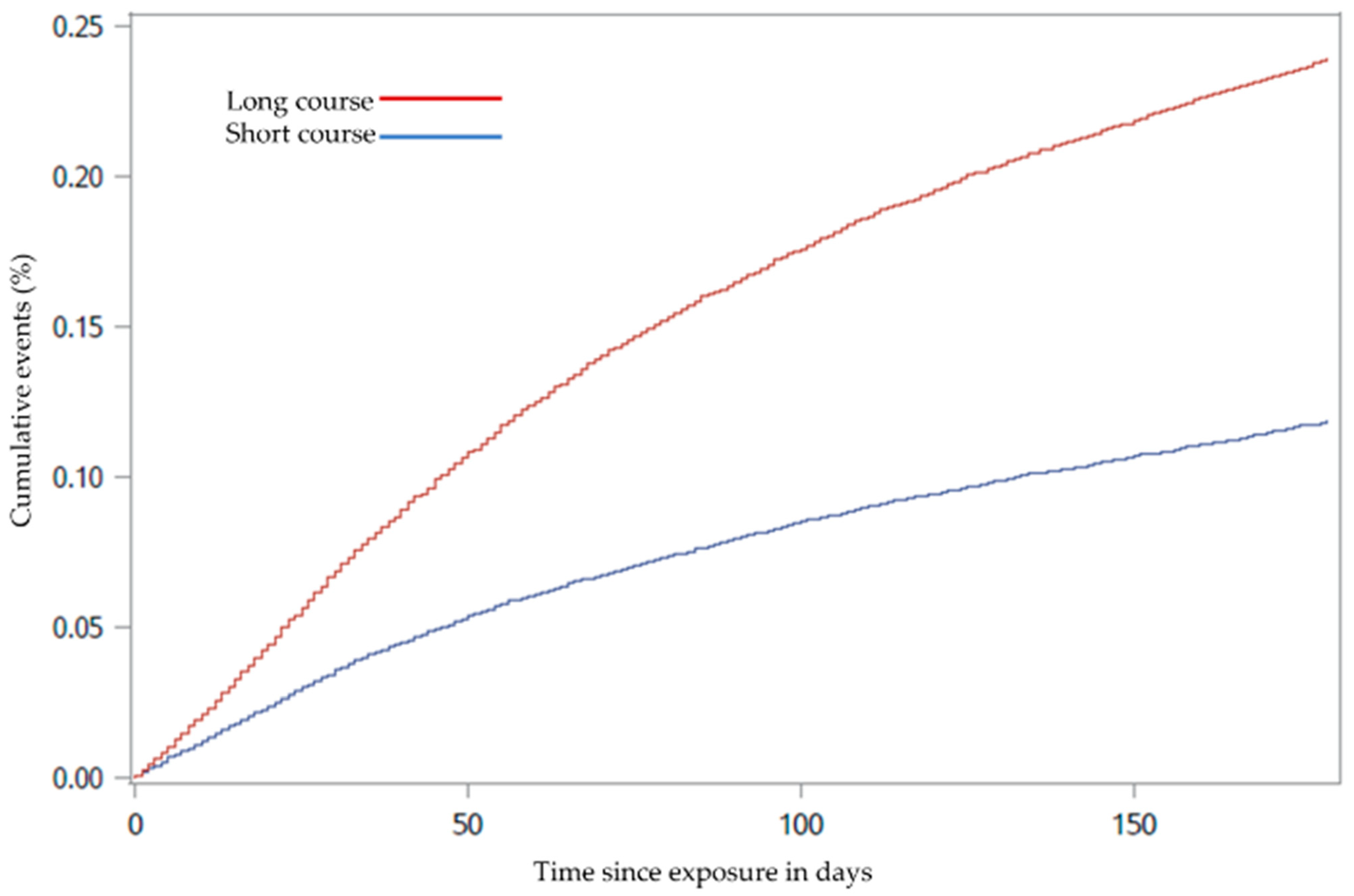
3.2. Statistical Analyses
3.2.1. Main Outcome Analysis
3.2.2. Sensitivity Analysis
4. Discussion
Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy For The Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, 2021 Report; p 106. Available online: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf (accessed on 20 July 2021).
- Leuppi, J.D.; Schuetz, P.; Bingisser, R.; Bodmer, M.; Briel, M.; Drescher, T.; Duerring, U.; Henzen, C.; Leibbrandt, Y.; Maier, S.; et al. Short-term vs. Conventional Glucocorticoid Therapy in Acute Exacerbations of Chronic Obstructive Pulmonary Disease. JAMA 2013, 309, 2223–2231. [Google Scholar] [CrossRef]
- Sivapalan, P.; Ingebrigtsen, T.S.; Rasmussen, D.B.; Sørensen, R.; Rasmussen, C.M.; Jensen, C.B.; Allin, K.; Eklöf, J.; Seersholm, N.; Vestbo, J.; et al. COPD exacerbations: The impact of long versus short courses of oral corticosteroids on mortality and pneumonia: Nationwide data on 67 000 patients with COPD followed for 12 months. BMJ Open Respir. Res. 2019, 6, e000407. [Google Scholar] [CrossRef] [PubMed]
- Waljee, A.K.; Rogers, M.; Lin, P.; Singal, A.G.; Stein, J.; Marks, R.M.; Ayanian, J.Z.; Nallamothu, B.K. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ 2017, 357, j1415. [Google Scholar] [CrossRef] [Green Version]
- Saeed, M.I.; Eklöf, J.; Achir, I.; Sivapalan, P.; Meteran, H.; Løkke, A.; Biering-Sørensen, T.; Knop, F.K.; Jensen, J.-U.S. Use of inhaled corticosteroids and the risk of developing type 2 diabetes in patients with chronic obstructive pulmonary disease. Diabetes Obes. Metab. 2020, 22, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Huerta, C.; Johansson, S.; Wallander, M.-A.; Rodríguez, L.A.G. Risk Factors and Short-term Mortality of Venous Thromboembolism Diagnosed in the Primary Care Setting in the United Kingdom. Arch. Intern. Med. 2007, 167, 935–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhassan, N.; Trepanier, M.; Sabapathy, C.; Chaudhury, P.; Liberman, A.S.; Charlebois, P.; Stein, B.L.; Lee, L. Risk factors for post-discharge venous thromboembolism in patients undergoing colorectal resection: A NSQIP analysis. Tech. Coloproctol. 2018, 22, 955–964. [Google Scholar] [CrossRef]
- Majoor, C.J.; Kamphuisen, P.W.; Zwinderman, A.H.; Brinke, A.T.; Amelink, M.; Rijssenbeek-Nouwens, L.; Sterk, P.J.; Büller, H.R.; Bel, E.H. Risk of deep vein thrombosis and pulmonary embolism in asthma. Eur. Respir. J. 2012, 42, 655–661. [Google Scholar] [CrossRef]
- Zöller, B.; PirouziFard, M.; Memon, A.A.; Sundquist, J.; Sundquist, K. Risk of pulmonary embolism and deep venous thrombosis in patients with asthma: A nationwide case−control study from Sweden. Eur. Respir. J. 2017, 49, 1601014. [Google Scholar] [CrossRef]
- Buchanan, I.A.; Lin, M.; Donoho, D.A.; Ding, L.; Giannotta, S.L.; Attenello, F.; Mack, W.J.; Liu, J.C. Venous Thromboembolism After Degenerative Spine Surgery: A Nationwide Readmissions Database Analysis. World Neurosurg. 2019, 125, e165–e174. [Google Scholar] [CrossRef]
- Kantar, R.S.; Haddad, A.G.; Tamim, H.; Jamali, F.; Taher, A.T. Venous thromboembolism and preoperative steroid use: Analysis of the NSQIP database to evaluate risk in surgical patients. Eur. J. Intern. Med. 2015, 26, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Aleva, F.E.; Voets, L.W.; Simons, S.O.; de Mast, Q.; van der Ven, A.J.; Heijdra, Y.F. Prevalence and Localization of Pulmonary Embolism in Unexplained Acute Exacerbations of COPD. Chest 2017, 151, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Rizkallah, J.; Man, S.F.P.; Sin, D.D. Prevalence of Pulmonary Embolism in Acute Exacerbations of COPD. Chest 2009, 135, 786–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zvezdin, B.; Milutinov, S.; Kojicic, M.V.; Hadnadjev, M.; Hromis, S.; Markovic, M.; Gajic, O. A Postmortem Analysis of Major Causes of Early Death in Patients Hospitalized With COPD Exacerbation. Chest 2009, 136, 376–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannesdottir, S.A.; Horváth-Puhó, E.; Dekkers, O.M.; Cannegieter, S.C.; Jørgensen, J.O.L.; Ehrenstein, V.; Vandenbroucke, J.P.; Pedersen, L.; Sørensen, H.T. Use of Glucocorticoids and Risk of Venous Thromboembolism: A nationwide populationbased case-control study. JAMA Intern. Med. 2013, 173, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Lange, P.; Tøttenborg, S.S.; Sorknæs, A.D.; Andersen, J.S.; Søgaard, M.; Nielsen, H.; Thomsen, R.; Nielsen, K.A. Danish Register of chronic obstructive pulmonary disease. Clin. Epidemiol. 2016, 8, 673–678. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Schmidt, S.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sørensen, H.T. The Danish National Patient Registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannesdottir, S.A.; Horváth-Puhó, E.; Ehrenstein, V.; Schmidt, M.; Pedersen, L.; Sørensen, H. Existing data sources for clinical epidemiology: The Danish National Database of Reimbursed Prescriptions. Clin. Epidemiol. 2012, 4, 303–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacoby, R.C.; Owings, J.T.; Ortega, T.; Gosselin, R.; Feldman, E.C. Biochemical Basis for the Hypercoagulable State Seen in Cushing Syndrome. Arch. Surg. 2001, 136, 1003–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, L.J.; Dunn, M.E.; Allegret, V.; Bédard, C. Effect of prednisone administration on coagulation variables in healthy Beagle dogs. Veter-Clin. Pathol. 2011, 40, 426–434. [Google Scholar] [CrossRef]
- Sneeboer, M.M.; Majoor, C.J.; de Kievit, A.; Meijers, J.C.; van der Poll, T.; Kamphuisen, P.; Bel, E.H. Prothrombotic state in patients with severe and prednisolone-dependent asthma. J. Allergy Clin. Immunol. 2016, 137, 1727–1732. [Google Scholar] [CrossRef] [Green Version]
- Bertoletti, L.; Quenet, S.; Mismetti, P.; Hernández, L.; Martín-Villasclaras, J.; Tolosa, C.; Valdés, M.; Barrón, M.; Todolí, J.; Monreal, M.; et al. Clinical presentation and outcome of venous thromboembolism in COPD. Eur. Respir. J. 2011, 39, 862–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourmand, A.; Robinson, H.; Mazer-Amirshahi, M.; Pines, J.M. Pulmonary Embolism Among Patients With Acute Exacerbation Of Chronic Obstructive Pulmonary Disease: Implications For Emergency Medicine. J. Emerg. Med. 2018, 55, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Monreal, M.; Muñoz-Torrero, J.F.S.; Naraine, V.S.; Jiménez, D.; Soler, S.; Rabuñal, R.; Gallego, P. Pulmonary Embolism in Patients with Chronic Obstructive Pulmonary Disease or Congestive Heart Failure. Am. J. Med. 2006, 119, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.; Goel, N.; Gangar, M.J.; Zhao, H.; Ciccolella, D.E.; Silverman, E.K.; Crapo, J.D.; Criner, G.J. Risk Factors for Venous Thromboembolism in Chronic Obstructive Pulmonary Disease. Chronic Obstr. Pulm. Dis. J. COPD Found. 2014, 1, 239–249. [Google Scholar] [CrossRef]
- Gunen, H.; Gulbas, G.; In, E.; Yetkin, O.; Hacievliyagil, S.S. Venous thromboemboli and exacerbations of COPD. Eur. Respir. J. 2009, 35, 1243–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillie-Leblond, I.; Marquette, C.-H.; Perez, T.; Scherpereel, A.; Zanetti, C.; Tonnel, A.-B.; Remy-Jardin, M. Pulmonary Embolism in Patients with Unexplained Exacerbation of Chronic Obstructive Pulmonary Disease: Prevalence and Risk Factors. Ann. Intern. Med. 2006, 144, 390–396. [Google Scholar] [CrossRef]
- Couturaud, F.; Bertoletti, L.; Pastre, J.; Roy, P.-M.; Le Mao, R.; Gagnadoux, F.; Paleiron, N.; Schmidt, J.; Sanchez, O.; De Magalhaes, E.; et al. Prevalence of Pulmonary Embolism Among Patients With COPD Hospitalized With Acutely Worsening Respiratory Symptoms. JAMA 2021, 325, 59–68. [Google Scholar] [CrossRef]
- Stuijver, D.J.F.; Majoor, C.; van Zaane, B.; Souverein, P.; de Boer, A.; Dekkers, O.; Büller, H.R.; Gerdes, V.E.A. Use of Oral Glucocorticoids and the Risk of Pulmonary Embolism. Chest 2013, 143, 1337–1342. [Google Scholar] [CrossRef]
- Lieber, B.A.; Han, J.; Appelboom, G.; Taylor, B.E.; Han, B.; Agarwal, N.; Connolly, E.S. Association of Steroid Use with Deep Venous Thrombosis and Pulmonary Embolism in Neurosurgical Patients: A National Database Analysis. World Neurosurg. 2016, 89, 126–132. [Google Scholar] [CrossRef]
- Torres-Macho, J.; Mancebo-Plaza, A.B.; Crespo-Giménez, A.; de Barros, M.R.S.; Bibiano-Guillén, C.; Fallos-Martí, R.; Calderón-Parra, J.; de Miguel-Yanes, J.M. Clinical features of patients inappropriately undiagnosed of pulmonary embolism. Am. J. Emerg. Med. 2013, 31, 1646–1650. [Google Scholar] [CrossRef] [PubMed]
- Pineda, L.A.; Hathwar, V.S.; Grant, B.J. Clinical suspicion of fatal pulmonary embolism. Chest 2001, 120, 791–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| N (Number of Participants) | Total (n = 30,473) | Short OCS Treatment Course (n = 18,302) | Long OCS Treatment Course (n = 12,171) |
|---|---|---|---|
| Age, years, median (IQR) | 73 (66–80) | 73 (66–80) | 73 (66–79) |
| Age | |||
| ≤62 years | 6275 (20.6%) | 3781 (20.7%) | 2494 (20.5%) |
| 63–70 years | 8081 (26.5%) | 4713 (25.8%) | 3368 (27.7%) |
| 71–77 years | 7977 (26.2%) | 4745 (25.9%) | 3232 (26.6%) |
| ≥78 years | 8140 (26.7%) | 5063 (27.7%) | 3077 (25.3%) |
| Sex | |||
| Female | 16,074 (52.7%) | 9830 (53.7%) | 6244 (51.3%) |
| Male | 14,399 (47.3%) | 8472 (46.3%) | 5927 (48.7) |
| Smoking | |||
| Active | 11,553 (37.9%) | 7155 (39.1%) | 4398 (36.1%) |
| Former/never smoker | 18,219 (59.8%) | 10,702 (58.5%) | 7517 (61.8%) |
| Missing value | 701 (2.3%) | 445 (2.4%) | 256 (2.1%) |
| BMI, kg/m2, median (IQR) | 25 (21–29) | 25 (21–29) | 24 (21–28) |
| BMI | |||
| ≤18.4 kg/m2 | 3683 (12.1%) | 2098 (11.5%) | 1585 (13.0%) |
| 18.5–24.9 kg/m2 | 12,158 (39.9%) | 7142 (39.0%) | 5016 (41.2%) |
| 25–30 kg/m2 | 8291 (27.2%) | 5093 (27.8%) | 3198 (26.3%) |
| >30 kg/m2 | 6341 (20.8%) | 3969 (21.7%) | 2372 (19.5%) |
| FEV1 | |||
| ≥80% | 1211 (3.9%) | 760 (4.2%) | 451 (3.7%) |
| <80–50% | 9989 (32.8%) | 6405 (35.0%) | 3584 (29.4%) |
| <50–30% | 10,906 (35.8%) | 6561 (35.8%) | 4345 (35.7%) |
| <30% | 8367 (27.5%) | 4576 (25.0%) | 3791 (31.1%) |
| Exacerbations 12 months prior to baseline | 0 | 10,947 (59.8%) | 6080 (50.0%) |
| 1 | 2798 (15.3%) | 2186 (18.0%) | |
| ≥2 | 4557 (24.9%) | 3905 (32.1%) | |
| Total dose of oral corticosteroids | 250 (12.5–250) | 500 (500–7500) | |
| Comorbidities * | |||
| Pulmonary embolism | 1284 (4.2%) | 716 (3.9%) | 568 (4.7%) |
| Deep vein thrombosis | 962 (3.2%) | 566 (3.1%) | 396 (3.3%) |
| Inflammatory Bowel disease | 581 (1.9%) | 303 (1.7%) | 278 (2.3%) |
| Malignancy | 5633 (18.5%) | 2829 (15.5%) | 2804 (23.0%) |
| Diabetes mellitus | 3854 (12.6%) | 2322 (12.7%) | 1532 (12.6%) |
| Hypertension | 10,791 (35.4%) | 6517 (35.6%) | 4274 (35.1%) |
| Myocardial infarction | 2533 (8.3%) | 1506 (8.2%) | 1027 (8.4%) |
| Peripheral vascular disease | 3837 (12.5%) | 2354 (12.9%) | 1483 (12.2%) |
| Cerebrovascular disease | 3065 (10.1%) | 1837 (10.0%) | 1228 (10.1%) |
| Renal failure | 1690 (5.5%) | 996 (5.4%) | 694 (5.7%) |
| Heart failure | 5094 (16.7%) | 3008 (16.4%) | 2086 (17.1%) |
| Depression | 1072 (3.5%) | 672 (3.7%) | 400 (3.3%) |
| Atrial fibrillation | 5773 (18.9%) | 3397 (18.6%) | 2376 (19.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastoder, E.; Sivapalan, P.; Eklöf, J.; Saeed, M.I.; Jordan, A.S.; Meteran, H.; Tønnesen, L.; Biering-Sørensen, T.; Løkke, A.; Seersholm, N.; et al. Systemic Corticosteroids and the Risk of Venous Thromboembolism in Patients with Severe COPD: A Nationwide Study of 30,473 Outpatients. Biomedicines 2021, 9, 874. https://doi.org/10.3390/biomedicines9080874
Rastoder E, Sivapalan P, Eklöf J, Saeed MI, Jordan AS, Meteran H, Tønnesen L, Biering-Sørensen T, Løkke A, Seersholm N, et al. Systemic Corticosteroids and the Risk of Venous Thromboembolism in Patients with Severe COPD: A Nationwide Study of 30,473 Outpatients. Biomedicines. 2021; 9(8):874. https://doi.org/10.3390/biomedicines9080874
Chicago/Turabian StyleRastoder, Ema, Pradeesh Sivapalan, Josefin Eklöf, Mohamad Isam Saeed, Alexander Svorre Jordan, Howraman Meteran, Louise Tønnesen, Tor Biering-Sørensen, Anders Løkke, Niels Seersholm, and et al. 2021. "Systemic Corticosteroids and the Risk of Venous Thromboembolism in Patients with Severe COPD: A Nationwide Study of 30,473 Outpatients" Biomedicines 9, no. 8: 874. https://doi.org/10.3390/biomedicines9080874
APA StyleRastoder, E., Sivapalan, P., Eklöf, J., Saeed, M. I., Jordan, A. S., Meteran, H., Tønnesen, L., Biering-Sørensen, T., Løkke, A., Seersholm, N., Lynghøj Nielsen, T., Carlsen, J., Janner, J., Godtfredsen, N., Bodtger, U., Laursen, C. B., Hilberg, O., Knop, F. K., Priemé, H., ... Stæhr Jensen, J. U. (2021). Systemic Corticosteroids and the Risk of Venous Thromboembolism in Patients with Severe COPD: A Nationwide Study of 30,473 Outpatients. Biomedicines, 9(8), 874. https://doi.org/10.3390/biomedicines9080874








