What Do We Know about Thromboprophylaxis and Its Monitoring in Critically Ill Patients?
Abstract
:1. Introduction
2. Thromboprophylaxis in Critically Ill Patients: Clinical Data
3. LMWH: Schema of Thromboprophylaxis
Head-to-Head Comparison of Once- or Twice-Daily LMWH Injections
4. Monitoring of Thromboprophylaxis: Peak or Trough or Peak and Trough?
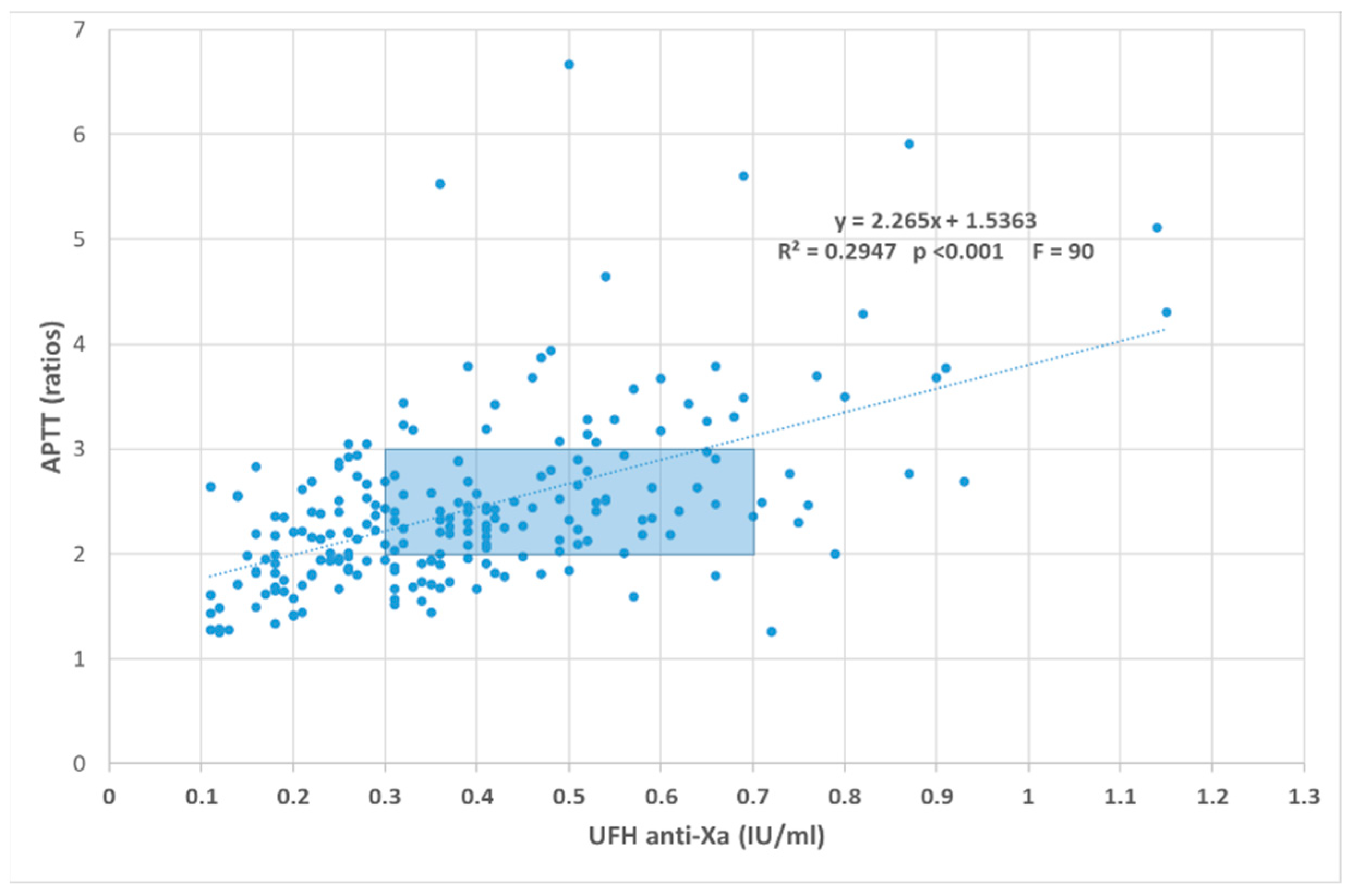
5. UFH in ICU
5.1. UFH Monitoring and APTT: The Too-Much Job?
5.2. UFH Monitoring and Anti-Xa Activity
5.3. Heparin Resistance and AT and Dextran Discussions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spyropoulos, A.C.; Anderson, F.A., Jr.; FitzGerald, G.; Decousus, H.; Pini, M.; Chong, B.H.; Zotz, R.B.; Bergmann, J.F.; Tapson, V.; Froehlich, J.B.; et al. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest 2011, 140, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.C.; Cortes, J.; Altshuler, D.; Papadopoulos, J. Venous Thromboembolism Prophylaxis: A Narrative Review with a Focus on the High-Risk Critically Ill Patient. J. Intensiv. Care Med. 2019, 34, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.F.; Wong, C.A.; Milligan, P.E.; Thoelke, M.S.; Woeltje, K.F.; Gage, B.F. Risk factors for inpatient venous thromboembolism despite thromboprophylaxis. Thromb. Res. 2014, 133, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. Coagulopathy of Coronavirus Disease 2019. Crit Care Med. 2020, 48, 1358–1364. [Google Scholar] [CrossRef]
- Samama, M.M.; Cohen, A.T.; Darmon, J.Y.; Desjardins, L.; Eldor, A.; Janbon, C.; Leizorovicz, A.; Nguyen, H.; Olsson, C.G.; Turpie, A.G.; et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N. Engl. J. Med. 1999, 341, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.-L. Give your patient a fast hug (at least) once a day*. Crit. Care Med. 2005, 33, 1225–1229. [Google Scholar] [CrossRef]
- Lauzier, F.; Muscedere, J.; Deland, E.; Kutsogiannis, D.J.; Jacka, M.; Heels-Ansdell, D.; Crowther, M.; Cartin-Ceba, R.; Cox, M.J.; Zytaruk, N.; et al. Thromboprophylaxis patterns and determinants in critically ill patients: A multicenter audit. Crit. Care 2014, 18, R82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group; Cook, D.; Meade, M.; Guyatt, G.; Walter, S.; Heels-Ansdell, D.; Warkentin, T.E.; Zytaruk, N.; Crowther, M.; Geerts, W.; et al. Dalteparin versus Unfractionated Heparin in Critically Ill Patients. N. Engl. J. Med. 2011, 364, 1305–1314. [Google Scholar] [CrossRef] [Green Version]
- Fowler, R.A.; Mittmann, N.; Geerts, W.; Heels-Ansdell, D.; Gould, M.K.; Guyatt, G.; Krahn, M.; Finfer, S.; Pinto, R.; Chan, B.; et al. Cost-effectiveness of Dalteparin vs Unfractionated Heparin for the Prevention of Venous Thromboembolism in Critically Ill Patients. JAMA 2014, 312, 2135–2145. [Google Scholar] [CrossRef]
- Pai, M.; Adhikari, N.K.J.; Ostermann, M.; Heels-Ansdell, D.; Douketis, J.D.; Skrobik, Y.; Qushmaq, I.; Meade, M.; Guyatt, G.; Geerts, W.; et al. Low-molecular-weight heparin venous thromboprophylaxis in critically ill patients with renal dysfunction: A subgroup analysis of the PROTECT trial. PLoS ONE 2018, 13, e0198285. [Google Scholar] [CrossRef]
- Beitland, S.; Wimmer, H.; Lorentsen, T.; Jacobsen, D.; Draegni, T.; Brunborg, C.; Kløw, N.E.; Sandset, P.M.; Sunde, K. Venous thromboembolism in the critically ill: A prospective observational study of occurrence, risk factors and outcome. Acta Anaesthesiol. Scand. 2019, 63, 630–638. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Mi, J.; Wang, X.; Zou, Y.; Chen, X.; Nie, Z.; Luo, X.; Gan, R. The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine 2019, 98, e15833. [Google Scholar] [CrossRef]
- Hamada, S.R.; Espina, C.; Guedj, T.; Buaron, R.; Harrois, A.; Figueiredo, S.; Duranteau, J. High level of venous thromboembolism in critically ill trauma patients despite early and well-driven thromboprophylaxis protocol. Ann. Intensiv. Care 2017, 7, 97. [Google Scholar] [CrossRef]
- Hanify, J.M.; Dupree, L.H.; Johnson, D.W.; Ferreira, J.A. Failure of chemical thromboprophylaxis in critically ill medical and surgical patients with sepsis. J. Crit. Care 2017, 37, 206–210. [Google Scholar] [CrossRef]
- Wang, T.F.; Milligan, P.; Wong, C.A.; Deal, E.; Thoelke, M.S.; Gage, B.F. Efficacy and safety of high-dose thromboprophylaxis in morbidly obese inpatients. Thromb. Haemost. 2014, 111, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Lim, W.; Meade, M.; Lauzier, F.; Zarychanski, R.; Mehta, S.; Lamontagne, F.; Dodek, P.; McIntyre, L.; Hall, R.; Heels-Ansdell, D.; et al. Failure of Anticoagulant Thromboprophylaxis: Risk factors in medical-surgical critically ill patients*. Crit. Care Med. 2015, 43, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Burns, K.E.A.; Alsolamy, S.J.; Alshahrani, M.S.; Al-Hameed, F.M.; Arshad, Z.; Almaani, M.; Hawa, H.; Mandourah, Y.; Almekhlafi, G.A.; et al. Surveillance or no surveillance ultrasonography for deep vein thrombosis and outcomes of critically ill patients: A pre-planned sub-study of the PREVENT trial. Intensiv. Care Med. 2020, 46, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Lopaciuk, S.; Bielska-Falda, H.; Noszczyk, W.; Bielawiec, M.; Witkiewicz, W.; Filipecki, S.; Michalak, J.; Ciesielski, L.; Mackiewicz, Z.; Czestochowska, E.; et al. Low molecular weight heparin versus acenocoumarol in the secondary prophylaxis of deep vein thrombosis. Thromb. Haemost. 1999, 81, 26–31. [Google Scholar]
- Pelzer, U.; Opitz, B.; Deutschinoff, G.; Stauch, M.; Reitzig, P.C.; Hahnfeld, S.; Müller, L.; Grunewald, M.; Stieler, J.M.; Sinn, M.; et al. Efficacy of Prophylactic Low–Molecular Weight Heparin for Ambulatory Patients with Advanced Pancreatic Cancer: Outcomes From the CONKO-004 Trial. J. Clin. Oncol. 2015, 33, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.I.; Roopkumar, J.; Puligandla, M.; Schlechter, B.L.; Sharda, A.V.; Peereboom, D.; Joyce, R.; Bockorny, B.; Neuberg, D.; Bauer, K.A.; et al. Dose-adjusted enoxaparin thromboprophylaxis in hospitalized cancer patients: A randomized, double-blinded multicenter phase 2 trial. Blood Adv. 2020, 4, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Piagnerelli, M.; Cauchie, P.; Wautrecht, J.C. Optimizing the Risk-Benefit Balance of Thromboprophylaxis in Critically Ill Patients with Coronavirus Disease 2019. Crit. Care Med. 2020, 48, e988–e989. [Google Scholar] [CrossRef]
- Borkgren-Okonek, M.J.; Hart, R.W.; Pantano, J.E.; Rantis, P.C.; Guske, P.J.; Kane, J.M., Jr.; Gordon, N.; Sambol, N.C. Enoxaparin thromboprophylaxis in gastric bypass patients: Extended duration, dose stratification, and antifactor Xa activity. Surg. Obes. Relat. Dis. 2008, 4, 625–631. [Google Scholar] [CrossRef]
- Ludwig, K.P.; Simons, H.J.; Mone, M.C.; Barton, R.G.; Kimball, E.J. Implementation of an Enoxaparin Protocol for Venous Thromboembolism Prophylaxis in Obese Surgical Intensive Care Unit Patients. Ann. Pharmacother. 2011, 45, 1356–1362. [Google Scholar] [CrossRef]
- Bickford, A.; Majercik, S.; Bledsoe, J.; Smith, K.; Johnston, R.; Dickerson, J.; White, T. Weight-based enoxaparin dosing for venous thromboembolism prophylaxis in the obese trauma patient. Am. J. Surg. 2013, 206, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.B.; Majercik, S.; Sorensen, J.; Woller, S.C.; Stevens, S.M.; White, T.W.; Morris, D.S.; Baldwin, M.; Bledsoe, J.R. Weight-based enoxaparin dosing and deep vein thrombosis in hospitalized trauma patients: A double-blind, randomized, pilot study. Surgery 2018, 164. [Google Scholar] [CrossRef]
- Farkas, J. Mythbusting 40 Mg Enoxaparin Daily for DVT Prophylaxis in Critical Illness. 2020. Available online: https://emcrit.org/pulmcrit/40-enoxaparin/ (accessed on 21 March 2021).
- Bhutia, S.; Wong, P.F. Once versus twice daily low molecular weight heparin for the initial treatment of venous thromboembolism. Cochrane Database Syst. Rev. 2013, 7, CD003074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trujillo-Santos, J.; Bergmann, J.F.; Bortoluzzi, C.; López-Reyes, R.; Giorgi-Pierfranceschi, M.; López-Sáez, J.B.; Ferrazzi, P.; Bascuñana, J.; Suriñach, J.M.; Monreal, M. Once versus twice daily enoxaparin for the initial treatment of acute venous thromboembolism. J. Thromb. Haemost. 2017, 15, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Pannucci, C.J.; Hunt, M.M.; Fleming, K.I.; Prazak, A.M. Weight-Based Dosing for Once-Daily Enoxaparin Cannot Provide Adequate Anticoagulation for Venous Thromboembolism Prophylaxis. Plast. Reconstr. Surg. 2017, 140, 815–822. [Google Scholar] [CrossRef]
- Stephenson, M.L.; Serra, A.E.; Neeper, J.M.; Caballero, D.C.; McNulty, J. A randomized controlled trial of differing doses of postcesarean enoxaparin thromboprophylaxis in obese women. J. Perinatol. 2015, 36, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Huang, E.; Waller, J.; White, C.; Martinez-Quinones, P.; Robinson, T. Achievement of goal anti-Xa activity with weight-based enoxaparin dosing for venous thromboembolism prophylaxis in trauma patients. Pharmacotherapy 2021, 41, 508–514. [Google Scholar] [CrossRef]
- Samama, C.M.; Afshari, A. European guidelines on perioperative venous thromboembolism prophylaxis. Eur. J. Anaesthesiol. 2018, 35, 73–76. [Google Scholar] [CrossRef]
- Robinson, S.; Zincuk, A.; Larsen, U.L.; Ekstrøm, C.; Nybo, M.; Rasmussen, B.; Toft, P. A comparative study of varying doses of enoxaparin for thromboprophylaxis in critically ill patients: A double-blinded, randomised controlled trial. Crit. Care 2013, 17, R75. [Google Scholar] [CrossRef] [Green Version]
- Kopelman, T.R.; O’Neill, P.J.; Pieri, P.G.; Salomone, J.P.; Hall, S.T.; Quan, A.; Wells, J.R.; Pressman, M.S. Alternative dosing of prophylactic enoxaparin in the trauma patient: Is more the answer? Am. J. Surg. 2013, 206, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.N.; Planes, A.; Hirsh, J.; Goodyear, M.; Vochelle, N.; Gent, M. done to prevent deep vein thrombosis after hip replacement. Thromb. Haemost. 1989, 62, 940–944. [Google Scholar]
- Dhillon, N.K.; Smith, E.J.; Gillette, E.; Mason, R.; Barmparas, G.; Gewertz, B.L.; Ley, E.J. Trauma patients with lower extremity and pelvic fractures: Should anti-factor Xa trough level guide prophylactic enoxaparin dose? Int. J. Surg. 2018, 51, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.; Harada, M.Y.; Barmparas, G.; Chung, K.; Mason, R.; Yim, D.A.; Dhillon, N.; Margulies, D.R.; Gewertz, B.L.; Ley, E.J. Association between Enoxaparin Dosage Adjusted by Anti–Factor Xa Trough Level and Clinically Evident Venous Thromboembolism After Trauma. JAMA Surg. 2016, 151, 1006–1013. [Google Scholar] [CrossRef] [Green Version]
- Malinoski, D.; Jafari, F.; Ewing, T.; Ardary, C.; Conniff, H.; Baje, M.; Kong, A.; Lekawa, M.E.; Dolich, M.O.; Cinat, M.E.; et al. Standard Prophylactic Enoxaparin Dosing Leads to Inadequate Anti-Xa Levels and Increased Deep Venous Thrombosis Rates in Critically Ill Trauma and Surgical Patients. J. Trauma. 2010, 68, 874–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goland, S.; Schwartzenberg, S.; Fan, J.; Kozak, N.; Khatri, N.; Elkayam, U. Monitoring of Anti-Xa in Pregnant Patients With Mechanical Prosthetic Valves Receiving Low-Molecular-Weight Heparin: Peak or Trough Levels? J. Cardiovasc. Pharmacol. Ther. 2014, 19, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Berresheim, M.; Wilkie, J.; Nerenberg, K.A.; Ibrahim, Q.; Bungard, T.J. A case series of LMWH use in pregnancy: Should trough anti-Xa levels guide dosing? Thromb. Res. 2014, 134, 1234–1240. [Google Scholar] [CrossRef]
- Lin, A.; Vazquez, S.R.; Jones, A.E.; Witt, D.M. Description of anti-Xa monitoring practices during low molecular weight heparin use. J. Thromb. Thrombolysis 2019, 48, 623–628. [Google Scholar] [CrossRef]
- Hollestelle, M.J.; van der Meer, F.J.; Meijer, P. Quality performance for indirect Xa inhibitor monitoring in patients using international external quality data. Clin. Chem. Lab. Med. 2020, 58, 1921–1930. [Google Scholar] [CrossRef]
- King, C.S.; Holley, A.B.; Jackson, J.L.; Shorr, A.F.; Moores, L.K. Twice vs Three Times Daily Heparin Dosing for Thromboembolism Prophylaxis in the General Medical Population. Chest 2007, 131, 507–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, M.F.; Rosborough, T.K.; Schwartz, M.L. Heparin Thromboprophylaxis in Gastric Bypass Surgery. Obes. Surg. 2003, 13, 249–253. [Google Scholar] [CrossRef]
- Shepherd, M.F.; Rosborough, T.K.; Schwartz, M.L. Unfractionated heparin infusion for thromboprophylaxis in highest risk gastric bypass surgery. Obes. Surg. 2004, 14, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.P.; Delate, T.; Cleary, S.J.; Witt, D.M. Unfractionated Heparin Dose Requirements Targeting Intermediate Intensity Antifactor Xa Concentration During Pregnancy. Pharmacotherapy 2010, 30, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Marlar, R.A.; Clement, B.; Gausman, J. Activated Partial Thromboplastin Time Monitoring of Unfractionated Heparin Therapy: Issues and Recommendations. Semin. Thromb. Hemost. 2017, 43, 253–260. [Google Scholar] [CrossRef]
- Frère, C.; Philip-Joët, C.; Valadier, J.; Morange, P.-E.; Juhan-Vague, I.; Alessi, M.; Aillaud, M.-F. Evaluation du STA-Cephasreen (Diagnostica Stago), nouveau réactif liquide prêt à l’emploi pour le Temps de Céphaline + Activateur (TCA). Spectra Biol. 2006, 153, 20–22. [Google Scholar]
- Streng, A.S.; Delnoij, T.S.; Mulder, M.M.; Sels, J.W.E.; Wetzels, R.J.; Verhezen, P.W.; Olie, R.H.; Kooman, J.P.; Van Kuijk, S.M.; Brandts, L.; et al. Monitoring of Unfractionated Heparin in Severe COVID-19: An Observational Study of Patients on CRRT and ECMO. TH Open 2020, 4, e365–e375. [Google Scholar] [CrossRef]
- Favresse, J.; Lardinois, B.; Sabor, L.; Devalet, B.; Vandepapeliere, J.; Braibant, M.; Lessire, S.; Chatelain, B.; Jacqmin, H.; Douxfils, J.; et al. Evaluation of the DOAC-Stop® Procedure to Overcome the Effect of DOACs on Several Thrombophilia Screening Tests. TH Open 2018, 2, e202–e209. [Google Scholar] [CrossRef] [Green Version]
- Farkh, C.; Ellouze, S.; Gounelle, L.; Houari, M.S.; Duchemin, J.; Proulle, V.; Fontenay, M.; Delavenne, X.; Jourdi, G. A Diagnostic Solution for Lupus Anticoagulant Testing in Patients Taking Direct Oral FXa Inhibitors Using DOAC Filter. Front. Med. 2021, 8, 683357. [Google Scholar] [CrossRef]
- Lessire, S.; Douxfils, J.; Pochet, L.; Dincq, A.S.; Larock, A.S.; Gourdin, M.; Dogné, J.M.; Chatelain, B.; Mullier, F. Estimation of Rivaroxaban Plasma Concentrations in the Perioperative Setting in Patients with or Without Heparin Bridging. Clin. Appl. Thromb. Hemost. 2018, 24, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Isgrò, G.; Cazzaniga, A.; Ditta, A.; Boncilli, A.; Cotza, M.; Carboni, G.; Brozzi, S. Different patterns of heparin resistance: Therapeutic implications. Perfusion 2002, 17, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Lehman, C.M.; Rettmann, J.A.; Wilson, M.L.W.; Markewitz, B.A. Comparative Performance of Three Anti–Factor Xa Heparin Assays in Patients in a Medical Intensive Care Unit Receiving Intravenous, Unfractionated Heparin. Am. J. Clin. Pathol. 2006, 126, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Ignjatovic, V.; Summerhayes, R.; Gan, A.; Than, J.; Chan, A.; Cochrane, A.; Bennett, M.; Horton, S.; Shann, F.; Lane, G.; et al. Monitoring Unfractionated Heparin (UFH) therapy: Which Anti Factor Xa assay is appropriate? Thromb. Res. 2007, 120, 347–351. [Google Scholar] [CrossRef]
- Young, E.; Podor, T.J.; Venner, T.; Hirsh, J. Induction of the Acute-Phase Reaction Increases Heparin-Binding Proteins in Plasma. Arter. Thromb. Vasc. Biol. 1997, 17, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Mouton, C.; Calderon, J.; Janvier, G.; Vergnes, M.-C. Dextran sulfate included in factor Xa assay reagent overestimates heparin activity in patients after heparin reversal by protamine. Thromb. Res. 2003, 111, 273–279. [Google Scholar] [CrossRef]
- van Roessel, S.; Middeldorp, S.; Cheung, Y.W.; Zwinderman, A.H.; de Pont, A.C. Accuracy of aPTT monitoring in critically ill patients treated with unfractionated heparin. Net. J. Med. 2014, 72, 305–310. [Google Scholar]
- Uprichard, J.; Manning, R.A.; Laffan, M. Monitoring heparin anticoagulation in the acute phase response. Br. J. Haematol. 2010, 149, 613–619. [Google Scholar] [CrossRef]
- Toulon, P.; Smahi, M.; De Pooter, N. APTT therapeutic range for monitoring unfractionated heparin therapy. Significant impact of the anti-Xa reagent used for correlation. J. Thromb. Haemost. 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cauchie, P.; Piagnerelli, M. What Do We Know about Thromboprophylaxis and Its Monitoring in Critically Ill Patients? Biomedicines 2021, 9, 864. https://doi.org/10.3390/biomedicines9080864
Cauchie P, Piagnerelli M. What Do We Know about Thromboprophylaxis and Its Monitoring in Critically Ill Patients? Biomedicines. 2021; 9(8):864. https://doi.org/10.3390/biomedicines9080864
Chicago/Turabian StyleCauchie, Philippe, and Michael Piagnerelli. 2021. "What Do We Know about Thromboprophylaxis and Its Monitoring in Critically Ill Patients?" Biomedicines 9, no. 8: 864. https://doi.org/10.3390/biomedicines9080864
APA StyleCauchie, P., & Piagnerelli, M. (2021). What Do We Know about Thromboprophylaxis and Its Monitoring in Critically Ill Patients? Biomedicines, 9(8), 864. https://doi.org/10.3390/biomedicines9080864





