Stable Gastric Pentadecapeptide BPC 157 Therapy for Monocrotaline-Induced Pulmonary Hypertension in Rats Leads to Prevention and Reversal
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Drugs
2.3. Protocol
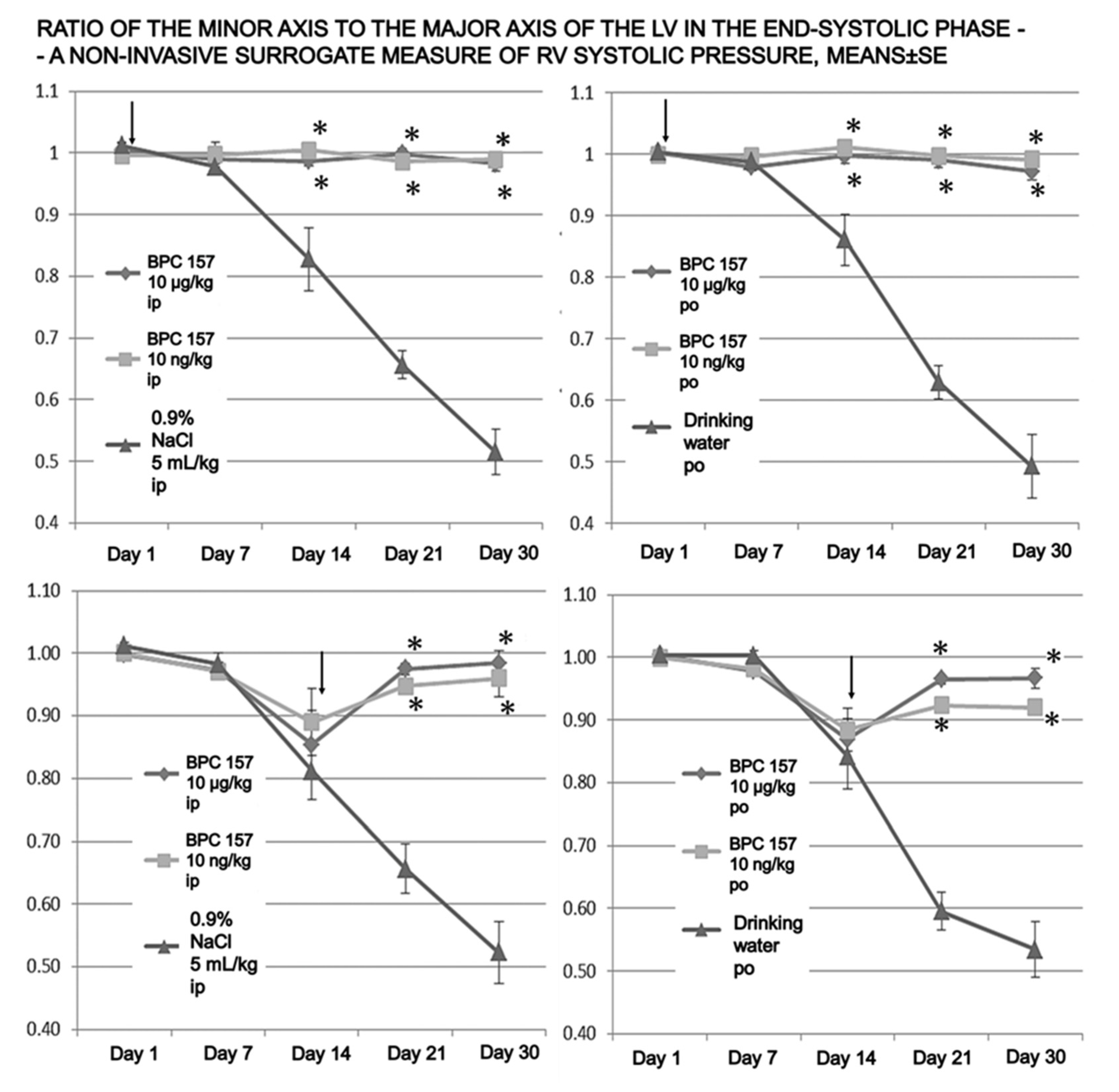
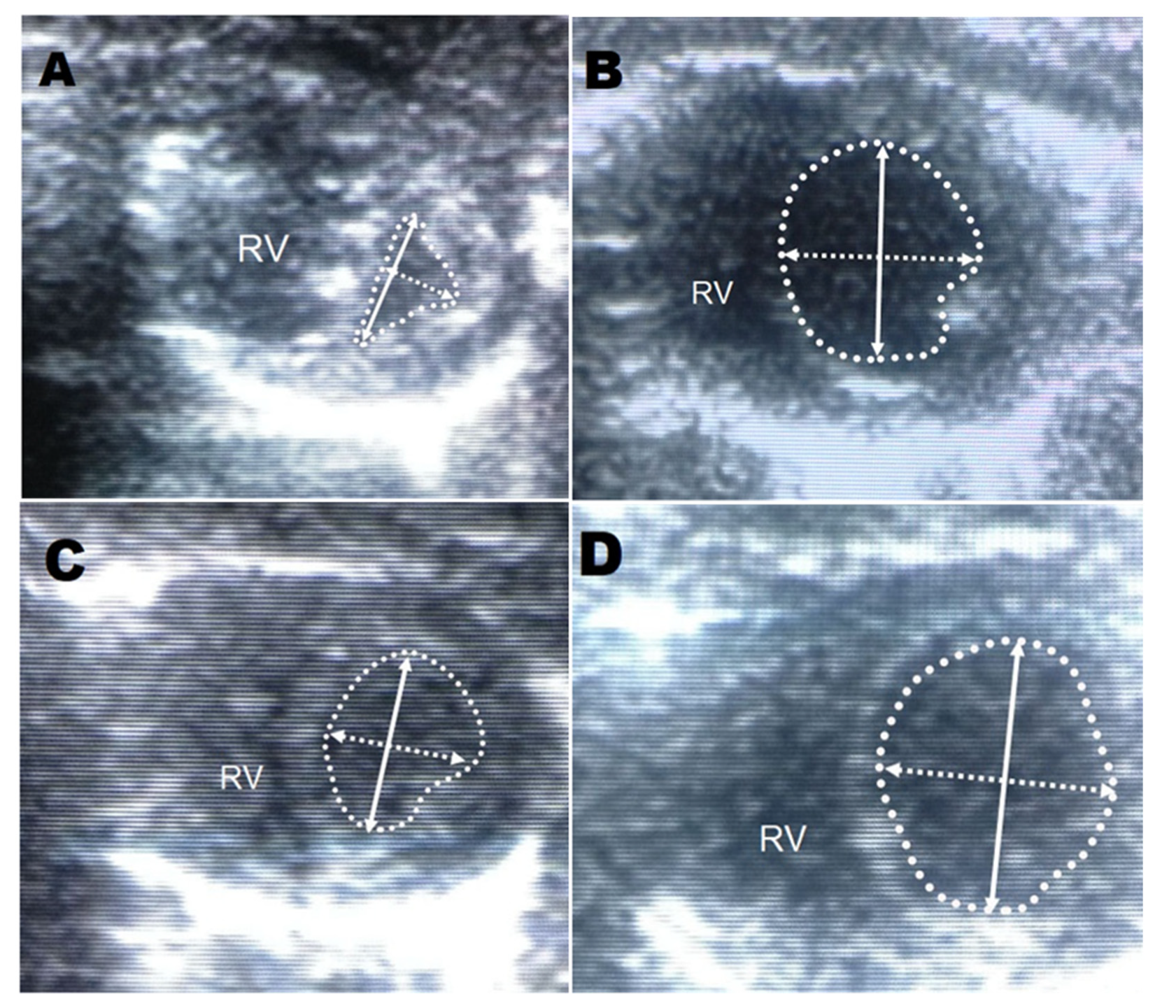
2.4. Echocardiography
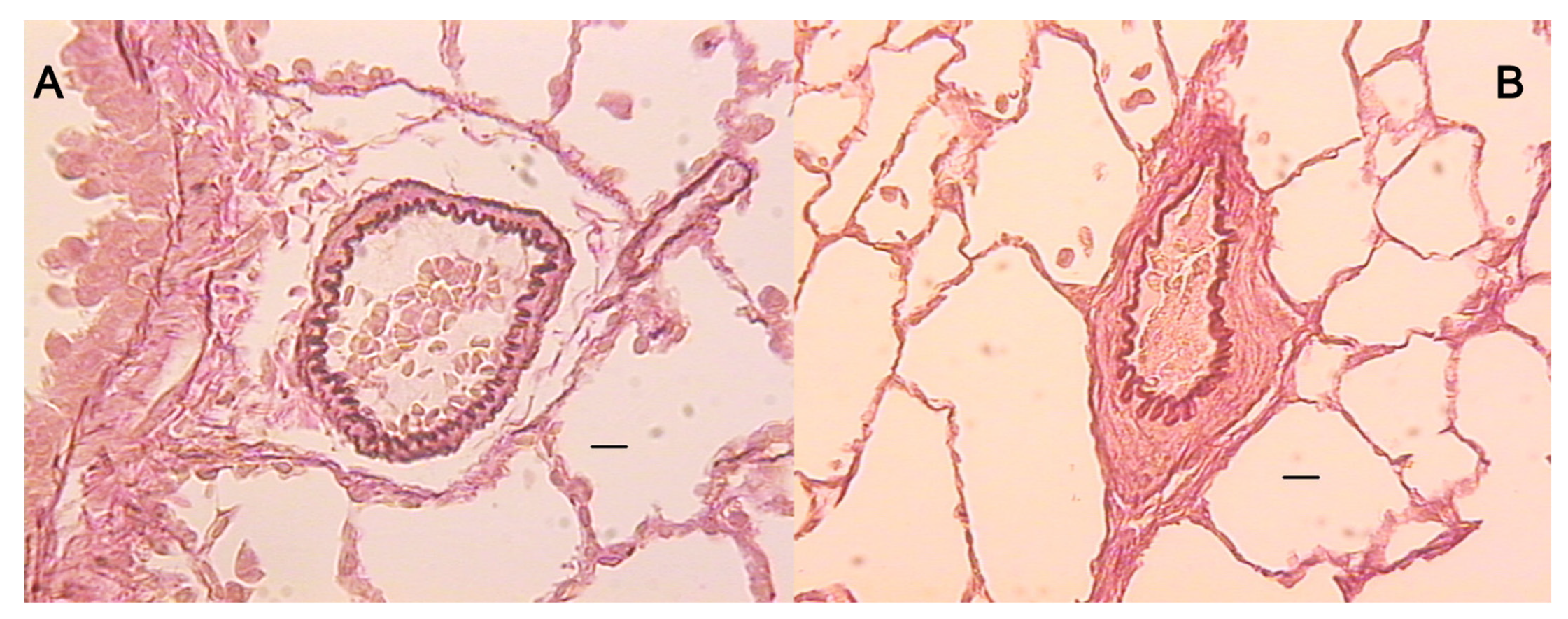
2.5. Microscopy
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sikiric, P.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. Novel cytoprotective mediator, stable gastric pentadecapeptide BPC 157. Vascular recruitment and gastrointestinal tract healing. Curr. Pharm. Des. 2018, 24, 1990–2001. [Google Scholar] [CrossRef]
- Seiwerth, S.; Brcic, L.; Vuletic, L.B.; Kolenc, D.; Aralica, G.; Misic, M.; Zenko, A.; Drmic, D.; Rucman, R.; Sikiric, P. BPC 157 and blood vessels. Curr. Pharm. Des. 2014, 20, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr. Pharm. Des. 2014, 20, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Hahm, K.B.; Blagaic, A.B.; Tvrdeic, A.; Pavlov, K.H.; Petrovic, A.; Kokot, A.; Gojkovic, S.; Krezic, I.; Drmic, D.; et al. Stable Gastric Pentadecapeptide BPC 157, Robert’s stomach cytoprotection/adaptive cytoprotection/organoprotection, and Selye’s stress coping response: Progress, achievements, and the future. Gut Liver 2020, 14, 153–167. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Ilic, S.; Kolenc, D. Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr. Pharm. Des. 2010, 16, 1224–1234. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Drmic, D.; Stupnisek, M.; Kokot, A.; Sever, M.; Zoricic, I.; Zoricic, Z.; Batelja, L.; et al. Stress in Gastrointestinal tract and stable gastric pentadecapeptide BPC 157. Finally, do we have a solution? Curr. Pharm. Des. 2017, 23, 4012–4028. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Grgic, T.; Strbe, S.; Zukanovic, G.; Crvenkovic, D.; et al. Brain-gut axis and pentadecapeptide BPC 157: Theoretical and practical implications. Curr. Neuropharmacol. 2016, 14, 857–865. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157: Novel therapy in gastrointestinal tract. Curr. Pharm. Des. 2011, 17, 1612–1632. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Ilic, S.; et al. Focus on ulcerative colitis: Stable gastric pentadecapeptide BPC 157. Curr. Med. Chem. 2012, 19, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr. Pharm. Des. 2013, 19, 76–83. [Google Scholar]
- Seiwerth, S.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. BPC 157 and standard angiogenic growth factors. Gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr. Pharm. Des. 2018, 24, 1972–1989. [Google Scholar] [CrossRef] [PubMed]
- Gwyer, D.; Wragg, N.M.; Wilson, S.L. Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell. Tissue Res. 2019, 377, 153–159. [Google Scholar] [CrossRef]
- Kang, E.A.; Han, Y.M.; An, J.M.; Park, Y.J.; Sikiric, P.; Kim, D.H.; Kwon, K.A.; Kim, Y.J.; Yang, D.; Tchah, H.; et al. BPC157 as potential agent rescuing from cancer cachexia. Curr. Pharm. Des. 2018, 24, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, H.J.; Sikiric, P.; Hahm, K.B. BPC 157 rescued NSAID-cytotoxicity via stabilizing intestinal permeability and enhancing cytoprotection. Curr. Pharm. Des. 2020, 26, 2971–2981. [Google Scholar] [CrossRef]
- Fukumitsu, M.; Suzuki, K. Mesenchymal stem/stromal cell therapy for pulmonary arterial hypertension: Comprehensive review of preclinical studies. J. Cardiol. 2019, 74, 304–312. [Google Scholar] [CrossRef]
- Nogueira-Ferreira, R.; Vitorino, R.; Ferreira, R.; Henriques-Coelho, T. Exploring the monocrotaline animal model for the study of pulmonary arterial hypertension: A network approach. Pulm. Pharmacol. Ther. 2015, 35, 8–16. [Google Scholar] [CrossRef]
- Hessel, M.H.; Steendijk, P.; den Adel, B.; Schutte, C.I.; van der Laarse, A. Characterizationof right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, 2424–2430. [Google Scholar] [CrossRef]
- Wilson, D.W.; Segall, H.J.; Pan, L.C.; Lame, M.W.; Estep, J.E.; Morin, D. Mechanisms and pathology of monocrotaline pulmonary toxicity. Crit. Rev. Toxicol. 1992, 22, 307–325. [Google Scholar] [CrossRef]
- Szabo, S.; Trier, J.S.; Brown, A.; Schnoor, J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology 1985, 88, 228–236. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Petek, M.; Rucman, R.; Turkovic, B.; Rotkvic, I.; Jagic, V.; Duvnjak, M.; Mise, S.; et al. The beneficial effect of BPC 157, a 15 amino acid peptide BPC fragment, on gastric and duodenal lesions induced by restraint stress, cysteamine and 96% ethanol in rats. A comparative study with H2 receptor antagonists, dopamine promotors and gut peptides. Life Sci. 1994, 54, 63–68. [Google Scholar] [CrossRef]
- Temple, I.P.; Monfredi, O.; Quigley, G.; Schneider, H.; Zi, M.; Cartwright, E.J.; Boyett, M.R.; Mahadevan, V.S.; Hart, G. Macitentan treatment retards the progression of established pulmonary arterial hypertension in an animal model. Int. J. Cardiol. 2014, 177, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Clozel, M.; Hess, P.; Rey, M.; Iglarz, M.; Binkert, C.; Qiu, C. Bosentan, sildenafil, and their combination in the monocrotaline model of pulmonary hypertension in rats. Exp. Biol. Med. 2006, 231, 967–973. [Google Scholar]
- Mouchaers, K.T.; Schalij, I.; de Boer, M.A.; Postmus, P.E.; van Hinsbergh, V.W.; van Nieuw Amerongen, G.P.; Vonk Noordegraaf, A.; van der Laarse, W.J. Fasudil reduces monocrotaline-induced pulmonary arterial hypertension: Comparison with bosentan and sildenafil. Eur. Respir. J. 2010, 36, 800–807. [Google Scholar] [CrossRef]
- McMurtry, M.S.; Bonnet, S.; Michelakis, E.D.; Bonnet, S.; Haromy, A.; Archer, S.L. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: The rapamcyin-atorvastatin-simvastatin study. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, 933–940. [Google Scholar] [CrossRef]
- Hamidi, S.A.; Lin, R.Z.; Szema, A.M.; Lyubsky, S.; Jiang, Y.P.; Said, S.I. VIP and endothelin receptor antagonist: An effective combination against experimental pulmonary arterial hypertension. Respir. Res. 2011, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Iglarz, M.; Binkert, C.; Morrison, K.; Fischli, W.; Gatfield, J.; Treiber, A.; Weller, T.; Bolli, M.H.; Boss, C.; Buchmann, S.; et al. Pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist. J. Pharmacol. Exp. Ther. 2008, 327, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Hrelec, M.; Klicek, R.; Brcic, L.; Brcic, I.; Cvjetko, I.; Seiwerth, S.; Sikiric, P. Abdominal aorta anastomosis in rats and stable gastric pentadecapeptide BPC 157, prophylaxis and therapy. J. Physiol. Pharmacol. 2009, 60, 161–165. [Google Scholar] [PubMed]
- Vukojevic, J.; Siroglavic, M.; Kasnik, K.; Kralj, T.; Stancic, D.; Kokot, A.; Kolaric, D.; Drmic, D.; Sever, A.Z.; Barisic, I.; et al. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vascul. Pharmacol. 2018, 106, 54–66. [Google Scholar] [CrossRef]
- Gojkovic, S.; Krezic, I.; Vrdoljak, B.; Malekinusic, D.; Barisic, I.; Petrovic, A.; Horvat Pavlov, K.; Kolovrat, M.; Duzel, A.; Knezevic, M.; et al. Pentadecapeptide BPC 157 resolves suprahepatic occlusion of the inferior caval vein, Budd-Chiari syndrome model in rats. World J. Gastrointest. Pathophysiol. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Kolovrat, M.; Gojkovic, S.; Krezic, I.; Malekinusic, D.; Vrdoljak, B.; Kasnik Kovac, K.; Kralj, T.; Drmic, D.; Barisic, I.; Horvat Pavlov, K.; et al. Pentadecapeptide BPC 157 resolves Pringle maneuver in rats, both ischemia and reperfusion. World J. Hepatol. 2020, 12, 184–206. [Google Scholar] [CrossRef]
- Duzel, A.; Vlainic, J.; Antunovic, M.; Malekinusic, D.; Vrdoljak, B.; Samara, M.; Gojkovic, S.; Krezic, I.; Vidovic, T.; Bilic, Z.; et al. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights. World J. Gastroenterol. 2017, 23, 8465–8488. [Google Scholar] [CrossRef]
- Amic, F.; Drmic, D.; Bilic, Z.; Krezic, I.; Zizek, H.; Peklic, M.; Klicek, R.; Pajtak, A.; Amic, E.; Vidovic, T.; et al. Bypassing major venous occlusion and duodenal lesions in rats, and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine. World J. Gastroenterol. 2018, 24, 5366–5378. [Google Scholar] [CrossRef] [PubMed]
- Drmic, D.; Samara, M.; Vidovic, T.; Malekinusic, D.; Antunovic, M.; Vrdoljak, B.; Ruzman, J.; Milkovic Perisa, M.; Horvat Pavlov, K.; Jeyakumar, J.; et al. Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World J. Gastroenterol. 2018, 24, 5462–5476. [Google Scholar] [CrossRef]
- Sever, A.Z.; Sever, M.; Vidovic, T.; Lojo, N.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Kokot, A.; Zoricic, I.; Coric, M.; et al. Stable gastric pentadecapeptide BPC 157 in the therapy of the rats with bile duct ligation. Eur. J. Pharmacol. 2019, 847, 130–142. [Google Scholar] [CrossRef]
- Vukojevic, J.; Vrdoljak, B.; Malekinusic, D.; Siroglavic, M.; Milavic, M.; Kolenc, D.; Boban Blagaic, A.; Bateljam, L.; Drmic, D.; Seiwerth, S.; et al. The effect of pentadecapeptide BPC 157 on hippocampal ischemia/reperfusion injuries in rats. Brain Behav. 2020, 10, e01726. [Google Scholar] [CrossRef]
- Stupnisek, M.; Franjic, S.; Drmic, D.; Hrelec, M.; Kolenc, D.; Radic, B.; Bojic, D.; Vcev, A.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 reduces bleeding time and thrombocytopenia after amputation in rats treated with heparin, warfarin or aspirin. Thromb Res. 2012, 129, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Stupnisek, M.; Kokot, A.; Drmic, D.; Hrelec Patrlj, M.; Zenko Sever, A.; Kolenc, D.; Radic, B.; Suran, J.; Bojic, D.; Vcev, A.; et al. Pentadecapeptide BPC 157 reduces bleeding and thrombocytopenia after amputation in rats treated with heparin, warfarin, L-NAME and L-arginine. PLoS ONE 2015, 10, e0123454. [Google Scholar] [CrossRef] [PubMed]
- Konosic, S.; Petricevic, M.; Ivancan, V.; Konosic, L.; Goluza, E.; Krtalic, B.; Drmic, D.; Stupnisek, M.; Seiwerth, S.; Sikiric, P. Intragastric application of aspirin, clopidogrel, cilostazol, and BPC 157 in rats: Platelet aggregation and blood clot. Oxidative Med. Cell. Longev. 2019, 2019, 9084643. [Google Scholar] [CrossRef]
- Chang, C.H.; Tsai, W.C.; Lin, M.S.; Hsu, Y.H.; Pang, J.H. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J. Appl. Physiol. 2011, 110, 774–780. [Google Scholar] [CrossRef]
- Chang, C.H.; Tsai, W.C.; Hsu, Y.H.; Pang, J.H. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules 2014, 19, 19066–19077. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, K.; Sun, L.; Xue, X.; Zhang, C.; Shu, Z.; Mu, N.; Gu, J.; Zhang, W.; Wang, Y.; et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des. Dev. Ther. 2015, 9, 2485–2499. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Liu, H.T.; Wang, C.N.; Huang, H.Y.; Lin, Y.; Ko, Y.S.; Wang, J.S.; Chang, V.H.; Pang, J.S. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J. Mol. Med. 2017, 95, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Tkalcevic, V.I.; Cuzic, S.; Brajsa, K.; Mildner, B.; Bokulic, A.; Situm, K.; Perovic, D.; Glojnaric, I.; Parnham, M.J. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur. J. Pharmacol. 2007, 570, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Cesarec, V.; Becejac, T.; Misic, M.; Djakovic, Z.; Olujic, D.; Drmic, D.; Brcic, L.; Rokotov, D.S.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur. J. Pharmacol. 2013, 701, 203–212. [Google Scholar] [CrossRef]
- Frasch, H.F.; Marshall, C.; Marshall, B.E. Endothelin-1 is elevated in monocrotaline pulmonary hypertension. Am. J. Physiol. Lung. Cell. Mol. Physiol. 1999, 276, 304–310. [Google Scholar] [CrossRef]
- Naeije, R.; Dewachter, L. Animal models of pulmonary arterial hypertension. Rev. Mal. Respir. 2007, 24, 481–496. [Google Scholar] [CrossRef]
- Tyler, R.C.; Muramatsu, M.; Abman, S.H.; Stelzner, T.J.; Rodman, D.M.; Bloch, K.D.; McMurtry, I.F. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am. J. Physiol. Lung. Cell. Mol. Physiol. 1999, 276, 297–303. [Google Scholar] [CrossRef]
- Lovric-Bencic, M.; Sikiric, P.; Hanzevacki, J.S.; Seiwerth, S.; Rogic, D.; Kusec, V.; Aralica, G.; Konjevoda, P.; Batelja, L.; Blagaic, A.B. Doxorubicine-congestive heart failure-increased big endothelin-1 plasma concentration: Reversal by amlodipine, losartan, and gastric pentadecapeptide BPC157 in rat and mouse. J. Pharmacol. Sci. 2004, 95, 19–26. [Google Scholar] [CrossRef]
- Kokot, A.; Zlatar, M.; Stupnisek, M.; Drmic, D.; Radic, R.; Vcev, A.; Seiwerth, S.; Sikiric, P. NO system dependence of atropine-induced mydriasis and L-NAME- and L-arginine-induced miosis: Reversal by the pentadecapeptide BPC 157 in rats and guinea pigs. Eur. J. Pharmacol. 2016, 771, 211–219. [Google Scholar] [CrossRef]
- Belosic, H.Z.; Vlainic, J.; Drmic, D.; Strinic, D.; Luetic, K.; Sucic, M.; Medvidovic-Grubisic, M.; Pavelic Turudic, T.; Petrovic, I.; Seiwerth, S.; et al. Class side effects: Decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and L-arginine. Inflammopharmacology 2017, 25, 511–522. [Google Scholar] [CrossRef]
- Grabarevic, Z.; Tisljar, M.; Artukovic, B.; Bratulic, M.; Dzaja, P.; Seiwerth, S.; Sikiric, P.; Peric, J.; Geres, D.; Kos, J. The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicks. J. Physiol. 1997, 91, 139–149. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Rucman, R.; Petek, M.; Jagic, V.; Turkovic, B.; Rotkvic, I.; Mise, S.; Zoricic, I.; et al. The influence of a novel pentadecapeptide BPC 157 on NG-nitro-L-arginine methylester and L-arginine effect on stomach mucosal integrity and blood pressure. Eur. J. Pharmacol. 1997, 332, 23–33. [Google Scholar] [CrossRef]
- Turkovic, B.; Sikiric, P.; Seiwerth, S.; Mise, S.; Anic, T.; Petek, M. Stable gastric pentadecapeptide BPC 157 studied for inflammatory bowel disease (PLD-116, PL14736, Pliva) induces nitric oxide synthesis. Gastroenterology 2004, 126, 287. [Google Scholar]
- Barisic, I.; Balenovic, D.; Klicek, R.; Radic, B.; Nikitovic, B.; Drmic, D.; Udovicic, M.; Strinic, D.; Bardak, D.; Berkopic, L.; et al. Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157. Regul. Pept. 2013, 181, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Medvidovic-Grubisic, M.; Stambolija, V.; Kolenc, D.; Katancic, J.; Murselovic, T.; Plestina-Borjan, I.; Strbe, S.; Drmic, D.; Barisic, I.; Sindic, A.; et al. Hypermagnesemia disturbances in rats, NO-related: Pentadecapeptide BPC 157 abrogates, L-NAME and L-arginine worsen. Inflammopharmacology 2017, 25, 439–449. [Google Scholar] [CrossRef]
- Balenovic, D.; Barisic, I.; Prkacin, I.; Horvat, I.; Udovicic, M.; Uzun, S.; Strinic, D.; Pevec, D.; Drmic, D.; Radic, B.; et al. Mortal. Furosemide-hypokalemia-disturbances in rats NO-system related. Shorten survival by L-NAME. Therapy benefit with BPC 157 more than with L-arginine. J. Clin. Exp. Cardiolog. 2012, 3, 201. [Google Scholar] [CrossRef]
- Lozic, M.; Stambolija, V.; Krezic, I.; Dugandzic, A.; Zivanovic-Posilovic, G.; Gojkovic, S.; Kovacevic, J.; Vrdoljak, L.; Mirkovic, I.; Kokot, A.; et al. In relation to NO-system, stable pentadecapeptide BPC 157 counteracts lidocaine-induced adverse effects in rats and depolarisation In vitro. Emerg. Med. Int. 2020, 2020, 6805354. [Google Scholar] [CrossRef]
- Balenovic, D.; Bencic, M.L.; Udovicic, M.; Simonji, K.; Hanzevacki, J.S.; Barisic, I.; Kranjcevic, S.; Prkacin, I.; Coric, V.; Brcic, L.; et al. Inhibition of methyldigoxin-induced arrhythmias by pentadecapeptide BPC 157: A relation with NO-system. Regul. Pept. 2009, 156, 83–89. [Google Scholar] [CrossRef]
- Strinic, D.; Belosic, H.Z.; Luetic, K.; Nedic, A.; Petrovic, I.; Sucic, M.; Zivanovic Posilovic, G.; Balenovic, D.; Strbe, S.; Udovicic, M.; et al. BPC 157 counteracts QTc prolongation induced by haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide in rats. Life Sci. 2017, 186, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Stambolija, V.; Stambolija, T.P.; Holjevac, J.K.; Murselovic, T.; Radonic, J.; Duzel, V.; Duplancic, B.; Uzun, S.; Zivanovic-Posilovic, G.; Kolenc, D.; et al. BPC 157: The counteraction of succinylcholine, hyperkalemia, and arrhythmias. Eur. J. Pharmacol. 2016, 781, 83–91. [Google Scholar] [CrossRef]
- Zivanovic-Posilovic, G.; Balenovic, D.; Barisic, I.; Strinic, D.; Stambolija, V.; Udovicic, M.; Uzun, S.; Drmic, D.; Vlainic, J.; Bencic, M.L.; et al. Stable gastric pentadecapeptide BPC 157 and bupivacaine. Eur. J. Pharmacol. 2016, 793, 56–65. [Google Scholar] [CrossRef]
- Ryan, J.J.; Huston, J.; Kutty, S.; Hatton, N.D.; Bowman, L.; Tian, L.; Herr, J.E.; Johri, A.M.; Archer, S.L. Right ventricular adaptation and failure in pulmonary arterial hypertension. Can. J. Cardiol. 2015, 31, 391–406. [Google Scholar] [CrossRef]
- Ueno, M.; Miyauchi, T.; Sakai, S.; Yamauchi-Kohno, R.; Goto, K.; Yamaguchi, I. A combination of oral endothelin-A receptor antagonist and oral prostacyclin analogue is superior to each drug alone in ameliorating pulmonary hypertension in rats. J. Am. Coll. Cardiol. 2002, 40, 175–181. [Google Scholar] [CrossRef][Green Version]
- Urboniene, D.; Haber, I.; Fang, Y.H.; Thenappan, T.; Archer, S.L. Validation of high resolution echocardiography and magnetic resonance imaging vs. high-fidelity catheterization in experimental pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, 401–412. [Google Scholar] [CrossRef]
- Julian, R.J.; Caston, L.J.; Leeson, S. The effect of dietary sodium on right ventricular failure-induced ascites, gain and fat deposition in meat-type chickens. Can. J. Vet. Res. 1992, 56, 214–219. [Google Scholar]
- Rich, J.D.; Thenappan, T.; Freed, B.; Patel, A.R.; Thisted, R.A.; Childers, R.; Archer, S.L. QTc prolongation is associated with impaired right ventricular function and predicts mortality in pulmonary hypertension. Int. J. Cardiol. 2013, 167, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Benoist, D.; Stones, R.; Drinkhill, M.; Bernus, O.; White, E. Arrhythmogenic substrate in hearts of rats with monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- Benoist, D.; Stones, R.; Drinkhill, M.J.; Benson, A.P.; Yang, Z.; Cassan, C.; Gilbert, S.H.; Saint, D.A.; Cazorla, O.; Steele, D.S.; et al. Cardiac arrhythmia mechanisms in rats with heart failure induced by pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, 2381–2395. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Lee, J.H.; de Lange, E.; Iorga, A.; Partow-Navid, R.; Bapat, A.; van der Laarse, A.; Saggar, R.; Saggar, R.; Ypey, D.L.; et al. Spontaneous ventricular fibrillation in right ventricular failure secondary to chronic pulmonary hypertension. Circ. Arrhythm. Electrophysiol. 2012, 5, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Galié, N.; Manes, A.; Branzi, A. The endothelin system in pulmonary arterial hypertension. Cardiovasc. Res. 2004, 61, 227–237. [Google Scholar] [CrossRef]
- Kay, J.M.; Harris, P.; Heath, D. Pulmonary hypertension produced in rats by ingestion of Crotalaria spectabilis seeds. Thorax 1967, 22, 176–179. [Google Scholar] [CrossRef]
- Rosenberg, H.C.; Rabinovitch, M. Endothelial injury and vascular reactivity in monocrotaline pulmonary hypertension. Am. J. Physiol. 1988, 255, 1484–1491. [Google Scholar] [CrossRef]
- Moreira-Gonçalves, D.; Padrão, A.I.; Ferreira, R.; Justino, J.; Nogueira-Ferreira, R.; Neuparth, M.J.; Vitorino, R.; Fonseca, H.; Silva, A.F.; Duarte, J.A.; et al. Signaling pathways underlying skeletal muscle wasting in experimental pulmonary arterial hypertension. Biochim. Biophys. Acta 2015, 1852, 2722–2731. [Google Scholar] [CrossRef]
- Estep, J.E.; Lame, M.W.; Morin, D.; Jones, A.D.; Wilson, D.W.; Segall, H.J. [14C]monocrotaline kinetics and metabolism in the rat. Drug metabolism and disposition: The biological fate of chemicals. Drug Metab. Dispos. 1991, 19, 135–139. [Google Scholar]
- Pan, L.C.; Lame, M.W.; Morin, D.; Wilson, D.W.; Segall, H.J. Red blood cells augment transport of reactive metabolites of monocrotaline from liver to lung in isolated and tandem liver and lung preparations. Toxicol. Appl. Pharmacol. 1991, 110, 336–346. [Google Scholar] [CrossRef]
- Wilson, D.W.; Lame, M.W.; Dunston, S.K.; Segall, H.J. DNA damage cell checkpoint activities are altered in monocrotaline pyrrole-induced cell cycle arrest in human pulmonary artery endothelial cells. Toxicol. Appl. Pharmacol. 2000, 166, 69–80. [Google Scholar] [CrossRef]
- Lai, Y.L.; Olson, J.W.; Gillespie, M.N. Ventilatory dysfunction precedes pulmonary vascular changes in monocrotaline-treated rats. J. Appl. Physiol. 1991, 70, 561–566. [Google Scholar] [CrossRef]
- Hill, N.S.; Warburton, R.R.; Pietras, L.; Klinger, J.R. Nonspecific endothelin-receptor antagonist blunts monocrotaline-induced pulmonary hypertension in rats. J. Appl. Physiol. 1997, 83, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.; Prié, S. The ET(A)-Receptor Antagonist LU 135252 Prevents the progression of established pulmonary hypertension induced by monocrotaline in rats. J. Cardiovasc. Pharmacol. Ther. 1999, 4, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Prié, S.; Leung, T.K.; Cernacek, P.; Ryan, J.W.; Dupuis, J. The orally active ET(A) receptor antagonist (+)-(S)-2-(4,6-dimethoxy-pyrimidin-2-yloxy)-3-methoxy-3,3-diphe nyl-propionic acid (LU 135252) prevents the development of pulmonary hypertension and endothelial metabolic dysfunction in monocrotaline-treated rats. J. Pharmacol. Exp. Ther. 1997, 282, 1312–1318. [Google Scholar]
- Hsieh, M.J.; Lee, C.H.; Chueh, H.Y.; Chang, G.J.; Huang, H.Y.; Lin, Y.; Pang, J.S. Modulatory effects of BPC 157 on vasomotor tone and the activation of Src-Caveolin-1-endothelial nitric oxide synthase pathway. Sci. Rep. 2020, 10, 17078. [Google Scholar] [CrossRef] [PubMed]
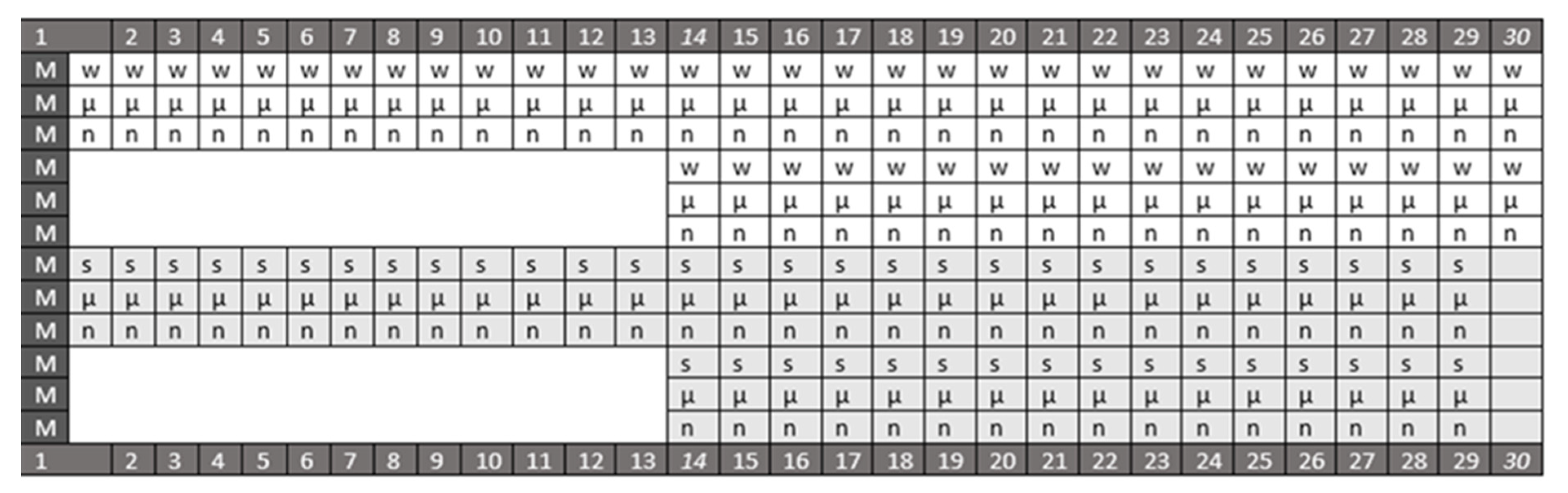
| Assessed Parameters | Therapy Started Immediately after Monocrotaline Application | |||||
|---|---|---|---|---|---|---|
| A. Medication: One Time Daily, Intraperitoneally * p ˂ 0.05, at Least, vs. Control | B. Medication: BPC 157 Continuously in Drinking Water until Sacrifice # p ˂ 0.05, at Least, vs. Control | |||||
| 0.9% NaCl 5 mL/kg | BPC 157 10 µg/kg | BPC 157 10 ng/kg i.p. | Drinking Water 12 mL/rat /day | BPC 157 10 µg/kg | BPC 157 10 ng/kg | |
| BW end (g) | 224.5 ± 3.46 | 252.5 ± 8.73 * | 258.33 ± 4.59 * | 226.75 ± 4.39 | 257.5 ± 5.28 # | 243.67 ± 4.18 # |
| heart (g) | 1.12 ± 0.04 | 0.88 ± 0.04 | 0.99 ± 0.06 | 1.19 ± 0.05 | 1.02 ± 0.09 | 0.95 ± 0.05 |
| RV (g) | 0.32 ± 0.03 | 0.15 ± 0.01 * | 0.18 ± 0.01 * | 0.34 ± 0.02 | 0.17 ± 0.01 # | 0.17 ± 0.01 # |
| LV + IVS (g) | 0.59 ± 0.03 | 0.53 ± 0.03 | 0.59 ± 0.05 | 0.62 ± 0.02 | 0.62 ± 0.06 | 0.58 ± 0.04 |
| liver (g) | 12.82 ± 0.43 | 8.72 ± 0.35 * | 8.68 ± 0.36 * | 12.3 ± 0.04 | 9.14 ± 0.44 # | 9.90 ± 0.74 # |
| RV/(LV + IVS) | 0.56 ± 0.07 | 0.29 ± 0.01 * | 0.3 ± 0.01 * | 0.54 ± 0.02 | 0.28 ± 0.01 # | 0.29 ± 0.02 # |
| RV/BW (mg/g) | 1.44 ± 0.14 | 0.61 ± 0.02 * | 0.68 ± 0.03 * | 1.42 ± 0.08 | 0.67 ± 0.04 # | 0.68 ± 0.04 # |
| liver/BW(mg/g) | 57.11 ± 3.76 | 34.6 ± 1.20 * | 33.68 ± 1.63 * | 52.37 ± 2.69 | 35.64 ± 2.07 # | 40.66 ± 3.05 # |
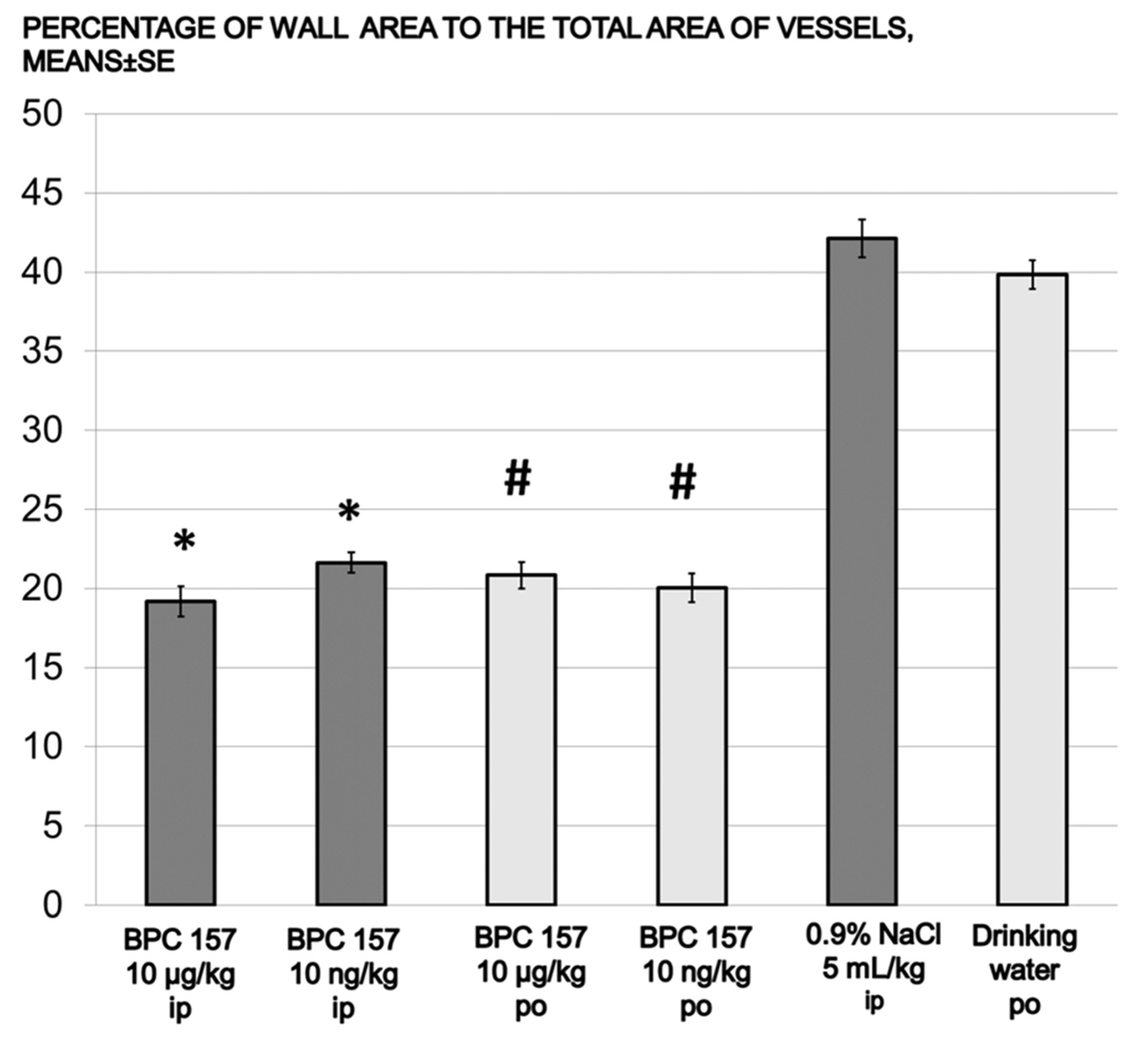
| media area % | 42.13 ± 1.19 | 19.19 ± 0.94 * | 21.63 ± 0.66 * | 39.83 ± 0.92 | 20.83 ± 0.84 # | 20.04 ± 0.92 # |
| heart rate | 319.2 ± 4.38 | 396.01 ± 1.46 * | 392.8 ± 2.51 * | 312.8 ± 2.68 | 400.4 ± 2.55 # | 387.9 ± 3.36 # |
| respiration rate | 139 ± 8.59 | 103.67 ± 1.74 * | 102.67 ± 1.32 * | 131 ± 7.41 | 102.67 ± 3.04 # | 102.00 ± 2.01 # |
| QT interval | 78 ± 2.32 | 44.67 ± 1.86 * | 46.67 ± 1.68 * | 83.58 ± 3.6 | 45.63 ± 2.09 # | 48.96 ± 3.67 # |
| Monocrotaline (80 mg/kg sc, Day 0) Rats | |||||||
|---|---|---|---|---|---|---|---|
| Assessed Parameters | Day 14 (before Therapy Initiation) § p < 0.05, at Least, vs. day 14 | Day 30 (at the End of Therapy Protocol) | |||||
| After Monocrotaline Application Therapy Started at Day 14 | |||||||
| A. Medication: One Time Daily, Intraperitoneally * p ˂ 0.05, at Least, vs. Control | B. Medication: BPC 157 Continuously in Drinking Water Until Sacrifice # p ˂ 0.05, at Least, vs. Control | ||||||
| 0.9% NaCl 5 mL/kg | BPC 157 10 µg/kg | BPC 157 10 ng/kg | Drinking Water 12 mL/rat /day | BPC 157 10 µg/kg water | BPC 157 10 ng/kg | ||
| BW end (g) | 201.33 ± 6.4 | 207.6 ± 5.19 | 233.17 ± 9.24 *§ | 232.17 ± 5.15 *§ | 205.75 ± 3.20 | 240.17 ± 7.41 #§ | 232.33 ± 4.45 #§ |
| heart (g) | 0.77 ± 0.05 | 1.02 ± 0.04 § | 0.85 ± 0.04 | 0.87 ± 0.03 | 1.04 ± 0.02 § | 0.90 ± 0.05 | 0.93 ± 0.03 |
| RV (g) | 0.15 ± 0.01 | 0.26 ± 0.02 § | 0.15 ± 0.01 *§ | 0.18 ± 0.01 *§ | 0.29 ± 0.01 § | 0.16 ± 0.01 #§ | 0.19 ± 0.01 #§ |
| LV + IVS (g) | 0.41 ± 0.03 | 0.49 ± 0.01 | 0.52 ± 0.04 | 0.5 ± 0.02 | 0.5 ± 0.02 | 0.54 ± 0.03 | 0.55 ± 0.02 |
| liver (g) | 6.39 ± 0.44 | 12.85 ± 0.31 § | 7.47 ± 0.58 *§ | 9.06 ± 0.33 *§ | 13.19 ± 0.22 § | 8.19 ± 0.50 #§ | 8.47 ± 0.62 #§ |
| RV/ (LV + IVS) | 0.37 ± 0.02 | 0.53 ± 0.03 § | 0.29 ± 0.01 *§ | 0.36 ± 0.02 *§ | 0.55 ± 0.02 § | 0.29 ± 0.01 #§ | 0.34 ± 0.01 #§ |
| RV/BW (mg/g) | 0.75 ± 0.04 | 1.27 ± 0.07 § | 0.64 ± 0.01 *§ | 0.77 ± 0.01 *§ | 1.39 ± 0.07 § | 0.66 ± 0.01 #§ | 0.80 ± 0.01 #§ |
| liver/BW (mg/g) | 31.74 ± 1.83 | 62.07 ± 2.35 § | 31.86 ± 1.54 *§ | 39.07 ± 1.44 *§ | 64.13 ± 0.99 § | 34.05 ± 1.59 #§ | 36.43 ± 2.47 #§ |
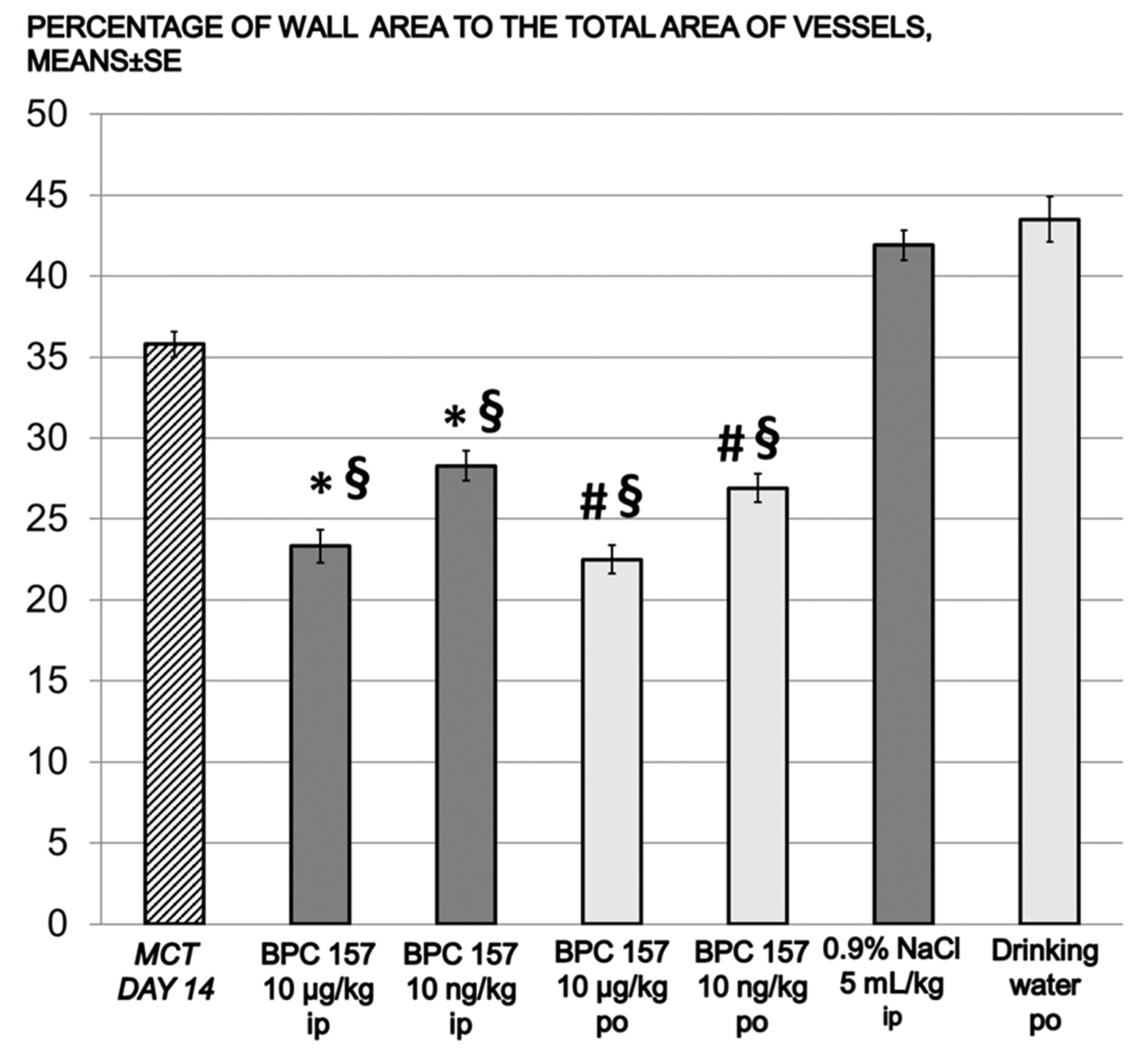
| media area % | 35.79 ± 0.79 | 41.92 ± 0.93 | 23.33 ± 1.02 *§ | 30.15 ± 0.86 | 43.51 ± 1.40 | 22.58 ± 0.86 #§ | 28.50 ± 0.91 #§ |
| heart rate | 366.00 ± 2.73 | 316.02 ± 6.81 § | 387.83 ± 4.59 *§ | 378.83 ± 6.74 *§ | 319.20 ± 7.14 § | 375.50 ± 4.19 #§ | 379.17 ± 4.73 #§ |
| respiration rate | 114.67 ± 3.00 | 139 ± 8.59 § | 98.67 ± 1.98 *§ | 100.7 ± 1.61 *§ | 131 ± 7.42 § | 112.67 ± 2.81 #§ | 111.33 ± 1.91 #§ |
| QT interval | 61 ± 5.94 | 80 ± 3.82 § | 48 ± 3.10 *§ | 52.7 ± 3.01 *§ | 89 ± 3.65 § | 45.5 ± 2.75 #§ | 56.33 ± 0.76 #§ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udovicic, M.; Sever, M.; Kavur, L.; Loncaric, K.; Barisic, I.; Balenovic, D.; Zivanovic Posilovic, G.; Strinic, D.; Uzun, S.; Batelja Vuletic, L.; et al. Stable Gastric Pentadecapeptide BPC 157 Therapy for Monocrotaline-Induced Pulmonary Hypertension in Rats Leads to Prevention and Reversal. Biomedicines 2021, 9, 822. https://doi.org/10.3390/biomedicines9070822
Udovicic M, Sever M, Kavur L, Loncaric K, Barisic I, Balenovic D, Zivanovic Posilovic G, Strinic D, Uzun S, Batelja Vuletic L, et al. Stable Gastric Pentadecapeptide BPC 157 Therapy for Monocrotaline-Induced Pulmonary Hypertension in Rats Leads to Prevention and Reversal. Biomedicines. 2021; 9(7):822. https://doi.org/10.3390/biomedicines9070822
Chicago/Turabian StyleUdovicic, Mario, Marko Sever, Lovro Kavur, Kristina Loncaric, Ivan Barisic, Diana Balenovic, Gordana Zivanovic Posilovic, Dean Strinic, Sandra Uzun, Lovorka Batelja Vuletic, and et al. 2021. "Stable Gastric Pentadecapeptide BPC 157 Therapy for Monocrotaline-Induced Pulmonary Hypertension in Rats Leads to Prevention and Reversal" Biomedicines 9, no. 7: 822. https://doi.org/10.3390/biomedicines9070822
APA StyleUdovicic, M., Sever, M., Kavur, L., Loncaric, K., Barisic, I., Balenovic, D., Zivanovic Posilovic, G., Strinic, D., Uzun, S., Batelja Vuletic, L., Sikiric, S., Skrtic, A., Drmic, D., Boban Blagaic, A., Lovric Bencic, M., Seiwerth, S., & Sikiric, P. (2021). Stable Gastric Pentadecapeptide BPC 157 Therapy for Monocrotaline-Induced Pulmonary Hypertension in Rats Leads to Prevention and Reversal. Biomedicines, 9(7), 822. https://doi.org/10.3390/biomedicines9070822






