β-Elemene Suppresses Obesity-Induced Imbalance in the Microbiota-Gut-Brain Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Brain Sample Preparation
2.3. 1H NMR-Based Metabolite Analysis
2.4. Fecal DNA Extraction and Intestinal Microbiota Analysis
2.5. Statistical Analysis
3. Results
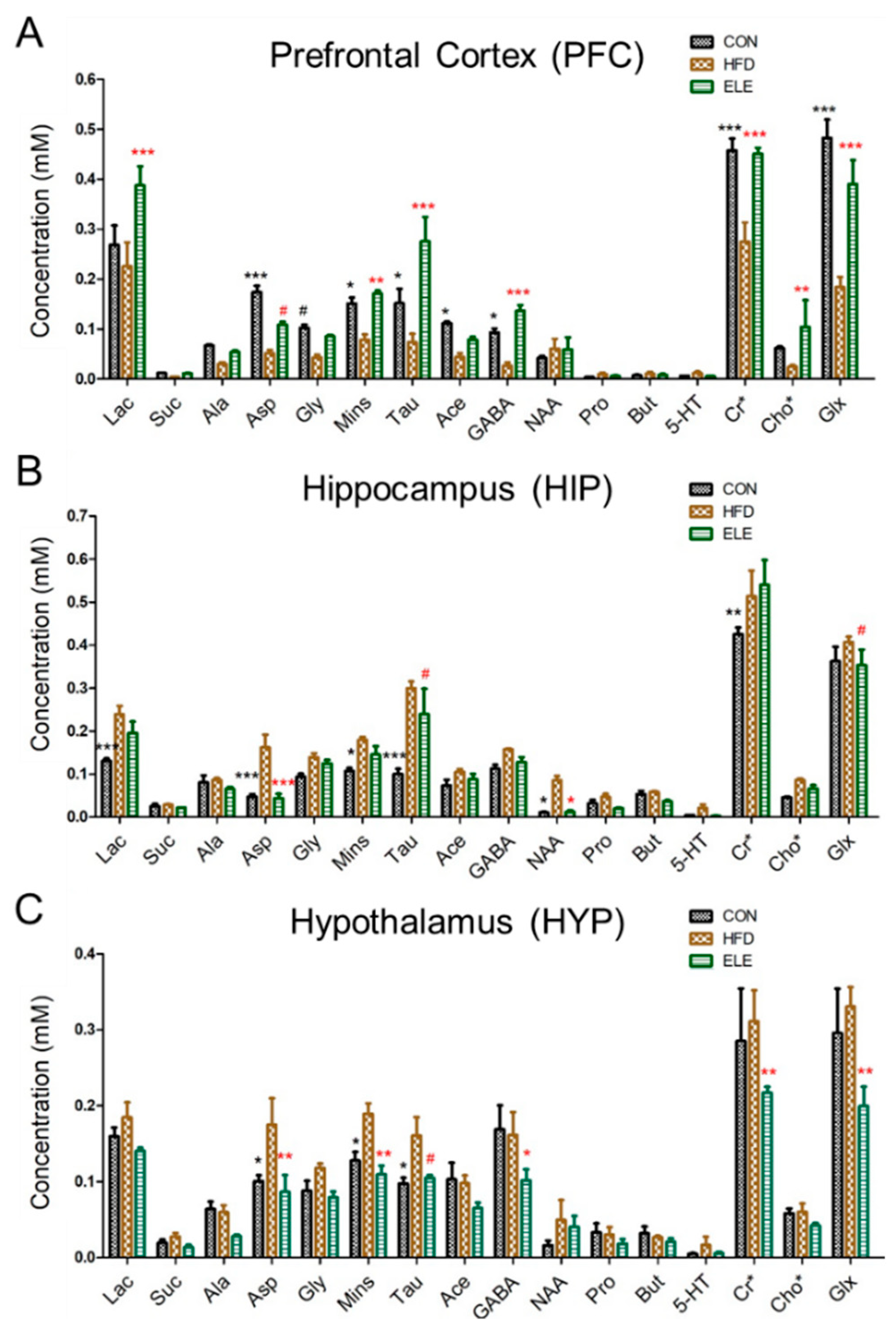
3.1. β-Elemene Regulated the Metabolite Contents of the PFC, HIP and HYP of Obese Mice
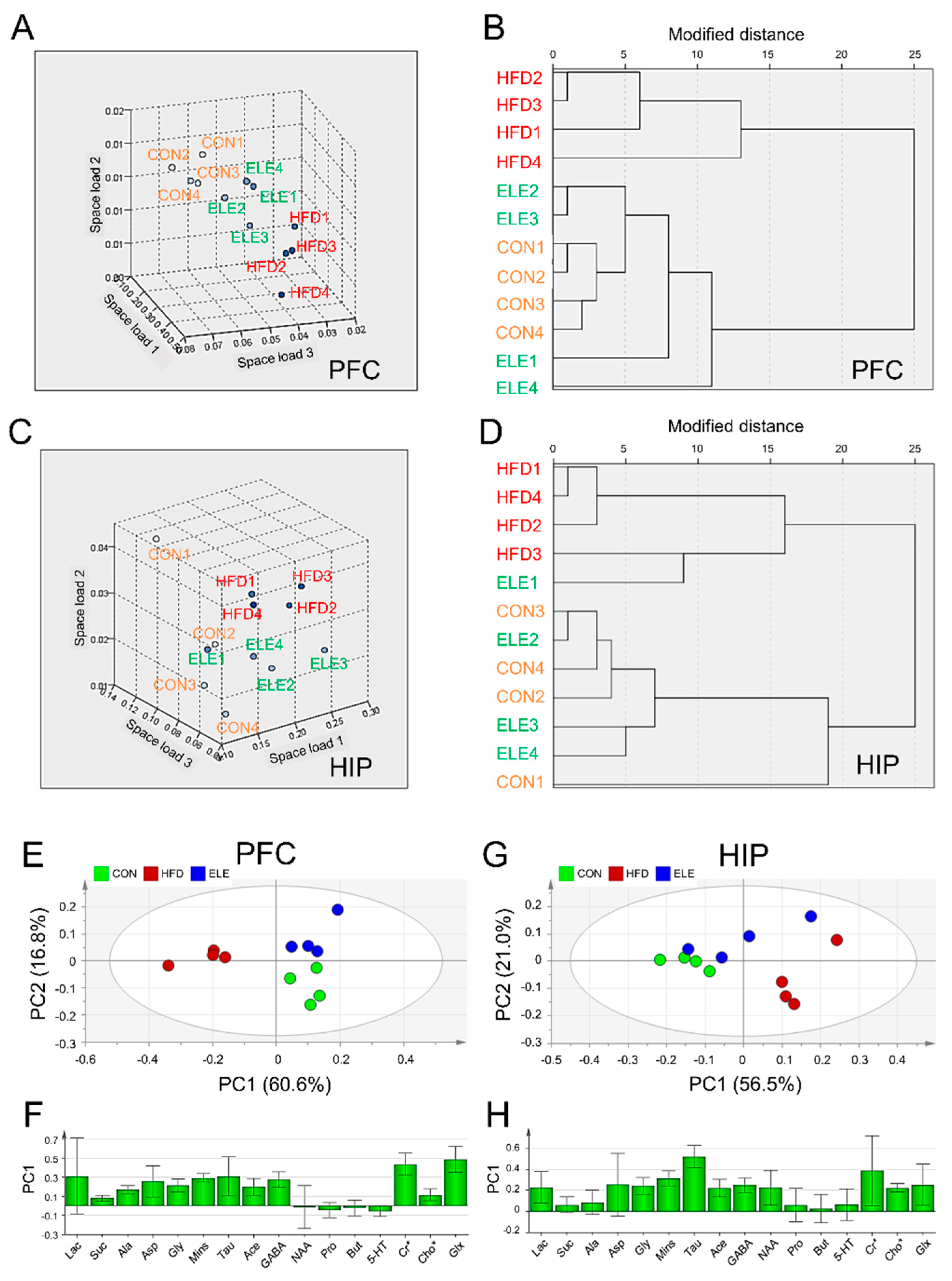
3.2. Regulatory Effects of β-Elemene on Obesity-Induced Imbalance of Cerebral Metabolites Using Clustering Analysis and PCA
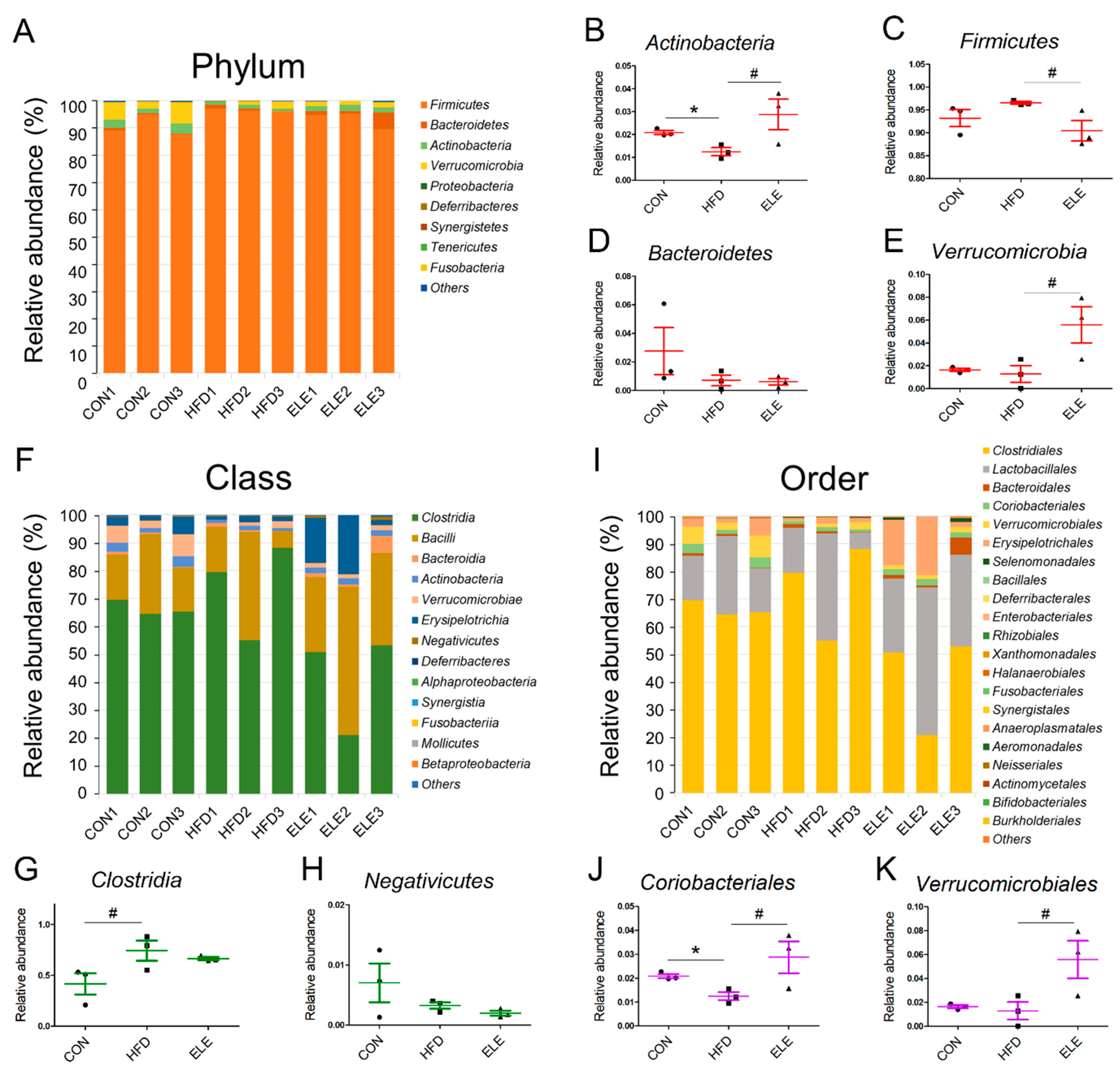
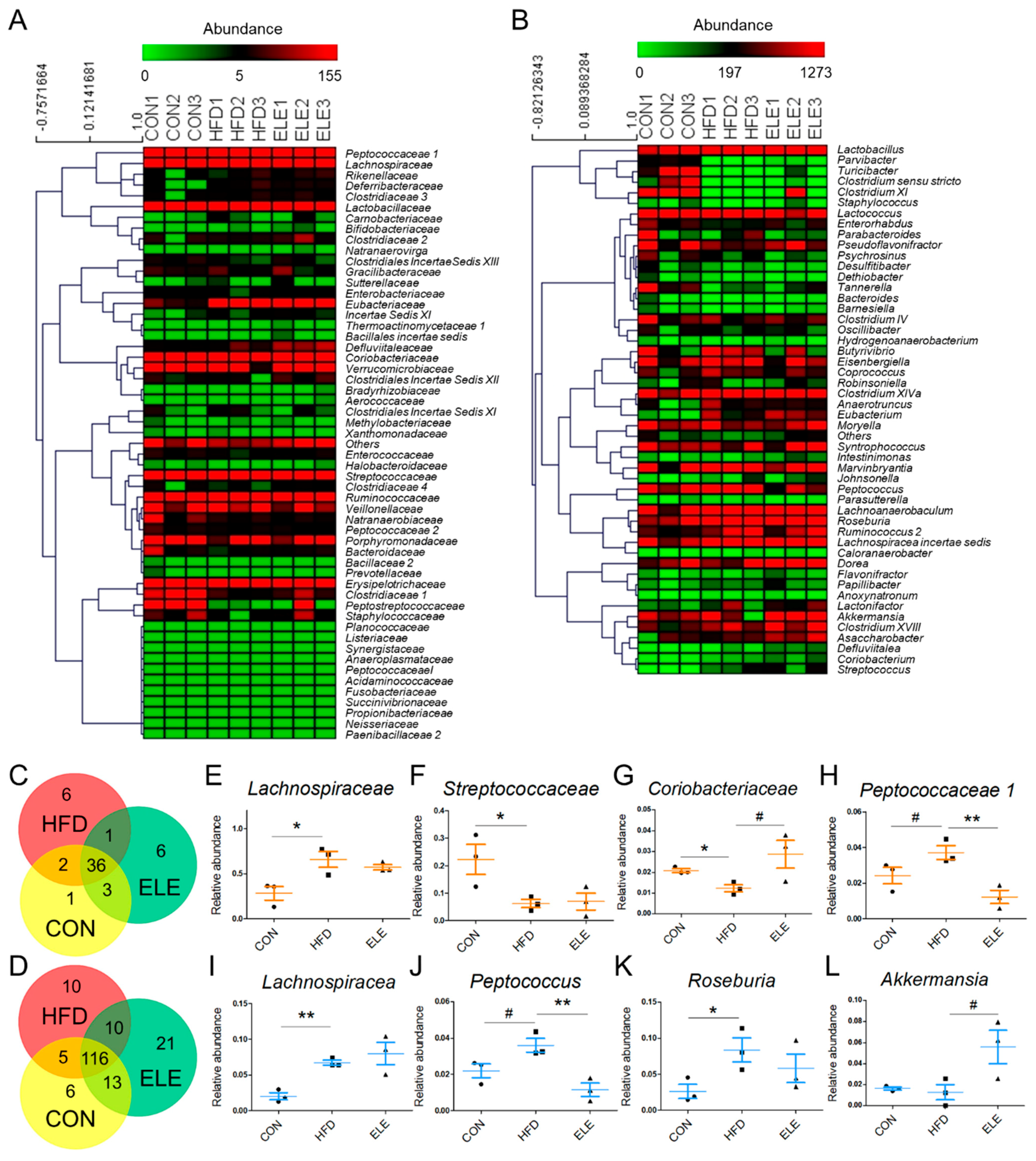
3.3. Effects of β-Elemene on Regulating Obesity-Induced Changes in the Composition of Intestinal Microbiota
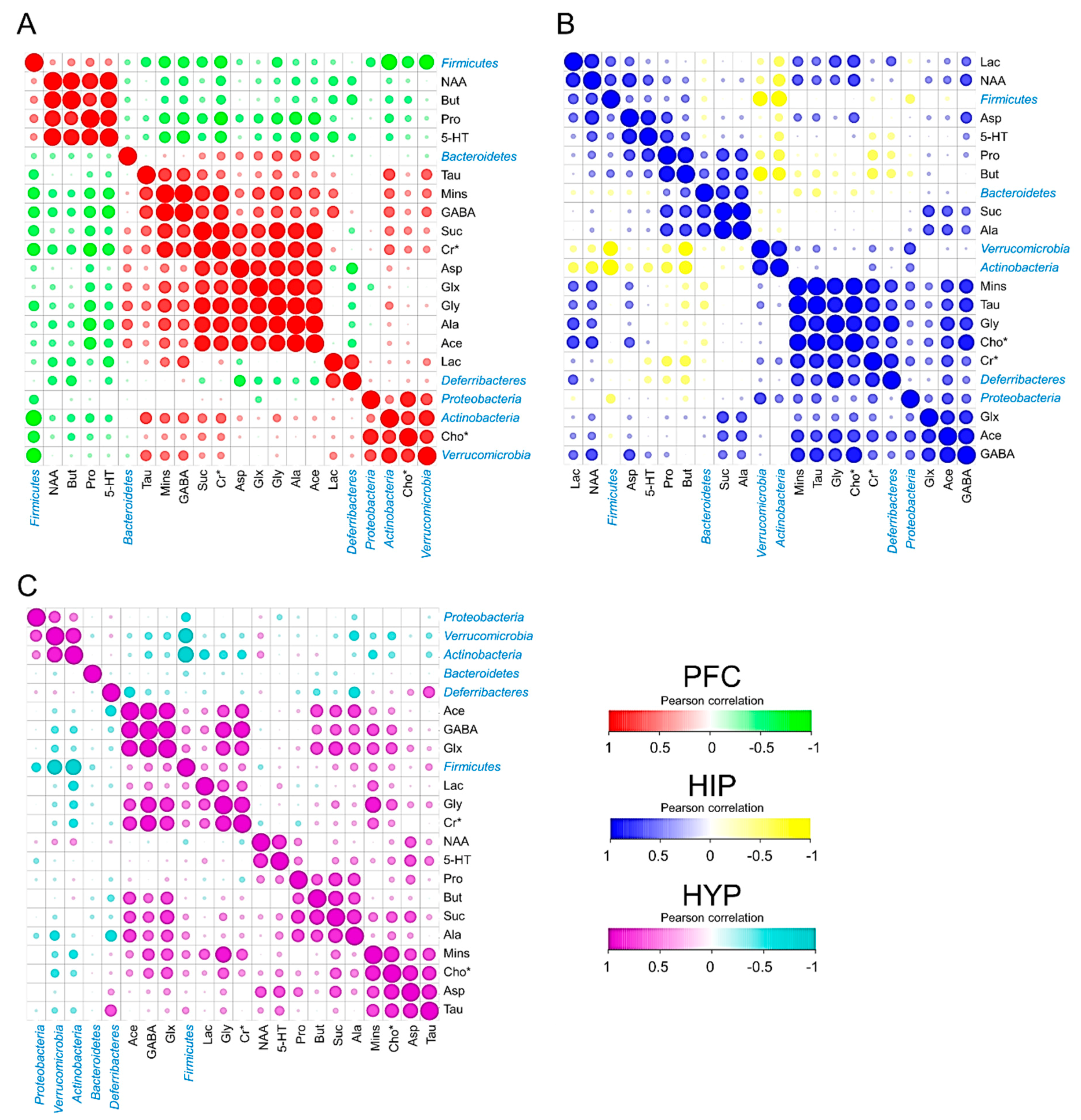
3.4. Cerebral Metabolites Correlated with Intestinal Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Low-grade systemic inflammation in overweight children. Pediatry 2001, 107, e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, M.; Castelhano, J.; Petrella, L.I.; Sereno, J.; Rodrigues, T.; Neves, C.; Letra, L.; Baptista, F.I.; Seiça, R.; Matafome, P.; et al. High-fat diet induces a neurometabolic state characterized by changes in glutamate and N-acetylaspartate pools associated with early glucose intolerance: An in vivo multimodal MRI study. J. Magn. Reson. Imaging 2018, 48, 757–766. [Google Scholar] [CrossRef]
- Castanon, N.; Lasselin, J.; Capuron, L. Neuropsychiatric Comorbidity in Obesity: Role of Inflammatory Processes. Front. Endocrinol. 2014, 5, 74. [Google Scholar] [CrossRef] [Green Version]
- Agustí, A.; García-Pardo, M.P.; López-Almela, I.; Campillo, I.; Maes, M.; Pérez, M.R.; Sanz, Y. Interplay Between the Gut-Brain Axis, Obesity and Cognitive Function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef]
- Huang, D.; Li, C.; Zhang, W.; Qin, J.; Jiang, W.; Hu, C. Dysfunction of astrocytic connexins 30 and 43 in the medial prefrontal cortex and hippocampus mediates depressive-like behaviours. Behav. Brain Res. 2019, 372, 111950. [Google Scholar] [CrossRef]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugon, P.; Lagier, J.-C.; Colson, P.; Bittar, F.; Raoult, D. Repertoire of human gut microbes. Microb. Pathog. 2017, 106, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Zeng, H. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J. Gastrointest. Oncol. 2014, 6, 41–51. [Google Scholar] [CrossRef]
- Wei, Y.; Melas, P.; Wegener, G.; Mathé, A.A.; Lavebratt, C. Antidepressant-Like Effect of Sodium Butyrate is Associated with an Increase in TET1 and in 5-Hydroxymethylation Levels in the Bdnf Gene. Int. J. Neuropsychopharmacol. 2014, 18, pyu032. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Dang, Y.-Y.; Huang, M.; Xu, W.-S.; Chen, X.-P.; Wang, Y.-T. Anti-cancer properties of terpenoids isolated from Rhizoma Curcumae—A review. J. Ethnopharmacol. 2012, 143, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Li, Q.Q.; Zhao, J.; Li, J.M.; Cuff, C.F.; Reed, E. β-elemene and taxanes synergistically induce cytotoxicity and inhibit proliferation in ovarian cancer and other tumor cells. Anticancer Res. 2013, 33, 929–940. [Google Scholar] [PubMed]
- Zhou, Y.; Takano, T.; Wang, Y.; Li, X.; Wang, R.; Wakatsuki, Y.; Nakajima-Adachi, H.; Tanokura, M.; Miyakawa, T.; Hachimura, S. Intestinal regulatory T cell induction by β-elemene alleviates the formation of fat tissue-related inflammation. iScience 2021, 24, 101883. [Google Scholar] [CrossRef]
- Zhang, R.; Tian, A.; Zhang, H.; Zhou, Z.; Yu, H.; Chen, L. Amelioration of Experimental Autoimmune Encephalomyelitis by β-elemene Treatment is Associated with Th17 and Treg Cell Balance. J. Mol. Neurosci. 2011, 44, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Pears, M.R.; Cooper, J.D.; Mitchison, H.M.; Mortishire-Smith, R.J.; Pearce, D.A.; Griffin, J.L. High Resolution 1H NMR-based Metabolomics Indicates a Neurotransmitter Cycling Deficit in Cerebral Tissue from a Mouse Model of Batten Disease. J. Biol. Chem. 2005, 280, 42508–42514. [Google Scholar] [CrossRef] [Green Version]
- Durante, M.; Manti, L. Human response to high-background radiation environments on Earth and in space. Adv. Space Res. 2008, 42, 999–1007. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Wu, J.; Zhang, X.; Liu, M.; Wang, Y. Metabonomic alterations in hippocampus, temporal and prefrontal cortex with age in rats. Neurochem. Int. 2009, 54, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xiang, Y.; Sun, N.; Zhu, H.; Wang, Y.; Liu, M.; Ma, Y.; Lei, H. Metabolic changes in rat prefrontal cortex and hippocampus induced by chronic morphine treatment studied ex vivo by high resolution 1H NMR spectroscopy. Neurochem. Int. 2007, 50, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Lv, L.; Yan, G.; Deng, P.; Wang, Y.; Zhou, J.; Yang, Y.; Li, Y.; Cen, X. NMR-based metabonomic in hippocampus, nucleus accumbens and prefrontal cortex of methamphetamine-sensitized rats. NeuroToxicology 2013, 36, 17–23. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a Prokaryotic Universal Primer for Simultaneous Analysis of Bacteria and Archaea Using Next-Generation Sequencing. PLOS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronesty, E. Comparison of Sequencing Utility Programs. Open Bioinform. J. 2013, 7, 1–8. [Google Scholar] [CrossRef]
- Gordon, A.; Hannon, G.J. Gordon FASTX-Toolkit. Available online: http://hannonlab.cshl.edu/fastx_toolkithttp://hannonlab.cshl.edu/fastx_toolkit2014 (accessed on 10 April 2020).
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Graham, S.F.; Hölscher, C.; McClean, P.; Elliott, C.T.; Green, B.D. 1H NMR metabolomics investigation of an Alzheimer’s disease (AD) mouse model pinpoints important biochemical disturbances in brain and plasma. Metabolomics 2013, 9, 974–983. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. Cell Biol. 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Mao, G.; Yan, L.; Hu, Y.; Ye, X.; Tian, D.; Linhardt, R.J.; Chen, S. Effect of the sulfation pattern of sea cucumber-derived fucoidan oligosaccharides on modulating metabolic syndromes and gut microbiota dysbiosis caused by HFD in mice. J. Funct. Foods 2019, 55, 193–210. [Google Scholar] [CrossRef]
- Fink, G.; Sumner, B.E.; McQueen, J.K.; Wilson, H.; Rosie, R. SEX STEROID CONTROL OF MOOD, MENTAL STATE AND MEMORY. Clin. Exp. Pharmacol. Physiol. 1998, 25, 764–775. [Google Scholar] [CrossRef]
- Sajja, S.; Perrine, S.A.; Ghoddoussi, F.; Hall, C.S.; Galloway, M.P.; VandeVord, P.J. Blast neurotrauma impairs working memory and disrupts prefrontal myo-inositol levels in rats. Mol. Cell. Neurosci. 2014, 59, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Menzie, J.; Pan, C.; Prentice, H.; Wu, J.-Y. Taurine and central nervous system disorders. Amino Acids 2014, 46, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Kurcyus, K.; Annac, E.; Hanning, N.M.; Harris, A.D.; Oeltzschner, G.; Edden, R.; Riedl, V. Opposite Dynamics of GABA and Glutamate Levels in the Occipital Cortex during Visual Processing. J. Neurosci. 2018, 38, 9967–9976. [Google Scholar] [CrossRef]
- Spoerri, P.E. Neurotrophic effects of GABA in cultures of embryonic chick brain and retina. Synapse 1988, 2, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Pow, D.V.; Sullivan, R.; Reye, P.; Hermanussen, S. Localization of taurine transporters, taurine, and (3)H taurine accumulation in the rat retina, pituitary, and brain. Glia 2001, 37, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Lizarbe, B.; Cherix, A.; Duarte, J.M.N.; Cardinaux, J.-R.; Gruetter, R. High-fat diet consumption alters energy metabolism in the mouse hypothalamus. Int. J. Obes. 2019, 43, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Dias, C.; Foerster, B.; Li, L.; Covolan, R. NAA and NAAG variation in neuronal activation during visual stimulation. Braz. J. Med. Biol. Res. 2012, 45, 1031–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.-I.; Song, K.-H.; Yoo, C.-H.; Woo, D.-C.; Choe, B.-Y. High-fat diet-induced hyperglutamatergic activation of the hippocampus in mice: A proton magnetic resonance spectroscopy study at 9.4T. Neurochem. Int. 2018, 114, 10–17. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.J.; Moloney, R.D.; Shanahan, F.; Dinan, T.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Barczynska, R.; Kapusniak, J.; Litwin, M.; Slizewska, K.; Szalecki, M. Dextrins from Maize Starch as Substances Activating the Growth of Bacteroidetes and Actinobacteria Simultaneously Inhibiting the Growth of Firmicutes, Responsible for the Occurrence of Obesity. Plant Foods Hum. Nutr. 2016, 71, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Soniwala, S.; Scinto, K.; Schott, E.; Stolarczyk, A.; Villani, D.; Dar, Q.-A.; Grier, A.; Ketz, J.; Gill, S.; Mooney, R.; et al. Oral hydrolyzed type 2 collagen protects against the OA of obesity and mitigates obese gut microbiome dysbiosis. Osteoarthr. Cartil. 2018, 26, S173–S174. [Google Scholar] [CrossRef]
- Bodogai, M.; O’Connell, J.; Kim, K.; Kim, Y.; Moritoh, K.; Chen, C.; Gusev, F.; Vaughan, K.; Shulzhenko, N.; Mattison, J.A.; et al. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci. Transl. Med. 2018, 10, eaat4271. [Google Scholar] [CrossRef]
- Wagoner, A.L.; Olson, J.D.; Westwood, B.M.; Fortunato, J.E.; Diz, D.I.; Shaltout, H.A. Children with orthostatic intolerance exhibit elevated markers of inflammation in the dorsal medulla. Am. J. Physiol. Circ. Physiol. 2019, 317, H323–H329. [Google Scholar] [CrossRef] [PubMed]
- Lizarbe, B.; Soares, A.F.; Larsson, S.; Duarte, J.M.N. Neurochemical Modifications in the Hippocampus, Cortex and Hypothalamus of Mice Exposed to Long-Term High-Fat Diet. Front. Neurosci. 2019, 12, 985. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Shungu, D.C.; Mao, X.; Nestadt, P.; Kelly, C.; Collins, K.A.; Murrough, J.W.; Charney, D.S.; Mathew, S.J. Amino Acid Neurotransmitters Assessed by Proton Magnetic Resonance Spectroscopy: Relationship to Treatment Resistance in Major Depressive Disorder. Biol. Psychiatry 2009, 65, 792–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindaraju, V.; Young, K.; Maudsley, A.A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000, 13, 129–153. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Qiu, W.; Wang, Y.; Wang, R.; Takano, T.; Li, X.; Zhu, Z.; Nakajima-Adachi, H.; Tanokura, M.; Hachimura, S.; et al. β-Elemene Suppresses Obesity-Induced Imbalance in the Microbiota-Gut-Brain Axis. Biomedicines 2021, 9, 704. https://doi.org/10.3390/biomedicines9070704
Zhou Y, Qiu W, Wang Y, Wang R, Takano T, Li X, Zhu Z, Nakajima-Adachi H, Tanokura M, Hachimura S, et al. β-Elemene Suppresses Obesity-Induced Imbalance in the Microbiota-Gut-Brain Axis. Biomedicines. 2021; 9(7):704. https://doi.org/10.3390/biomedicines9070704
Chicago/Turabian StyleZhou, Yingyu, Wanyi Qiu, Yimei Wang, Rong Wang, Tomohiro Takano, Xuyang Li, Zhangliang Zhu, Haruyo Nakajima-Adachi, Masaru Tanokura, Satoshi Hachimura, and et al. 2021. "β-Elemene Suppresses Obesity-Induced Imbalance in the Microbiota-Gut-Brain Axis" Biomedicines 9, no. 7: 704. https://doi.org/10.3390/biomedicines9070704
APA StyleZhou, Y., Qiu, W., Wang, Y., Wang, R., Takano, T., Li, X., Zhu, Z., Nakajima-Adachi, H., Tanokura, M., Hachimura, S., & Miyakawa, T. (2021). β-Elemene Suppresses Obesity-Induced Imbalance in the Microbiota-Gut-Brain Axis. Biomedicines, 9(7), 704. https://doi.org/10.3390/biomedicines9070704






