Calcium Regulation on the Atrial Regional Difference of Collagen Production Activity in Atrial Fibrogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of LA and RA Cardiac Fibroblasts from Healthy Rats
2.2. Intracellular Ca2+ Imaging
2.3. Patch Clamp Experiments
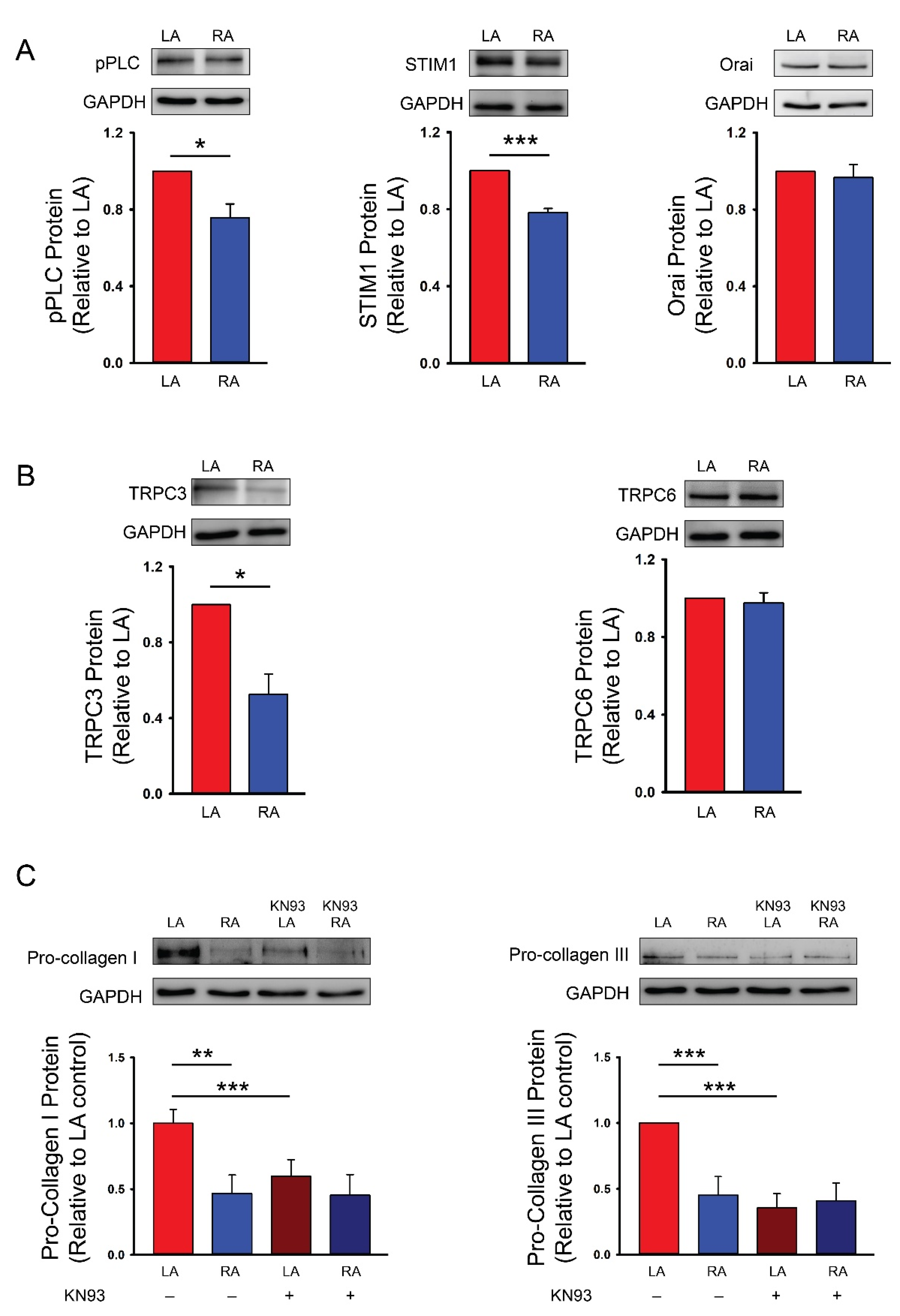
2.4. Western Blotting
2.5. Induction of Heart Failure
2.6. Statistical Analysis
3. Results
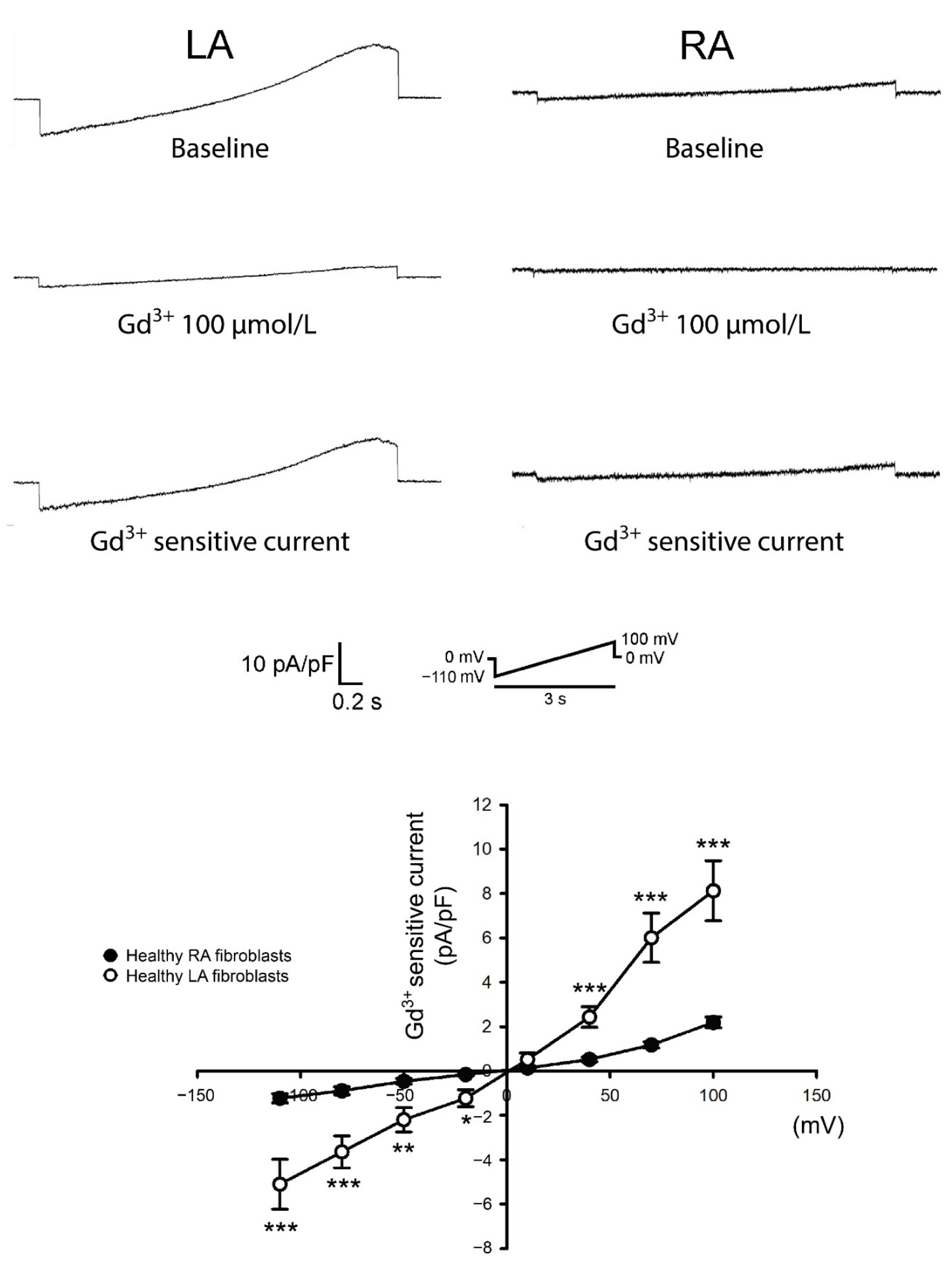
3.1. Diversity in Ca2+ Entry Between Isolated P0 LA and RA Fibroblasts from Healthy Rats
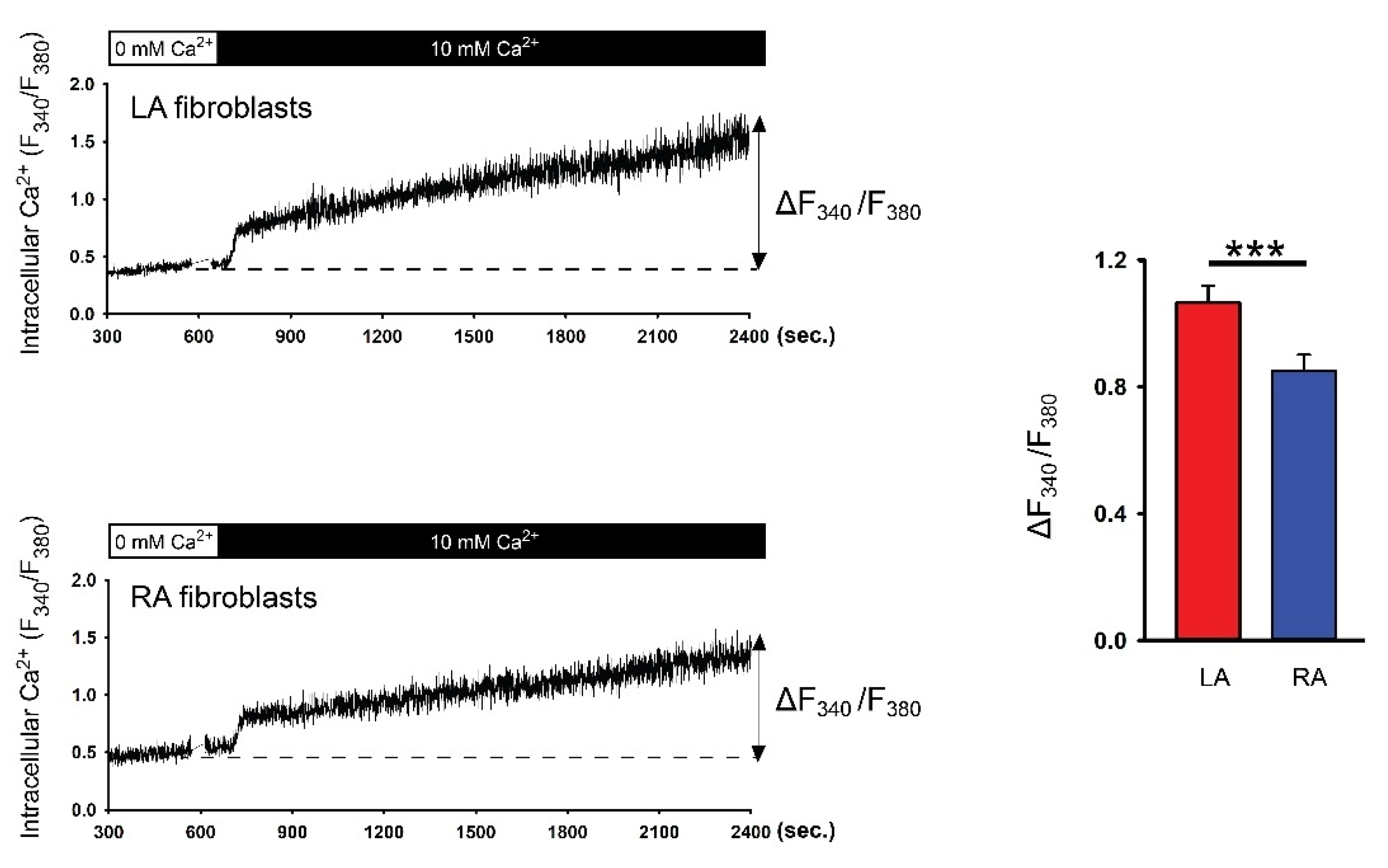
3.2. Differences in Ca2+ Signaling between Cultured P1 LA and RA Fibroblasts from Healthy Rats
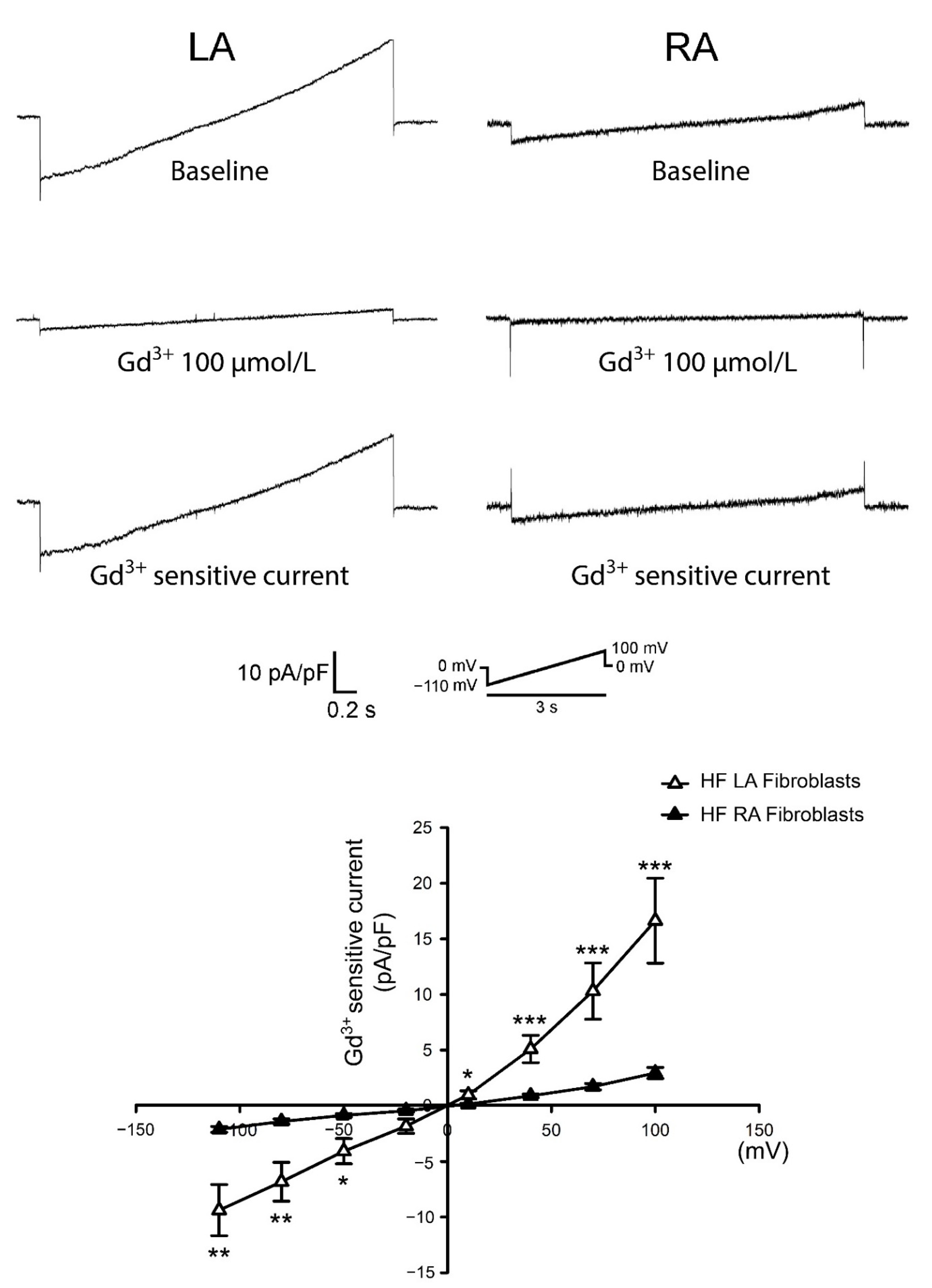
3.3. Differences in Gadolinium-Sensitive Currents between Isolated P0 LA and RA Fibroblasts from HF Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kostin, S.; Klein, G.; Szalay, Z.; Hein, S.; Bauer, E.P.; Schaper, J. Structural correlate of atrial fibrillation in human patients. Cardiovasc. Res. 2002, 54, 361–379. [Google Scholar] [CrossRef] [Green Version]
- Ohtani, K.; Yutani, C.; Nagata, S.; Koretsune, Y.; Hori, M.; Kamada, T. High prevalence of atrial fibrosis in patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 1995, 25, 1162–1169. [Google Scholar] [CrossRef] [Green Version]
- Azadani, P.N.; King, J.B.; Kheirkhahan, M.; Chang, L.; Marrouche, N.F.; Wilson, B.D. Left atrial fibrosis is associated with new-onset heart failure in patients with atrial fibrillation. Int. J. Cardiol. 2017, 248, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, K.; Zannad, F.; McMurray, J.J.V.; Krum, H.; van Veldhuisen, D.J.; Shi, H.; Vincent, J.; Pitt, B. Eplerenone and atrial fibrillation in mild systolic heart failure: Results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J. Am. Coll. Cardiol. 2012, 59, 1598–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabibiazar, R.; Wagner, R.A.; Liao, A.; Quertermous, T. Transcriptional profiling of the heart reveals chamber-specific gene expression patterns. Circ. Res. 2003, 93, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Kahr, P.C.; Piccini, I.; Fabritz, L.; Greber, B.; Schöler, H.; Scheld, H.H.; Hoffmeier, A.; Brown, N.A.; Kirchhof, P. Systematic analysis of gene expression differences between left and right atria in different mouse strains and in human atrial tissue. PLoS ONE 2011, 6, e26389. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, J.S.; Ko, Y.G.; Lee, S.H.; Lee, B.S.; Kang, S.M.; Chang, B.C.; Pak, H.N. Histological and biochemical comparisons between right atrium and left atrium in patients with mitral valvular atrial fibrillation. Korean Circ. J. 2014, 44, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasin, T.; Elhanani, O.; Abassi, Z.; Hai, T.; Aronheim, A. Angiotensin II signaling up-regulates the immediate early transcription factor ATF3 in the left but not the right atrium. Basic Res. Cardiol. 2011, 106, 175–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren, L.; Elhanani, O.; Kehat, I.; Hai, T.; Aronheim, A. Adult cardiac expression of the activating transcription factor 3, ATF3, promotes ventricular hypertrophy. PLoS ONE 2013, 8, e68396. [Google Scholar] [CrossRef]
- Jansen, H.J.; Mackasey, M.; Moghtadaei, M.; Belke, D.D.; Egom, E.E.; Tuomi, J.M.; Rafferty, S.A.; Kirkby, A.W.; Rose, R.A. Distinct patterns of atrial electrical and structural remodeling in angiotensin II mediated atrial fibrillation. J. Mol. Cell. Cardiol. 2018, 124, 12–25. [Google Scholar] [CrossRef]
- Lin, H.; Dolmatova, E.V.; Morley, M.P.; Lunetta, K.L.; McManus, D.D.; Magnani, J.W.; Margulies, K.B.; Hakonarson, H.; del Monte, F.; Benjamin, E.J.; et al. Gene expression and genetic variation in human atria. Heart Rhythm 2014, 11, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Seet, L.F.; Toh, L.Z.; Finger, S.N.; Chu, S.W.L.; Stefanovic, B.; Wong, T.T. Valproic acid suppresses collagen by selective regulation of Smads in conjunctival fibrosis. J. Mol. Med. (Berlin) 2016, 94, 321–334. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.C.; Kao, Y.H.; Yao, C.J.; Lin, Y.K.; Chen, Y.J. A comparison of left and right atrial fibroblasts reveals different collagen production activity and stress-induced mitogen-activated protein kinase signalling in rats. Acta Physiol. 2017, 220, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Kolb, M.R.J.; Duan, F.; Janssen, L.J. Transforming growth factor-β evokes Ca2+ waves and enhances gene expression in human pulmonary fibroblasts. Am. J. Respir. Cell Mol. Biol. 2012, 46, 757–764. [Google Scholar] [CrossRef]
- Mukherjee, S.; Duan, F.; Kolb, M.R.J.; Janssen, L.J. Platelet derived growth factor-evoked Ca2+ wave and matrix gene expression through phospholipase C in human pulmonary fibroblast. Int. J. Biochem. Cell Biol. 2013, 45, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Kahr, P.C.; Kaese, S.; Piccini, I.; Vokshi, I.; Scheld, H.H.; Rotering, H.; Fortmueller, L.; Laakmann, S.; Verheule, S.; et al. PITX2c is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. Circ. Cardiovasc. Genet. 2011, 4, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, Y.H.; Chung, C.C.; Cheng, W.L.; Lkhagva, B.; Chen, Y.J. Pitx2c inhibition increases atrial fibroblast activity: Implications in atrial arrhythmogenesis. Eur. J. Clin. Investig. 2019, 49, e13160. [Google Scholar] [CrossRef]
- Mookerjee, I.; Hewitson, T.D.; Halls, M.L.; Summers, R.J.; Mathai, M.L.; Bathgate, R.A.D.; Tregear, G.W.; Samuel, C.S. Relaxin inhibits renal myofibroblast differentiation via RXFP1, the nitric oxide pathway, and Smad2. FASEB J. 2009, 23, 1219–1229. [Google Scholar] [CrossRef] [Green Version]
- Mendes-Silverio, C.B.; Leiria, L.O.S.; Morganti, R.P.; Anhê, G.F.; Marcondes, S.; Mónica, F.Z.; De Nucci, G.; Antunes, E. Activation of haem-oxidized soluble guanylyl cyclase with BAY 60-2770 in human platelets lead to overstimulation of the cyclic GMP signaling pathway. PLoS ONE 2012, 7, e47223. [Google Scholar] [CrossRef] [Green Version]
- Brahmajothi, M.V.; Campbell, D.L. Heterogeneous expression of NO-activated soluble guanylyl cyclase in mammalian heart: Implications for NO- and redox-mediated indirect versus direct regulation of cardiac ion channel function. Channels (Austin Tex) 2007, 1, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Oronowicz, J.; Reinhard, J.; Reinach, P.S.; Ludwiczak, S.; Luo, H.; Omar Ba Salem, M.H.; Kraemer, M.M.; Biebermann, H.; Kakkassery, V.; Mergler, S. Ascorbate-induced oxidative stress mediates TRP channel activation and cytotoxicity in human etoposide-sensitive and -resistant retinoblastoma cells. Lab. Investig. 2021, 101, 70–88. [Google Scholar] [CrossRef]
- Du, J.; Xie, J.; Zhang, Z.; Tsujikawa, H.; Fusco, D.; Silverman, D.; Liang, B.; Yue, L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ. Res. 2010, 106, 992–1003. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, K.; Fiedler, S.; Vierkotten, S.; Weber, J.; Klee, S.; Jia, J.; Zwickenpflug, W.; Flockerzi, V.; Storch, U.; Yildirim, A.Ö.; et al. Classical transient receptor potential 6 (TRPC6) channels support myofibroblast differentiation and development of experimental pulmonary fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 560–568. [Google Scholar] [CrossRef]
- Harada, M.; Luo, X.; Qi, X.Y.; Tadevosyan, A.; Maguy, A.; Ordog, B.; Ledoux, J.; Kato, T.; Naud, P.; Voigt, N.; et al. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation 2012, 126, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Wedel, B.; Boyles, R.R.; Putney, J.W., Jr.; Bird, G.S. Role of the store-operated calcium entry proteins Stim1 and Orai1 in muscarinic cholinergic receptor-stimulated calcium oscillations in human embryonic kidney cells. J. Physiol. 2007, 579, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Obukhov, A.G.; Schaefer, M.; Harteneck, C.; Gudermann, T.; Schultz, G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 1999, 397, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Essen, L.O.; Perisic, O.; Katan, M.; Wu, Y.; Roberts, M.F.; Williams, R.L. Structural mapping of the catalytic mechanism for a mammalian phosphoinositide-specific phospholipase C. Biochemistry 1997, 36, 1704–1718. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Dragoni, S.; Lodola, F.; Bonetti, E.; Bottino, C.; Guerra, G.; Laforenza, U.; Rosti, V.; Tanzi, F. Store-dependent Ca2+ entry in endothelial progenitor cells as a perspective tool to enhance cell-based therapy and adverse tumour vascularization. Curr. Med. Chem. 2012, 19, 5802–5818. [Google Scholar] [CrossRef]
- Stathopulos, P.B.; Zheng, L.; Li, G.Y.; Plevin, M.J.; Ikura, M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell 2008, 135, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Manji, S.S.; Parker, N.J.; Williams, R.T.; van Stekelenburg, L.; Pearson, R.B.; Dziadek, M.; Smith, P.J. STIM1: A novel phosphoprotein located at the cell surface. Biochim. Biophys. Acta 2000, 1481, 147–155. [Google Scholar] [CrossRef]
- Williams, R.T.; Manji, S.S.; Parker, N.J.; Hancock, M.S.; Van Stekelenburg, L.; Eid, J.P.; Senior, P.V.; Kazenwadel, J.S.; Shandala, T.; Saint, R.; et al. Identification and characterization of the STIM (stromal interaction molecule) gene family: Coding for a novel class of transmembrane proteins. Biochem. J. 2001, 357, 673–685. [Google Scholar] [CrossRef]
- Muik, M.; Schindl, R.; Fahrner, M.; Romanin, C. Ca(2+) release-activated Ca(2+) (CRAC) current, structure, and function. Cell Mol. Life Sci. 2012, 69, 4163–4176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Jiang, J.; Yue, Z.; Liu, S.; Ma, Y.; Yu, N.; Gao, Y.; Sun, S.; Chen, S.; Liu, P. Store-Operated Ca2+ Entry (SOCE) contributes to angiotensin II-induced cardiac fibrosis in cardiac fibroblasts. J. Pharmacol. Sci. 2016, 132, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stathopulos, P.B.; Zheng, L.; Ikura, M. Stromal interaction molecule (STIM) 1 and STIM2 calcium sensing regions exhibit distinct unfolding and oligomerization kinetics. J. Biol. Chem. 2009, 284, 728–732. [Google Scholar] [CrossRef] [Green Version]
- Brandman, O.; Liou, J.; Park, W.S.; Meyer, T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 2007, 131, 1327–1339. [Google Scholar] [CrossRef] [Green Version]
- Brilla, C.G.; Scheer, C.; Rupp, H. Angiotensin II and intracellular calcium of adult cardiac fibroblasts. J. Mol. Cell Cardiol. 1998, 30, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.C.; Lin, Y.K.; Chen, Y.C.; Kao, Y.H.; Yeh, Y.H.; Chen, Y.J. Factor Xa inhibition by rivaroxaban regulates fibrogenesis in human atrial fibroblasts with modulation of nitric oxide synthesis and calcium homeostasis. J. Mol. Cell Cardiol. 2018, 123, 128–138. [Google Scholar] [CrossRef]
- Ziemba, B.P.; Falke, J.J. A PKC-MARCKS-PI3K regulatory module links Ca2+ and PIP3 signals at the leading edge of polarized macrophages. PLoS ONE 2018, 13, e0196678. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Zong, P.; Yan, J.; Yue, Z.; Li, X.; Smith, C.; Ai, X.; Yue, L. Upregulation of transient receptor potential melastatin 4 (TRPM4) in ventricular fibroblasts from heart failure patients. Pflugers Arch. 2021, 473, 521–531. [Google Scholar] [CrossRef]
- Akoum, N.; McGann, C.; Vergara, G.; Badger, T.; Ranjan, R.; Mahnkopf, C.; Kholmovski, E.; Macleod, R.O.B.; Marrouche, N. Atrial fibrosis quantified using late gadolinium enhancement MRI is associated with sinus node dysfunction requiring pacemaker implant. J. Cardiovasc. Electrophysiol. 2012, 23, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.Y.; Hsu, W.L.; Tsai, M.H.; Chai, C.Y.; Yen, C.J.; Chen, C.H.; Lu, J.H.; Yu, H.S.; Yoshioka, T. A potential new approach for treating systemic sclerosis: Dedifferentiation of SSc fibroblasts and change in the microenvironment by blocking store-operated Ca2+ entry. PLoS ONE 2019, 14, e0213400. [Google Scholar] [CrossRef] [PubMed]
- Gooch, J.L.; Gorin, Y.; Zhang, B.X.; Abboud, H.E. Involvement of calcineurin in transforming growth factor-beta-mediated regulation of extracellular matrix accumulation. J. Biol. Chem. 2004, 279, 15561–15570. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Stover, S.R.; Hoover, D.B. Regional localization and abundance of calcitonin gene-related peptide receptors in guinea pig heart. J. Mol. Cell Cardiol. 2001, 33, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubaiee, M.; Gangula, P.R.; Millis, R.M.; Walker, R.K.; Umoh, N.A.; Cousins, V.M.; Jeffress, M.A.; Haddad, G.E. Inotropic and lusitropic effects of calcitonin gene-related peptide in the heart. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1525–H1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Varagic, J.; Nagata, S.; Kon, N.D.; Ahmad, S.; VonCannon, J.L.; Wright, K.N.; Sun, X.; Deal, D.; Groban, L.; et al. Differential expression of the angiotensin-(1-12)/chymase axis in human atrial tissue. J. Surg. Res. 2020, 253, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.D.; Chang, S.F.; Wang, L.F.; Chen, C.M. Chymase mediates paraquat-induced collagen production in human lung fibroblasts. Toxicol. Lett. 2010, 193, 19–25. [Google Scholar] [CrossRef]
- Saito, K.; Muto, T.; Tomimori, Y.; Maruoka, H.; Tanaka, T.; Fukuda, Y. Human chymase stimulates Ca2+ signaling in human polymorphonuclear cells. Immunol. Lett. 2003, 89, 161–165. [Google Scholar] [CrossRef]
- Kucich, U.; Rosenbloom, J.C.; Shen, G.; Abrams, W.R.; Hamilton, A.D.; Sebti, S.M.; Rosenbloom, J. TGF-beta1 stimulation of fibronectin transcription in cultured human lung fibroblasts requires active geranylgeranyl transferase I, phosphatidylcholine-specific phospholipase C, protein kinase C-delta, and p38, but not erk1/erk2. Arch. Biochem. Biophys. 2000, 374, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-de-Lara, L.G.; Tlatelpa-Romero, B.; Romero, Y.; Fernández-Tamayo, N.; Vazquez-de-Lara, F.; Justo-Janeiro, J.M.; Garcia-Carrasco, M.; de-la-Rosa, P.R.; Cisneros-Lira, J.G.; Mendoza-Milla, C.; et al. Phosphatidylethanolamine Induces an Antifibrotic Phenotype in Normal Human Lung Fibroblasts and Ameliorates Bleomycin-Induced Lung Fibrosis in Mice. Int. J. Mol. Sci. 2018, 19, 2758. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.M.; Cabrera, C.P.; Finlay, M.; Lall, K.; Nobles, M.; Schilling, R.J.; Wood, K.; Mein, C.A.; Barnes, M.R.; Munroe, P.B.; et al. Differentially expressed genes for atrial fibrillation identified by RNA sequencing from paired human left and right atrial appendages. Physiol. Genomics 2019, 51, 323–332. [Google Scholar] [CrossRef]
- Zhang, J.; Chandrasekaran, G.; Li, W.; Kim, D.Y.; Jeong, I.Y.; Lee, S.H.; Liang, T.; Bae, J.Y.; Choi, I.; Kang, H.; et al. Wnt-PLC-IP 3-Connexin-Ca2+ axis maintains ependymal motile cilia in zebrafish spinal cord. Nat. Commun. 2020, 11, 1860. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.B.; Sonowal, H.; Shukla, K.; Srivastava, S.K.; Ramana, K.V. Aldose reductase mediates NLRP3 inflammasome-initiated innate immune response in hyperglycemia-induced Thp1 monocytes and male mice. Endocrinology 2017, 158, 3661–3675. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; La, L.; Feng, H.; Yang, Q.; Wu, F.; Wang, C.; Wu, J.; Hou, L.; Hou, C.; Liu, W. Aldose reductase inhibitor engeletin suppresses pelvic inflammatory disease by blocking the phospholipase C/protein kinase C-dependent/NF-κB and MAPK cascades. J. Agric. Food Chem. 2020, 68, 11747–11757. [Google Scholar] [CrossRef]
- Lipovsky, C.E.; Jimenez, J.; Guo, Q.; Li, G.; Yin, T.; Hicks, S.C.; Bhatnagar, S.; Takahashi, K.; Zhang, D.M.; Brumback, B.D.; et al. Chamber-specific transcriptional responses in atrial fibrillation. JCI Insight 2020, 5, e135319. [Google Scholar] [CrossRef]
- Song, S.; Babicheva, A.; Zhao, T.; Ayon, R.J.; Rodriguez, M.; Rahimi, S.; Balistrieri, F.; Harrington, A.; Shyy, J.Y.J.; Thistlethwaite, P.A.; et al. Notch enhances Ca2+ entry by activating calcium-sensing receptors and inhibiting voltage-gated K+ channels. Am. J. Physiol. Cell Physiol. 2020, 318, C954–C968. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, T.; Wang, Y.; Chen, Z.; Hua, D.; Yao, X.; Ma, X.; Zhang, P. Orai1 is critical for Notch-driven aggressiveness under hypoxic conditions in triple-negative breast cancers. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.R.; Bajwa, T., Jr.; Edwards, S.; Emelyanova, L.; Rizvi, F.; Holmuhamedov, E.L.; Werner, P.; Downey, F.X.; Tajik, A.J.; Jahangir, A. Enhanced store-operated Ca 2+ influx and ORAI1 expression in ventricular fibroblasts from human failing heart. Biol. Open 2017, 6, 326–332. [Google Scholar]
- Numaga-Tomita, T.; Kitajima, N.; Kuroda, T.; Nishimura, A.; Miyano, K.; Yasuda, S.; Kuwahara, K.; Sato, Y.; Ide, T.; Birnbaumer, L.; et al. TRPC3-GEF-H1 axis mediates pressure overload-induced cardiac fibrosis. Sci. Rep. 2016, 6, 39383. [Google Scholar] [CrossRef]
- Tai, C.T.; Lo, L.W.; Lin, Y.J.; Chen, S.A. Arrhythmogenic difference between the left and right atria in a canine ventricular pacing-induced heart failure model of atrial fibrillation. Pacing Clin. Electrophysiol. 2012, 35, 188–195. [Google Scholar] [CrossRef]
- Swartz, M.F.; Fink, G.W.; Lutz, C.J.; Taffet, S.M.; Berenfeld, O.; Vikstrom, K.L.; Kasprowicz, K.; Bhatta, L.; Puskas, F.; Kalifa, J.; et al. Left versus right atrial difference in dominant frequency, K(+) channel transcripts, and fibrosis in patients developing atrial fibrillation after cardiac surgery. Heart Rhythm 2009, 6, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.T.; Qi, X.Y.; Huang, H.; Naud, P.; Dawson, K.; Yeh, Y.H.; Harada, M.; Kuo, C.T.; Nattel, S. Disease and region-related cardiac fibroblast potassium current variations and potential functional significance. Cardiovasc. Res. 2014, 102, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giachini, F.R.C.; Chiao, C.W.; Carneiro, F.S.; Lima, V.V.; Carneiro, Z.N.; Dorrance, A.M.; Tostes, R.C.; Webb, R.C. Increased activation of stromal interaction molecule-1/Orai-1 in aorta from hypertensive rats: A novel insight into vascular dysfunction. Hypertension 2009, 53, 409–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauvet, S.; Jarvis, L.; Chevallet, M.; Shrestha, N.; Groschner, K.; Bouron, A. Pharmacological characterization of the native store-operated calcium channels of cortical neurons from embryonic mouse brain. Front. Pharmacol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Means, A.R. Regulatory cascades involving calmodulin-dependent protein kinases. Mol. Endocrinol. 2000, 14, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Voigt, N.; Li, N.; Wang, Q.; Wang, W.; Trafford, A.W.; Abu-Taha, I.; Sun, Q.; Wieland, T.; Ravens, U.; Nattel, S.; et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012, 125, 2059–2070. [Google Scholar] [CrossRef] [Green Version]
- Hoch, B.; Meyer, R.; Hetzer, R.; Krause, E.G.; Karczewski, P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ. Res. 1999, 84, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Chen, D.Q.; Qi, F.; Wang, J.; Xiao, W.Y.; Zhu, W.Z. Inhibition of calcium-calmodulin-dependent kinase II suppresses cardiac fibroblast proliferation and extracellular matrix secretion. J. Cardiovasc. Pharmacol. 2010, 55, 96–105. [Google Scholar] [CrossRef]
- Kreusser, M.M.; Lehmann, L.H.; Wolf, N.; Keranov, S.; Jungmann, A.; Gröne, H.J.; Müller, O.J.; Katus, H.A.; Backs, J. Inducible cardiomyocyte-specific deletion of CaM kinase II protects from pressure overload-induced heart failure. Basic Res. Cardiol. 2016, 111, 65. [Google Scholar] [CrossRef]
- Zhong, P.; Quan, D.; Peng, J.; Xiong, X.; Liu, Y.; Kong, B.; Huang, H. Role of CaMKII in free fatty acid/hyperlipidemia-induced cardiac remodeling both in vitro and in vivo. J. Mol. Cell Cardiol. 2017, 109, 1–16. [Google Scholar] [CrossRef]
- Masuoka, T.; Yamashita, Y.; Yoshida, J.; Nakano, K.; Tawa, M.; Nishio, M.; Ishibashi, T. Sensitization of glutamate receptor-mediated pain behaviour via nerve growth factor-dependent phosphorylation of transient receptor potential V1 under inflammatory conditions. Br. J. Pharmacol. 2020, 177, 4223–4241. [Google Scholar] [CrossRef] [PubMed]
- Siri-Angkul, N.; Song, Z.; Fefelova, N.; Gwathmey, J.K.; Chattipakorn, S.C.; Qu, Z.; Chattipakorn, N.; Xie, L.H. Activation of TRPC (transient receptor potential canonical) channel currents in iron overloaded cardiac myocytes. Circ. Arrhythm Electrophysiol. 2021, 14, e009291. [Google Scholar] [CrossRef] [PubMed]
- Germinario, E.; Esposito, A.; Midrio, M.; Peron, S.; Palade, P.T.; Betto, R.; Danieli-Betto, D. High-frequency fatigue of skeletal muscle: Role of extracellular Ca(2+). Eur. J. Appl. Physiol. 2008, 104, 445–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nejime, N.; Kagota, S.; Tada, Y.; Nakamura, K.; Hashimoto, M.; Kunitomo, M.; Shinozuka, K. Possible participation of chloride ion channels in ATP release from cancer cells in suspension. Clin. Exp. Pharmacol. Physiol. 2009, 36, 278–282. [Google Scholar] [CrossRef]
- Chen, J.B.; Tao, R.; Sun, H.Y.; Tse, H.F.; Lau, C.P.; Li, G.R. Multiple Ca2+ signaling pathways regulate intracellular Ca2+ activity in human cardiac fibroblasts. J. Cell Physiol. 2010, 223, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Wang, B.; Lin, W. Cl-channel blockers inhibit cell proliferation and arrest the cell cycle of human ovarian cancer cells. Eur. J. Gynaecol. Oncol. 2008, 29, 267–271. [Google Scholar]
- Kim, D.S.; Li, B.; Rhew, K.Y.; Oh, H.W.; Lim, H.D.; Lee, W.; Chae, H.J.; Kim, H.R. The regulatory mechanism of 4-phenylbutyric acid against ER stress-induced autophagy in human gingival fibroblasts. Arch. Pharm. Res. 2012, 35, 1269–1278. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, C.-C.; Lin, Y.-K.; Chen, Y.-C.; Kao, Y.-H.; Yeh, Y.-H.; Chen, Y.-J. Calcium Regulation on the Atrial Regional Difference of Collagen Production Activity in Atrial Fibrogenesis. Biomedicines 2021, 9, 686. https://doi.org/10.3390/biomedicines9060686
Chung C-C, Lin Y-K, Chen Y-C, Kao Y-H, Yeh Y-H, Chen Y-J. Calcium Regulation on the Atrial Regional Difference of Collagen Production Activity in Atrial Fibrogenesis. Biomedicines. 2021; 9(6):686. https://doi.org/10.3390/biomedicines9060686
Chicago/Turabian StyleChung, Cheng-Chih, Yung-Kuo Lin, Yao-Chang Chen, Yu-Hsun Kao, Yung-Hsin Yeh, and Yi-Jen Chen. 2021. "Calcium Regulation on the Atrial Regional Difference of Collagen Production Activity in Atrial Fibrogenesis" Biomedicines 9, no. 6: 686. https://doi.org/10.3390/biomedicines9060686
APA StyleChung, C.-C., Lin, Y.-K., Chen, Y.-C., Kao, Y.-H., Yeh, Y.-H., & Chen, Y.-J. (2021). Calcium Regulation on the Atrial Regional Difference of Collagen Production Activity in Atrial Fibrogenesis. Biomedicines, 9(6), 686. https://doi.org/10.3390/biomedicines9060686





