Brain-Specific Gene Expression and Quantitative Traits Association Analysis for Mild Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Samples
2.3. Genotype and Image Data Pre–Processing
2.4. Correspondences among GTEx Models, Anatomical Regions, and Freesurfer–Defined Structures
2.5. Correlation between Predictive Gene Expression and Quantitative Traits
2.6. Conversion Analysis Based on Quantitative Traits and SNPs
3. Results
3.1. Sample Characteristics
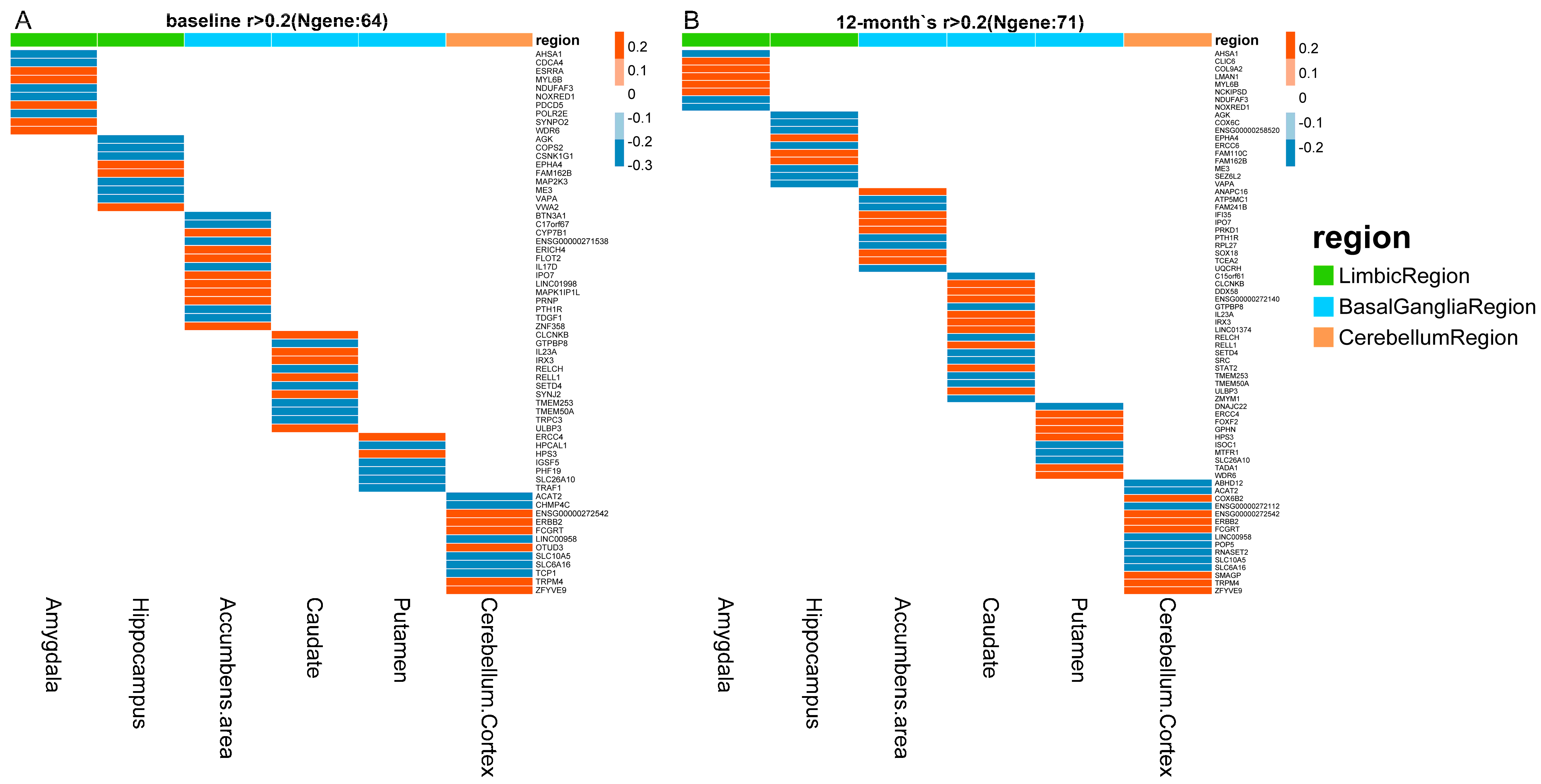
3.2. Identification of Quantitative Traits–Related Genes
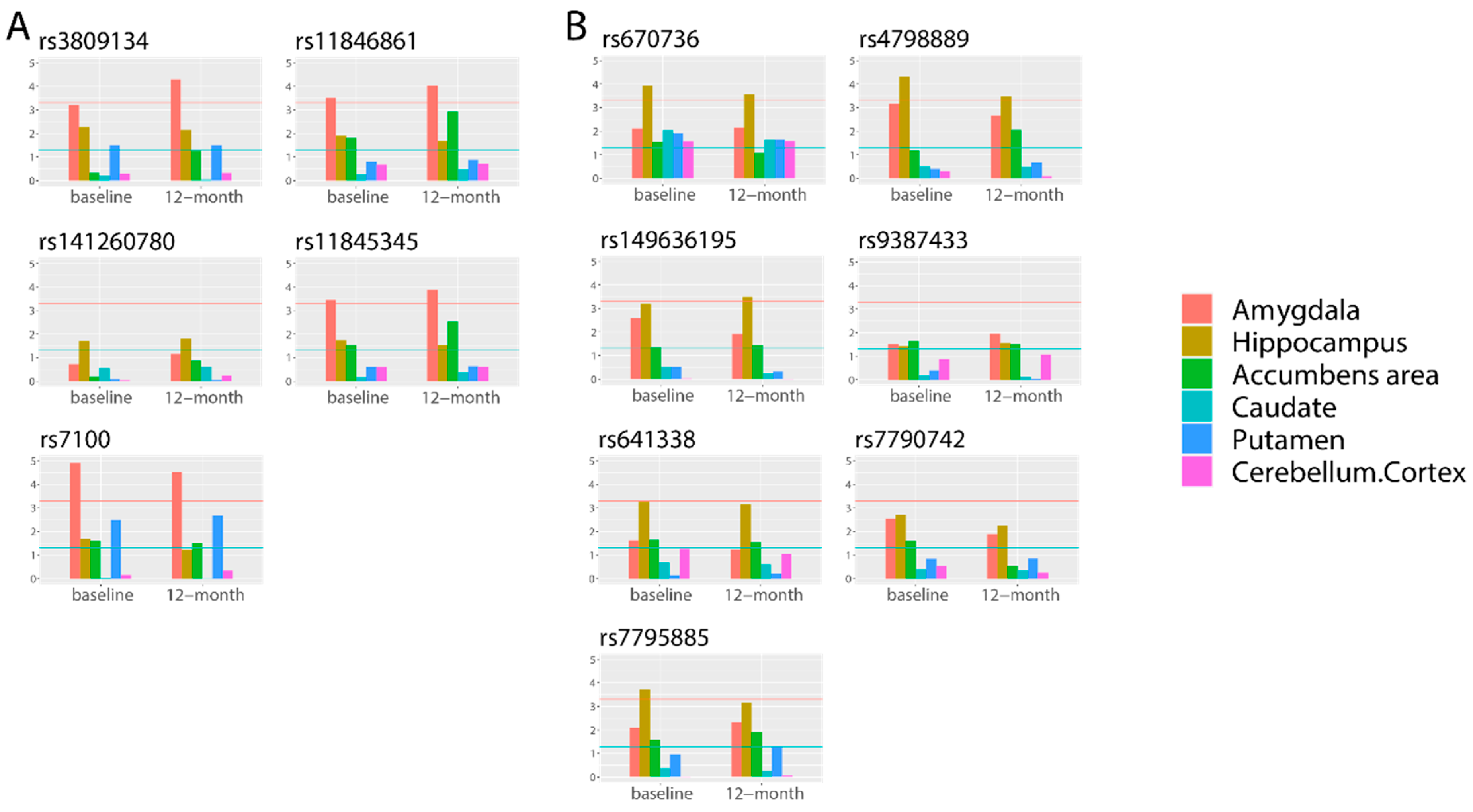
3.3. Fine-Mapping Analyses of Gene Expression-Determined Cis-eQTL SNPs
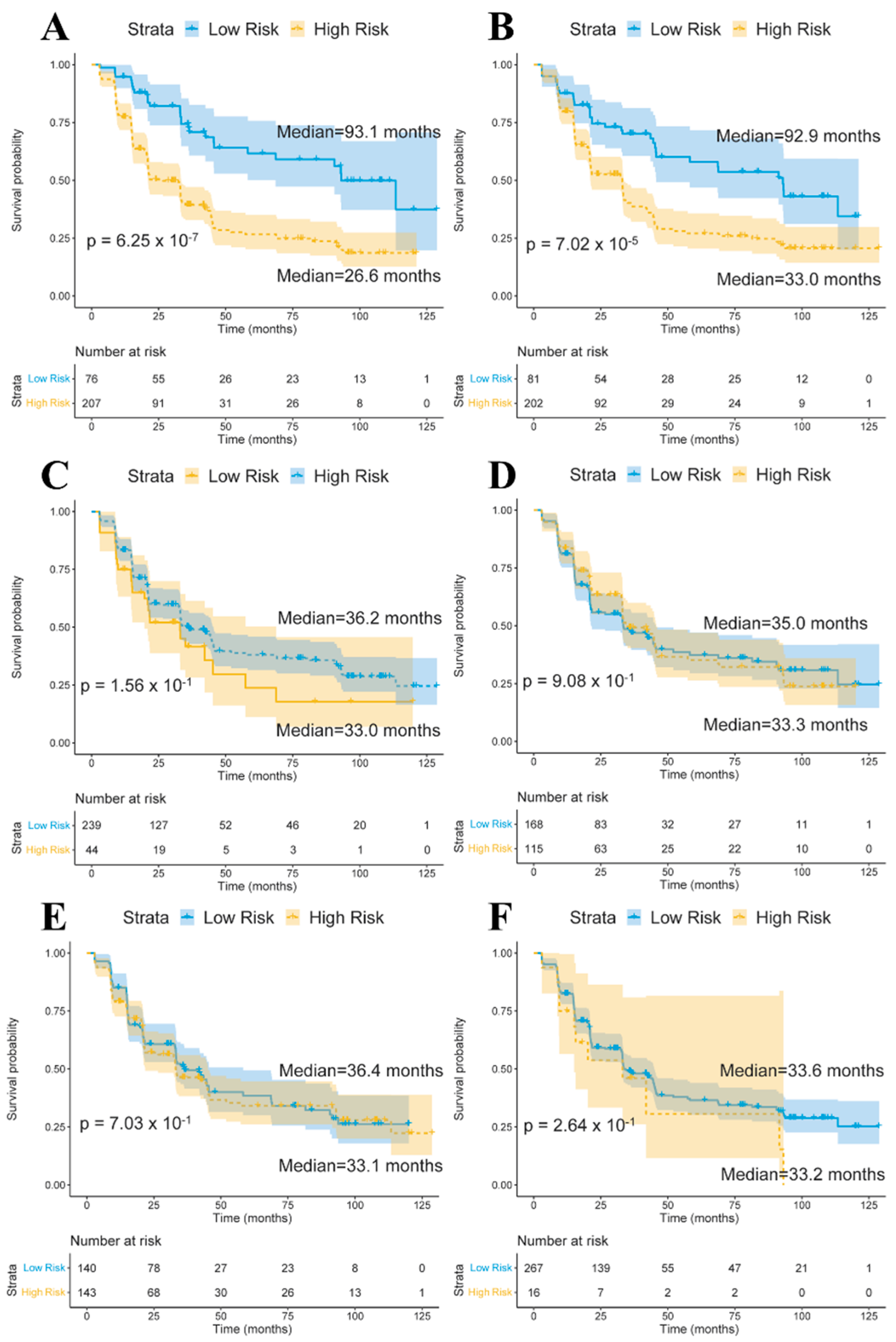
3.4. Conversion Analysis Based on Quantitative Traits and SNPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Giau, V.; Bagyinszky, E.; An, S.S.A.; Kim, S. Clinical Genetic Strategies for Early Onset Neurodegenerative Diseases. Mol. Cell. Toxicol. 2018, 14, 123–142. [Google Scholar] [CrossRef]
- Haines, J.L. Alzheimer Disease: Perspectives from Epidemiology and Genetics. J. Law Med. Ethics 2018, 46, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.F.; Snitz, B.E.; Ganguli, M. Should Mild Cognitive Impairment Be Subtyped? Curr. Opin. Psychiatry 2011, 24, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.-C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; Jun, G.; DeStefano, A.L.; Bis, J.C.; Beecham, G.W.; et al. Meta-Analysis of 74,046 Individuals Identifies 11 New Susceptibility Loci for Alzheimer’s Disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Jansen, I.E.; Savage, J.E.; Watanabe, K.; Bryois, J.; Williams, D.M.; Steinberg, S.; Sealock, J.; Karlsson, I.K.; Hägg, S.; Athanasiu, L.; et al. Genome-Wide Meta-Analysis Identifies New Loci and Functional Pathways Influencing Alzheimer’s Disease Risk. Nat. Genet. 2019, 51, 404–413. [Google Scholar] [CrossRef]
- Schwartzentruber, J.; Cooper, S.; Liu, J.Z.; Barrio-Hernandez, I.; Bello, E.; Kumasaka, N.; Young, A.M.; Franklin, R.J.; Johnson, T.; Estrada, K.; et al. Genome-Wide Meta-Analysis, Fine-Mapping and Integrative Prioritization Implicate New Alzheimer’s Disease Risk Genes. Nat. Genet. 2021, 53, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Kim, S.; Risacher, S.L.; Nho, K.; Swaminathan, S.; West, J.D.; Foroud, T.; Pankratz, N.; Moore, J.H.; Sloan, C.D.; et al. Whole Genome Association Study of Brain-Wide Imaging Phenotypes for Identifying Quantitative Trait Loci in MCI and AD: A Study of the ADNI Cohort. NeuroImage 2010, 53, 1051–1063. [Google Scholar] [CrossRef]
- Albert, F.W.; Kruglyak, L. The Role of Regulatory Variation in Complex Traits and Disease. Nat. Rev. Genet. 2015, 16, 197–212. [Google Scholar] [CrossRef]
- Lappalainen, T.; Sammeth, M.; Friedländer, M.R.; AC‘t Hoen, P.; Monlong, J.; Rivas, M.A.; Gonzalez-Porta, M.; Kurbatova, N.; Griebel, T.; Ferreira, P.G.; et al. Transcriptome and Genome Sequencing Uncovers Functional Variation in Humans. Nature 2013, 501, 506–511. [Google Scholar] [CrossRef]
- Gusev, A.; Ko, A.; Shi, H.; Bhatia, G.; Chung, W.; Penninx, B.W.; Jansen, R.; De Geus, E.J.; Boomsma, D.I.; Wright, F.A.; et al. Integrative Approaches for Large-Scale Transcriptome-Wide Association Studies. Nat. Genet. 2016, 48, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Gamazon, E.R.; Wheeler, H.E.; Shah, K.P.; Mozaffari, S.V.; Aquino-Michaels, K.; Carroll, R.J.; Eyler, A.E.; Denny, J.C.; Nicolae, D.L.; Cox, N.J.; et al. A Gene-Based Association Method for Mapping Traits Using Reference Transcriptome Data. Nat. Genet. 2015, 47, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Raj, T.; Li, Y.I.; Wong, G.; Humphrey, J.; Wang, M.; Ramdhani, S.; Wang, Y.-C.; Ng, B.; Gupta, I.; Haroutunian, V.; et al. Integrative Transcriptome Analyses of the Aging Brain Implicate Altered Splicing in Alzheimer’s Disease Susceptibility. Nat. Genet. 2018, 50, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Wang, R.; Zhang, Y.; Zhan, H. Prediction of Alzheimer’s Disease-Associated Genes by Integration of GWAS Summary Data and Expression Data. Front. Genet. 2019, 9, 653. [Google Scholar] [CrossRef]
- Jung, S.-H.; Nho, K.; Kim, D.; Won, H.-H.; Initiative, A.D.N. Genetic Risk Prediction of Late-Onset Alzheimer’s Disease Based on Tissue-Specific Transcriptomic Analysis and Polygenic Risk Scores: Genetics/Genetic Factors of Alzheimer’s Disease. Alzheimer’s Dement. 2020, 16, e045184. [Google Scholar] [CrossRef]
- Gerring, Z.F.; Lupton, M.K.; Edey, D.; Gamazon, E.R.; Derks, E.M. An Analysis of Genetically Regulated Gene Expression across Multiple Tissues Implicates Novel Gene Candidates in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- GTEx Consortium. Genetic Effects on Gene Expression across Human Tissues. Nature 2017, 550, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Boyes, R.G.; Gunter, J.L.; Frost, C.; Janke, A.L.; Yeatman, T.; Hill, D.L.; Bernstein, M.A.; Thompson, P.M.; Weiner, M.W.; Schuff, N.; et al. Intensity Non-Uniformity Correction Using N3 on 3-T Scanners with Multichannel Phased Array Coils. Neuroimage 2008, 39, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Howie, B.N.; Donnelly, P.; Marchini, J. A Flexible and Accurate Genotype Imputation Method for the next Generation of Genome-Wide Association Studies. PLoS Genet. 2009, 5, e1000529. [Google Scholar] [CrossRef] [PubMed]
- Dayem Ullah, A.Z.; Lemoine, N.R.; Chelala, C. SNPnexus: A Web Server for Functional Annotation of Novel and Publicly Known Genetic Variants (2012 Update). Nucleic Acids Res. 2012, 40, W65–W70. [Google Scholar] [CrossRef]
- Ward, L.D.; Kellis, M. HaploReg: A Resource for Exploring Chromatin States, Conservation, and Regulatory Motif Alterations within Sets of Genetically Linked Variants. Nucleic Acids Res. 2012, 40, D930–D934. [Google Scholar] [CrossRef]
- Boyle, A.P.; Hong, E.L.; Hariharan, M.; Cheng, Y.; Schaub, M.A.; Kasowski, M.; Karczewski, K.J.; Park, J.; Hitz, B.C.; Weng, S.; et al. Annotation of Functional Variation in Personal Genomes Using RegulomeDB. Genome Res. 2012, 22, 1790–1797. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, Y.-J.; Bai, X.-F.; Han, X.-L.; Jiang, Y.; Ai, B.; Shi, S.-S.; Wang, F.; Xu, M.-C.; Wang, Y.-Z.; et al. VARAdb: A Comprehensive Variation Annotation Database for Human. Nucleic Acids Res. 2021, 49, D1431–D1444. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, F.; Bai, X.; Liu, Y.; Wang, Q.; Ai, B.; Han, X.; Shi, S.; Zhang, J.; Li, X.; et al. SEdb: A Comprehensive Human Super-Enhancer Database. Nucleic Acids Res. 2019, 47, D235–D243. [Google Scholar] [CrossRef]
- Barnes, D.E.; Cenzer, I.S.; Yaffe, K.; Ritchie, C.S.; Lee, S.J.; Alzheimer’s Disease Neuroimaging Initiative. A Point-Based Tool to Predict Conversion from Mild Cognitive Impairment to Probable Alzheimer’s Disease. Alzheimer’s Dement. 2014, 10, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-M.; Zeng, H.-D.; Zhang, C.-Y.; Xu, J.-W. Identification of a Six-Gene Signature Predicting Overall Survival for Hepatocellular Carcinoma. Cancer Cell Int. 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, A.F.; Rozemuller, A.J.; van der Flier, W.M.; Scheltens, P.; van der Vies, S.M.; Hoozemans, J.J. Altered Distribution of the EphA4 Kinase in Hippocampal Brain Tissue of Patients with Alzheimer’s Disease Correlates with Pathology. Acta Neuropathol. Commun. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Tamura, K.; Chiu, Y.-W.; Shiohara, A.; Hori, Y.; Tomita, T. EphA4 Regulates Aβ Production via BACE1 Expression in Neurons. FASEB J. 2020, 34, 16383–16396. [Google Scholar] [CrossRef]
- Gavin, C.F.; Rubio, M.D.; Young, E.; Miller, C.; Rumbaugh, G. Myosin II Motor Activity in the Lateral Amygdala Is Required for Fear Memory Consolidation. Learn. Mem. 2012, 19, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, R.; Margulies, D.; Farb, C.; Hou, M.; Johnson, L.; LeDoux, J. Myosin Light Chain Kinase Regulates Synaptic Plasticity and Fear Learning in the Lateral Amygdala. Neuroscience 2006, 139, 821–829. [Google Scholar] [CrossRef]
- Kneussel, M.; Wagner, W. Myosin Motors at Neuronal Synapses: Drivers of Membrane Transport and Actin Dynamics. Nat. Rev. Neurosci. 2013, 14, 233–247. [Google Scholar] [CrossRef]
- Ishii, M.; Iadecola, C. Risk Factor for Alzheimer’s Disease Breaks the Blood–Brain Barrier; Nature Publishing Group: Berlin, Germany, 2020. [Google Scholar]
- Gill, G. Regulation of the Initiation of Eukaryotic Transcription. Essays Biochem. 2001, 37, 33–44. [Google Scholar] [PubMed]
- Khurana, E.; Fu, Y.; Chakravarty, D.; Demichelis, F.; Rubin, M.A.; Gerstein, M. Role of Non-Coding Sequence Variants in Cancer. Nat. Rev. Genet. 2016, 17, 93–108. [Google Scholar] [CrossRef]
- Kikuchi, M.; Hara, N.; Hasegawa, M.; Miyashita, A.; Kuwano, R.; Ikeuchi, T.; Nakaya, A. Enhancer Variants Associated with Alzheimer’s Disease Affect Gene Expression via Chromatin Looping. BMC Med. Genom. 2019, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Lee, J.J.; Gunasekaran, T.I.; Kang, S.; Lee, W.; Jeong, J.; Lim, H.J.; Zhang, X.; Zhu, C.; Won, S.-Y.; et al. APOE Promoter Polymorphism-219T/G Is an Effect Modifier of the Influence of APOE Ε4 on Alzheimer’s Disease Risk in a Multiracial Sample. J. Clin. Med. 2019, 8, 1236. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.K.; Hung, K.-W.; Huang, H.; Gu, S.; Shen, Y.; Cheng, E.Y.; Ip, F.C.; Huang, X.; Fu, W.-Y.; Ip, N.Y. Blockade of EphA4 Signaling Ameliorates Hippocampal Synaptic Dysfunctions in Mouse Models of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2014, 111, 9959–9964. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.; Cerpa, W.; Muñoz, F.; Zanlungo, S.; Alvarez, A. Amyloid-β Oligomers Synaptotoxicity: The Emerging Role of EphA4/c-Abl Signaling in Alzheimer’s Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1148–1159. [Google Scholar] [CrossRef]
- Blair, L.J.; Sabbagh, J.J.; Dickey, C.A. Targeting Hsp90 and Its Co-Chaperones to Treat Alzheimer’s Disease. Expert Opin. Ther. Targets 2014, 18, 1219–1232. [Google Scholar] [CrossRef]
- Rudolf, R.; Bittins, C.M.; Gerdes, H.-H. The Role of Myosin V in Exocytosis and Synaptic Plasticity. J. Neurochem. 2011, 116, 177–191. [Google Scholar] [CrossRef]
- Matteoli, M.; Coco, S.; Schenk, U.; Verderio, C. Vesicle Turnover in Developing Neurons: How to Build a Presynaptic Terminal. Trends Cell Biol. 2004, 14, 133–140. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Guan, X.; Nathan, P.J.; Wren, P. BDNF-Based Synaptic Repair as a Disease-Modifying Strategy for Neurodegenerative Diseases. Nat. Rev. Neurosci. 2013, 14, 401–416. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative Stress, Dysfunctional Glucose Metabolism and Alzheimer Disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Rottkamp, C.A.; Nunomura, A.; Raina, A.K.; Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative Stress, Antioxidants, and Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2000, 14, S62–S66. [Google Scholar] [CrossRef]
- Parkinson, M.H.; Schulz, J.B.; Giunti, P. Co-Enzyme Q10 and Idebenone Use in Friedreich’s Ataxia. J. Neurochem. 2013, 126, 125–141. [Google Scholar] [CrossRef]
- Qian, L.; Liu, R.; Qin, R.; Zhao, H.; Xu, Y. The Associated Volumes of Sub-Cortical Structures and Cognitive Domain in Patients of Mild Cognitive Impairment. J. Clin. Neurosci. 2018, 56, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wu, X.; Li, R.; Chen, K.; Long, Z.; Zhang, J.; Guo, X.; Yao, L. Prediction of Progressive Mild Cognitive Impairment by Multi-Modal Neuroimaging Biomarkers. J. Alzheimer’s Dis. 2016, 51, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-A.; Möller, C.; Dieleman, N.; Bouwman, F.H.; Barkhof, F.; Scheltens, P.; van der Flier, W.M.; Vrenken, H. Relation between Subcortical Grey Matter Atrophy and Conversion from Mild Cognitive Impairment to Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2016, 87, 425–432. [Google Scholar] [CrossRef] [PubMed]

| GTEx Model | Region | Subcortical Structures |
|---|---|---|
| Brain Amygdala | Limbic | Amygdala |
| Brain Hippocampus | Hippocampus | |
| Brain Caudate basal ganglia | Basal Ganglia | Caudate |
| Brain Putamen basal ganglia | Putamen | |
| Brain Nucleus accumbens basal ganglia | Accumbens area | |
| Brain Cerebellum | Cerebellum | Cerebellum cortex |
| Characteristic | Number (%) or Mean ± SD |
|---|---|
| Demographic | |
| Age, years | 74.85 ± 6.97 |
| Gender, female | 97 (33.9) |
| Education, ≤12 years | 53 (18.5) |
| Neuropsychological measures | |
| CDRSB | 1.53 ± 0.85 |
| MMSE | 27.04 ± 1.78 |
| FAQ | 3.89 ± 4.49 |
| ADAS11 | 11.66 ± 4.40 |
| ASAS13 | 4.40 ± 6.38 |
| ADASQ4 | 18.91 ± 2.23 |
| Conversion MCI | 167 (58.4) |
| Conversion period | 25.05 ± 21.76 |
| Non–conversion MCI | 119 (41.6) |
| With <3 years of follow–up data | 45 (37.8) |
| With ≥3 years of follow–up data | 71 (59.7) |
| With only 1 follow–up visit | 3 (0.03) |
| Structures | N | n | Overlap Genes | SNPs | Ranks | Annotations |
|---|---|---|---|---|---|---|
| Limbic Region | ||||||
| Amygdala | 10/8 | 4 | NDUFAF3 (−) | rs7100 | 1/1 | MCI |
| NOXRED1 (−) | rs141260780 a, rs11846861 a | 2/3 | - | |||
| AHSA1 (−) | rs11845345 a | 5/4 | AD/MCI | |||
| MYL6B (+) | rs3809134 ab | 9/2 | - | |||
| Hippocampus | 9/10 | 5 | VAPA (−) | rs4798889 ab | 1/5 | AD/MCI |
| ME3 (−) | rs670736 ab | 2/1 | MCI | |||
| AGK (−) | rs7790742 a, rs7795885 a | 3/9 | AD/MCI | |||
| FAM162B (+) | rs9387433, rs641338 a | 6/7 | - | |||
| EPHA4 (+) | rs149636195 ab | 8/3 | AD/MCI | |||
| Basal ganglia Region | ||||||
| Accumbens Area | 14/11 | 2 | PTH1R (−) | rs2168442 ab, rs144645644 b | 1/7 | AD/MCI |
| IPO7 (+) | rs75955853 ab, rs12363308 b | 3/1 | AD | |||
| Caudate | 12/17 | 10 | GTPBP8 (−) | rs114429530 ab | 1/1 | AD |
| RELCH (−) | rs3752091 a, rs9958695 | 2/8 | - | |||
| IRX3 (+) | rs191251428 ab | 4/3 | - | |||
| CLCNKB (+) | rs75909377 ab | 5/5 | MCI | |||
| IL23A (+) | rs79824801 ab | 6/10 | AD/MCI | |||
| RELL1 (+) | rs3832308, rs4832933 ab | 7/7 | - | |||
| TMEM50A (−) | rs3093586 b, rs3091243 b, rs8876 b | 8/4 | - | |||
| SETD4 (−) | rs2835263, rs142847892 a | 9/11 | - | |||
| ULBP3 (+) | rs1537648 a | 10/16 | AD | |||
| TMEM253 (−) | rs10872886 | 11/14 | - | |||
| Putamen | 7/10 | 3 | ERCC4 (+) | rs6498486 a, rs3136042 a, rs1799798 a | 1/1 | AD/MCI |
| HPS3 (+) | rs13089410 a, rs7643410 a | 3/4 | - | |||
| SLC26A10 (−) | rs10747780, rs10437954 | 5/5 | - | |||
| Cerebellum Region | ||||||
| Cerebellum Cortex | 12/15 | 9 | SLC6A16 (−) | rs8102658 a | 1/1 | - |
| SLC10A5 (−) | rs2955002, rs58379275, rs75348453 | 2/2 | - | |||
| ACAT2 (−) | rs2025187 ab | 3/5 | AD/MCI | |||
| ZFYVE9 (+) | rs627011 ab | 4/4 | MCI | |||
| ENSG00000272542 (+) | rs1886087, rs9518861, rs9554903 | 5/3 | - | |||
| ERBB2 (+) | rs2517955 ab, rs75849983 ab | 7/6 | AD/MCI | |||
| LINC00958 (−) | rs111880988, rs4756736 | 8/15 | - | |||
| FCGRT (+) | rs2946865 ab, rs1132990 b | 9/13 | - | |||
| TRPM4 (+) | rs11882563 ab, rs11083963 b, rs73048855 | 12/9 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.-X.; Li, H.-T.; Gu, Y.; Sun, X. Brain-Specific Gene Expression and Quantitative Traits Association Analysis for Mild Cognitive Impairment. Biomedicines 2021, 9, 658. https://doi.org/10.3390/biomedicines9060658
Yuan S-X, Li H-T, Gu Y, Sun X. Brain-Specific Gene Expression and Quantitative Traits Association Analysis for Mild Cognitive Impairment. Biomedicines. 2021; 9(6):658. https://doi.org/10.3390/biomedicines9060658
Chicago/Turabian StyleYuan, Shao-Xun, Hai-Tao Li, Yu Gu, and Xiao Sun. 2021. "Brain-Specific Gene Expression and Quantitative Traits Association Analysis for Mild Cognitive Impairment" Biomedicines 9, no. 6: 658. https://doi.org/10.3390/biomedicines9060658
APA StyleYuan, S.-X., Li, H.-T., Gu, Y., & Sun, X. (2021). Brain-Specific Gene Expression and Quantitative Traits Association Analysis for Mild Cognitive Impairment. Biomedicines, 9(6), 658. https://doi.org/10.3390/biomedicines9060658






