The Core Stem Genes SOX2, POU5F1/OCT4, and NANOG Are Expressed in Human Parathyroid Tumors and Modulated by MEN1, YAP1, and β-catenin Pathways Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Immunohistochemistry
2.3. RNA Isolation and Real-Time Quantitative Reverse Transcription (qRT-PCR)
2.4. Primary Parathyroid Adenoma Cell Isolation and Culture
2.5. DNA Extraction and Array Comparative Genomic Hybridization (aCGH) Analysis
2.6. Treatment of PAds-Derived Primary Cell Preparations with Lithium Chloride
2.7. Cell Transfection and RNA Interference
2.8. Statistical Analysis
3. Results
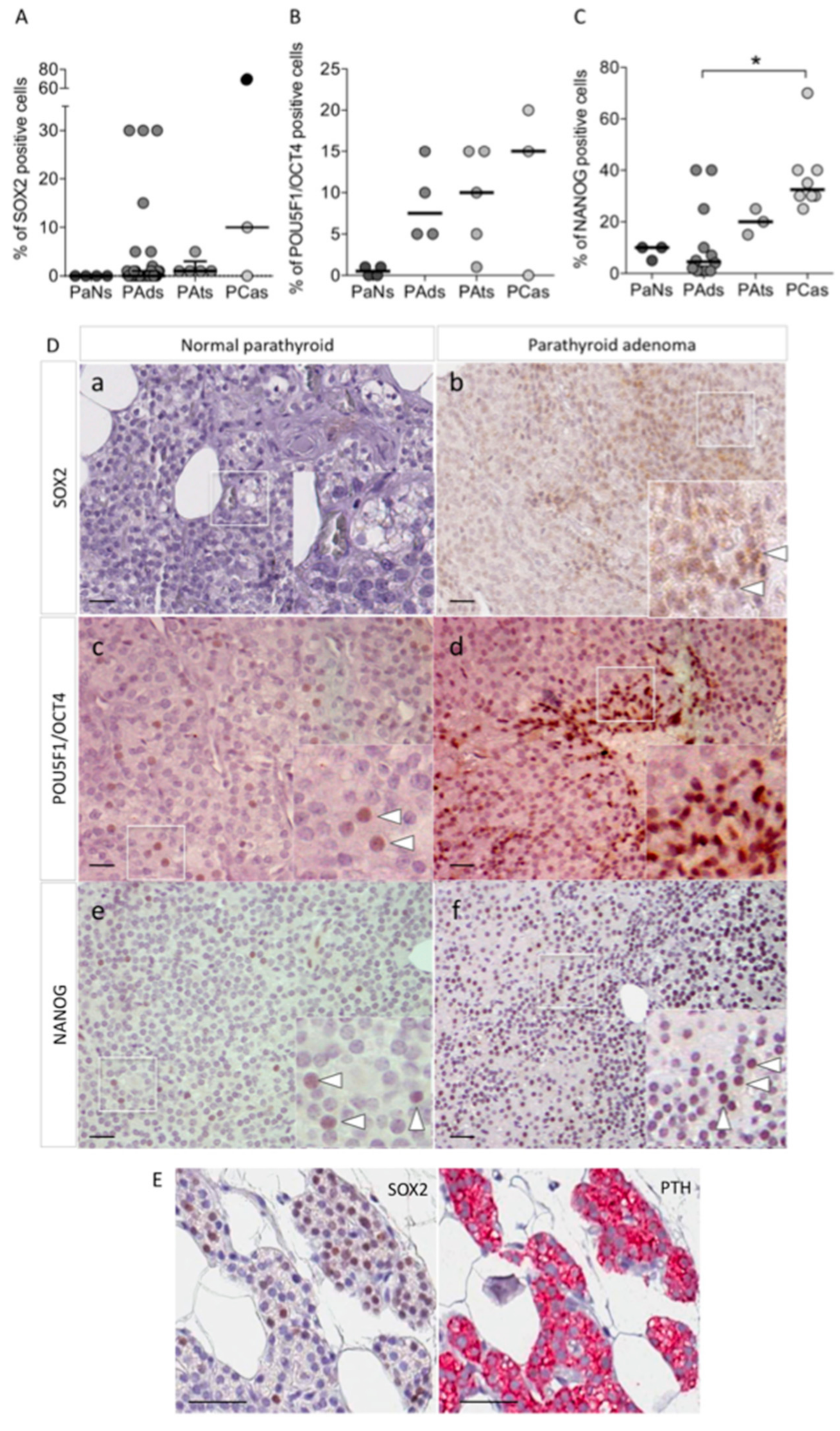
3.1. The Core Stem Cell Genes SOX2, POU5F1/OCT4, and NANOG Are Expressed in Parathyroid Tumors
3.2. Modulation of Core Stem Genes Expression in Sporadic Parathyroid Tumors
3.2.1. Role of MEN1
3.2.2. Role of CASR-Stimulated YAP1 Signaling
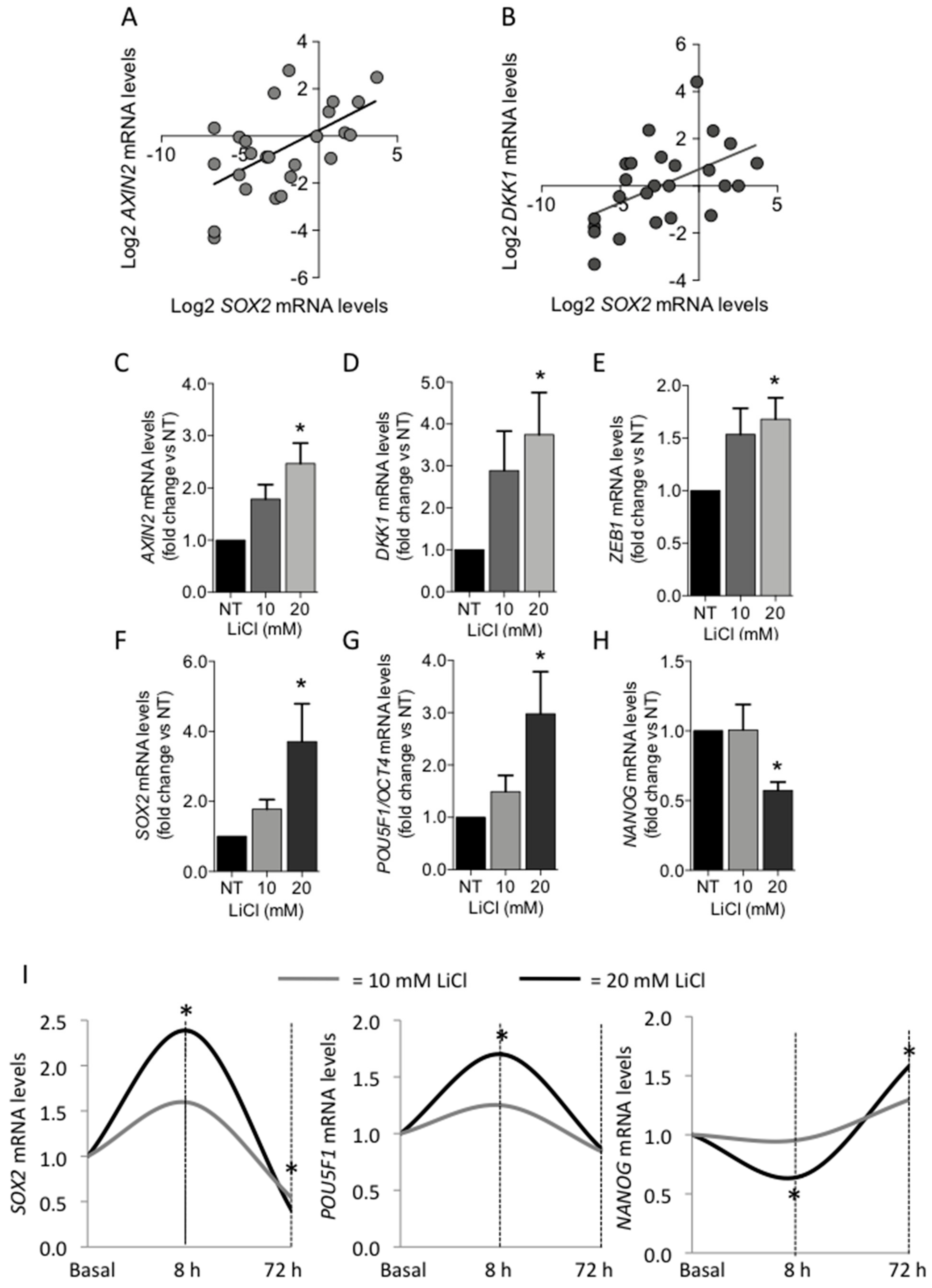
3.2.3. Role of Canonical WNT/β-Catenin Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peissig, K.; Condie, B.G.; Manley, N.R. Embryology of the parathyroid glands. Endocrinol. Metab. Clin. North. Am. 2018, 47, 733–742. [Google Scholar] [CrossRef]
- Shen, H.C.; Rosen, J.E.; Yang, L.M.; Savage, S.A.; Burns, A.L.; Mateo, C.M.; Agarwal, S.K.; Chandrasekharappa, S.C.; Spiegel, A.M.; Collins, F.S.; et al. Parathyroid tumor development involves deregulation of homeobox genes. Endocr. Relat. Cancer 2008, 15, 267–275. [Google Scholar] [CrossRef]
- D’Agruma, L.; Coco, M.; Guarnieri, V.; Battista, C.; Canaff, L.; Salcuni, A.S.; Corbetta, S.; Cetani, F.; Minisola, S.; Chiodini, I.; et al. Increased prevalence of the GCM2 polymorphism, Y282D, in primary hyperparathyroidism: Analysis of three Italian cohorts. J. Clin. Endocrinol. Metab. 2014, 99, E2794–E2798. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Welch, J.M.; Sapp, J.C.; Ling, H.; Li, Y.; Johnston, J.J.; Kebebew, E.; Biesecker, L.G.; Simonds, W.F.; Marx, S.J.; et al. GCM2-activating mutations in familial isolated hyperparathyroidism. Am. J. Hum. Genet. 2016, 99, 1034–1044. [Google Scholar] [CrossRef]
- Riccardi, A.; Aspir, T.; Shen, L.; Kuo, C.L.; Brown, T.C.; Korah, R.; Murtha, T.D.; Bellizzi, J.; Parham, K.; Carling, T.; et al. Analysis of activating GCM2 sequence variants in sporadic parathyroid adenomas. J. Clin. Endocrinol. Metab. 2019, 104, 1948–1952. [Google Scholar] [CrossRef]
- Verdelli, C.; Avagliano, L.; Guarnieri, V.; Cetani, F.; Ferrero, S.; Vicentini, L.; Beretta, E.; Scillitani, A.; Creo, P.; Bulfamante, G.P.; et al. Expression, function, and regulation of the embryonic transcription factor TBX1 in parathyroid tumors. Lab. Investig. 2017, 97, 1488–1499. [Google Scholar] [CrossRef]
- Condello, V.; Cetani, F.; Denaro, M.; Torregrossa, L.; Pardi, E.; Piaggi, P.; Borsari, S.; Poma, A.M.; Muscarella, L.A.; Graziano, P.; et al. Gene expression profile in metastatic and non-metastatic parathyroid carcinoma. Endocr. Relat. Cancer 2021, 28, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Verdelli, C.; Forno, I.; Vaira, V.; Corbetta, S. MicroRNA deregulation in parathyroid tumours suggests an embryonic signature. J. Endocrinol. Investig. 2015, 38, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Vaira, V.; Elli, F.; Forno, I.; Guarnieri, V.; Verdelli, C.; Ferrero, S.; Scillitani, A.; Vicentini, L.; Cetani, F.; Mantovani, G.; et al. The microRNA cluster C19MC is deregulated in parathyroid tumours. J. Mol. Endocrinol. 2012, 49, 115–124. [Google Scholar] [CrossRef]
- Brafman, D.A.; Moya, N.; Allen-Soltero, S.; Fellner, T.; Robinson, M.; McMillen, Z.L.; Gaasterland, T.; Willert, K. Analysis of SOX2-expressing cell populations derived from human pluripotent stem cells. Stem Cell Rep. 2013, 1, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Fathi, F.; Mobalegi, J.; Sofimajidpour, H.; Ghadimi, T. The expressions of stem cell markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3, Dppa4, and Esrrb in bladder, colon, and prostate cancer, and certain cancer cell lines. Anat. Cell Biol. 2014, 47, 1–11. [Google Scholar] [CrossRef]
- Najafzadeh, B.; Asadzadeh, Z.; Motafakker, A.R.; Mokhtarzadeh, A.; Baghbanzadeh, A.; Alemohammad, H.; Abdoli Shadbad, M.; Vasefifar, P.; Najafi, S.; Baradaran, B. The oncogenic potential of NANOG: An important cancer induction mediator. J. Cell Physiol. 2021, 236, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Amaya, C.N.; Bryan, B.A. Enrichment of the embryonic stem cell reprogramming factors Oct4, Nanog, Myc, and Sox2 in benign and malignant vascular tumors. BMC Clin. Pathol. 2015, 15, 18. [Google Scholar] [CrossRef]
- Chang, C.V.; Araujo, R.V.; Cirqueira, C.S.; Cani, C.M.; Matushita, H.; Cescato, V.A.; Fragoso, M.C.; Bronstein, M.D.; Zerbini, M.C.; Mendonca, B.B.; et al. Differential expression of stem cell markers in human adamantinomatous craniopharyngioma and pituitary adenoma. Neuroendocrinology 2017, 104, 183–193. [Google Scholar] [CrossRef]
- Peverelli, E.; Giardino, E.; Treppiedi, D.; Meregalli, M.; Belicchi, M.; Vaira, V.; Corbetta, S.; Verdelli, C.; Verrua, E.; Serban, A.L.; et al. Dopamine receptor type 2 (DRD2) and somatostatin receptor type 2 (SSTR2) agonists are effective in inhibiting proliferation of progenitor/stem-like cells isolated from nonfunctioning pituitary tumors. Int. J. Cancer 2017, 140, 1870–1880. [Google Scholar] [CrossRef]
- Mong, E.F.; Yang, Y.; Akat, K.M.; Canfield, J.; VanWye, J.; Lockhart, J.; Tsibris, J.C.M.; Schatz, F.; Lockwood, C.J.; Tuschl, T.; et al. Chromosome 19 microRNA cluster enhances cell reprogramming by inhibiting epithelial-to-mesenchymal transition. Sci. Rep. 2020, 10, 3029. [Google Scholar] [CrossRef]
- Bhuiyan, M.M.; Sato, M.; Murao, K.; Imachi, H.; Namihira, H.; Takahara, J. Expression of menin in parathyroid tumors. J. Clin. Endocrinol. Metab. 2000, 85, 2615–2619. [Google Scholar] [CrossRef]
- Tavanti, G.S.; Verdelli, C.; Morotti, A.; Maroni, P.; Guarnieri, V.; Scillitani, A.; Silipigni, R.; Guerneri, S.; Maggiore, R.; Mari, G.; et al. Yes-Associated protein 1 is a novel calcium sensing receptor target in human parathyroid tumors. Int. J. Mol. Sci. 2021, 22, 2016. [Google Scholar] [CrossRef]
- Chung, H.; Lee, B.K.; Uprety, N.; Shen, W.; Lee, J.; Kim, J. Yap1 is dispensable for self-renewal but required for proper differentiation of mouse embryonic stem (ES) cells. EMBO Rep. 2016, 17, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, L.; Zhu, F.; Tan, W.; Lin, W.; Chen, D.; Sun, Q.; Xia, Z. Critical POU domain residues confer Oct4 uniqueness in somatic cell reprogramming. Sci. Rep. 2016, 6, 20818. [Google Scholar] [CrossRef]
- Sun, X.; Ren, Z.; Cun, Y.; Zhao, C.; Huang, X.; Zhou, J.; Hu, R.; Su, X.; Ji, L.; Li, P.; et al. Hippo-YAP signaling controls lineage differentiation of mouse embryonic stem cells through modulating the formation of super-enhancers. Nucleic Acids Res. 2020, 48, 7182–7196. [Google Scholar] [CrossRef]
- Takao, Y.; Yokota, T.; Koide, H. β-catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem. Biophyisical Res. Commun. 2007, 353, 699–705. [Google Scholar] [CrossRef]
- Forno, I.; Ferrero, S.; Russo, M.V.; Gazzano, G.; Giangiobbe, S.; Montanari, E.; Del Nero, A.; Rocco, B.; Albo, G.; Languino, L.R.; et al. Deregulation of MiR-34b/Sox2 predicts prostate cancer progression. PLoS ONE 2015, 10, e0310060. [Google Scholar] [CrossRef]
- Verdelli, C.; Avagliano, L.; Creo, P.; Guarnieri, V.; Scillitani, A.; Vicentini, L.; Steffano, G.B.; Beretta, E.; Soldati, L.; Costa, E.; et al. Tumour-associated fibroblasts contribute to neoangiogenesis in human parathyroid neoplasia. Endocr. Relat. Cancer. 2015, 22, 87–98. [Google Scholar] [CrossRef]
- Morotti, A.; Forno, I.; Verdelli, C.; Guarnieri, V.; Cetani, F.; Terrasi, A.; Silipigni, R.; Guerneri, S.; Andrè, V.; Scillitani, A.; et al. The oncosuppressors MEN1 and CDC73 are involved in lncRNA deregulation in human parathyroid tumors. J. Bone Miner. Res. 2020, 35, 2423–2431. [Google Scholar] [CrossRef]
- Novak, D.; Hüser, L.; Elton, J.J.; Umansky, V.; Altevogt, P.; Utikal, J. SOX2 in development and cancer biology. Semin. Cancer Biol. 2020, 67, 74–82. [Google Scholar] [CrossRef]
- She, S.; Wei, Q.; Kang, B.; Wang, Y.J. Cell cycle and pluripotency: Convergence on octamer-binding transcription factor 4 (Review). Mol. Med. Rep. 2017, 16, 6459–6466. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, V.; Rezende, N.C.; Scotland, K.B.; Shaffer, S.M.; Persson, J.L.; Gudas, L.J.; Mongan, N.P. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 2009, 18, 1093–1108. [Google Scholar] [CrossRef]
- Swain, N.; Thakur, M.; Pathak, J.; Swain, B. SOX2, OCT4 and NANOG: The core embryonic stem cell pluripotency regulators in oral carcinogenesis. J. Oral Maxillofac. Pathol. 2020, 24, 368–373. [Google Scholar] [CrossRef]
- Brewer, K.; Costa-Guda, J.; Arnold, A. Molecular genetic insights into sporadic primary hyperparathyroidism. Endocr. Relat. Cancer 2019, 26, R53–R72. [Google Scholar] [CrossRef]
- Mingione, A.; Verdelli, C.; Terranegra, A.; Soldati, L.; Corbetta, S. Molecular and clinical aspects of the target therapy with the calcimimetic cinacalcet in the treatment of parathyroid tumors. Curr. Cancer Drug Targets 2015, 15, 563–574. [Google Scholar] [CrossRef]
- Dwight, T.; Twigg, S.; Delbridge, L.; Wong, F.K.; Farnebo, F.; Richardson, A.L.; Nelson, A.; Zedenius, J.; Philips, J.; Larsson, C.; et al. Loss of heterozygosity in sporadic parathyroid tumours: Involvement of chromosome 1 and the MEN1 gene locus in 11q13. Clin. Endocrinol. (Oxf) 2000, 53, 85–92. [Google Scholar] [CrossRef]
- Falchetti, A. Genetics of parathyroids disorders: Overview. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 781–790. [Google Scholar] [CrossRef]
- Verma, N.K.; Gadi, A.; Maurizi, G.; Roy, U.B.; Mansukhani, A.; Basilico, C. Myeloid zinc finger 1 and GA Binding Protein co-operate with Sox2 in regulating the expression of Yes-Associated Protein 1 in cancer cells. Stem Cells 2017, 35, 2340–2350. [Google Scholar] [CrossRef]
- Bora-Singhal, N.; Nguyen, J.; Schaal, C.; Perumal, D.; Singh, S.; Coppola, D.; Chellappan, S. YAP1 regulates OCT4 activity and SOX2 expression to facilitate self-renewal and vascular mimicry of stem-like cells. Stem Cells 2015, 33, 1705–1718. [Google Scholar] [CrossRef]
- Metz, E.P.; Rizzino, A. Sox2 dosage: A critical determinant in the functions of Sox2 in both normal and tumor cells. J. Cell Physiol. 2019, 234, 19298–19306. [Google Scholar] [CrossRef]
- Kim, C.G.; Chung, I.Y.; Lim, Y.; Lee, Y.H.; Shin, S.Y. A Tcf/Lef element within the enhancer region of the human NANOG gene plays a role in promoter activation. Biochem. Biophys. Res. Commun. 2011, 410, 637–642. [Google Scholar] [CrossRef]
- Ye, X.; Wu, F.; Wu, C.; Wang, P.; Jung, K.; Gopal, K.; Ma, Y.; Li, L.; Lai, R. β-catenin, a Sox2 binding partner, regulates the DNA binding and transcriptional activity of Sox2 in breast cancer cells. Cell. Signal. 2014, 26, 492–501. [Google Scholar] [CrossRef]
- Ikeda, S.; Ishizaki, Y.; Shimizu, Y.; Fujimori, M.; Ojima, Y.; Okajima, M.; Sugino, K.; Asahara, T. Immunohistochemistry of cyclin D1 and beta-catenin, and mutational analysis of exon 3 of beta-catenin gene in parathyroid adenomas. Int. J. Oncol. 2002, 20, 463–466. [Google Scholar]
- Juhlin, C.C.; Haglund, F.; Villablanca, A.; Forsberg, L.; Sandelin, K.; Bränström, R.; Larsson, C.; Höög, A. Loss of expression for the Wnt pathway components adenomatous polyposis coli and glycogen synthase kinase 3-beta in parathyroid carcinomas. Int. J. Oncol. 2009, 34, 481–492. [Google Scholar]
- Cetani, F.; Pardi, E.; Banti, C.; Collecchi, P.; Viacava, P.; Borsari, S.; Fanelli, G.; Naccarato, A.G.; Saponaro, F.; Berti, P.; et al. Beta-catenin activation is not involved in sporadic parathyroid carcinomas and adenomas. Endocr. Relat. Cancer 2010, 17, 1–6. [Google Scholar] [CrossRef]
- Guarnieri, V.; Baorda, F.; Battista, C.; Bisceglia, M.; Balsamo, T.; Gruppioni, E.; Fiorentino, M.; Muscarella, L.A.; Coco, M.; Barbano, R.; et al. A rare S33C mutation of CTNNB1 encoding β-catenin in a parathyroid adenoma found in an Italian primary hyperparathyroid cohort. Endocrine 2012, 41, 152–155. [Google Scholar] [CrossRef]
- Juhlin, C.C.; Kiss, N.B.; Villablanca, A.; Haglund, F.; Nordenström, J.; Höög, A.; Larsson, C. Frequent promoter hypermethylation of the APC and RASSF1A tumour suppressors in parathyroid tumours. PLoS ONE 2010, 5, e9472. [Google Scholar] [CrossRef]
- Sulaiman, L.; Juhlin, C.C.; Nilsson, I.L.; Fotouhi, O.; Larsson, C.; Hashemi, J. Global and gene-specific promoter methylation analysis in primary hyperparathyroidism. Epigenetics 2013, 8, 646–655. [Google Scholar] [CrossRef]
- Dahl, E.; Wiesmann, F.; Woenckhaus, M.; Stoehr, R.; Wild, P.J.; Veeck, J.; Knüchel, R.; Klopocki, E.; Sauter, G.; Simon, R.; et al. Frequent loss of SFRP1 expression in multiple human solid tumours: Association with aberrant promoter methylation in renal cell carcinoma. Oncogene 2007, 26, 5680–5691. [Google Scholar] [CrossRef]
- Starker, L.F.; Svedlund, J.; Udelsman, R.; Dralle, H.; Åkerström, G.; Westin, G.; Lufton, R.P.; Björklund, P.; Carling, T. The DNA methylome of benign and malignant parathyroid tumors. Genes Chromosomes Cancer 2011, 50, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Svedlund, J.; Aurén, M.; Sundström, M.; Dralle, H.; Åkerström, G.; Björklund, P.; Westin, G. Aberrant WNT/β-catenin signaling in parathyroid carcinoma. Mol. Cancer 2010, 9, 924. [Google Scholar] [CrossRef]
- Sveldlund, J.; Barazeghi, E.; Stålbrg, P.; Hellman, P.; Åkerström, G.; Björklund, P.; Westin, G. The histone methyltransferase EZH2, an oncogene common to benign and malignant parathyroid tumors. Endocr. Relat. Cancer 2014, 21, 231–239. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef]
- Lustig, B.; Jerchow, B.; Sachs, M.; Weiler, S.; Pietsch, T.; Karsten, U.; van de Wetering, M.; Clevers, H.; Schlag, P.M.; Birchmeier, W.; et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell Biol. 2002, 22, 1184–1193. [Google Scholar] [CrossRef]

| Parameters | PAds Co-Expressing Core Stem Genes mRNAs | PAds with Undetectable Core Stem Genes mRNAs | p |
|---|---|---|---|
| n | 6 | 10 | |
| SOX2 mRNA levels | 5.964 ± 1.836 | 0.024 ± 0.023 | 0.0008 |
| POU5F1/OCT4 mRNA levels | 2.842 ± 0.527 | −0.2920 ± 0.227 | <0.0001 |
| NANOG mRNA levels | 3.727 ± 0.453 | 0.065 ± 0.070 | <0.0001 |
| Age (years) | 51.0 ± 6.1 | 59.0 ± 2.8 | 0.197 |
| Sex (female/male) | 6/0 | 7/3 | 0.250 |
| Plasma Ca2+ (mmol/L) | 1.51 ± 0.02 | 1.61 ± 0.03 | 0.039 |
| Serum total Ca (mg/dl) | 11.5 ± 0.1 | 11.6 ± 0.3 | 0.977 |
| Serum PTH (pg/ml) | 231.2 ± 44.8 | 228.5 ± 47.8 | 0.971 |
| Tumor size (cm) | 1.70 ± 0.15 | 2.24 ± 0.40 | 0.312 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdelli, C.; Morotti, A.; Tavanti, G.S.; Silipigni, R.; Guerneri, S.; Ferrero, S.; Vicentini, L.; Vaira, V.; Corbetta, S. The Core Stem Genes SOX2, POU5F1/OCT4, and NANOG Are Expressed in Human Parathyroid Tumors and Modulated by MEN1, YAP1, and β-catenin Pathways Activation. Biomedicines 2021, 9, 637. https://doi.org/10.3390/biomedicines9060637
Verdelli C, Morotti A, Tavanti GS, Silipigni R, Guerneri S, Ferrero S, Vicentini L, Vaira V, Corbetta S. The Core Stem Genes SOX2, POU5F1/OCT4, and NANOG Are Expressed in Human Parathyroid Tumors and Modulated by MEN1, YAP1, and β-catenin Pathways Activation. Biomedicines. 2021; 9(6):637. https://doi.org/10.3390/biomedicines9060637
Chicago/Turabian StyleVerdelli, Chiara, Annamaria Morotti, Giulia Stefania Tavanti, Rosamaria Silipigni, Silvana Guerneri, Stefano Ferrero, Leonardo Vicentini, Valentina Vaira, and Sabrina Corbetta. 2021. "The Core Stem Genes SOX2, POU5F1/OCT4, and NANOG Are Expressed in Human Parathyroid Tumors and Modulated by MEN1, YAP1, and β-catenin Pathways Activation" Biomedicines 9, no. 6: 637. https://doi.org/10.3390/biomedicines9060637
APA StyleVerdelli, C., Morotti, A., Tavanti, G. S., Silipigni, R., Guerneri, S., Ferrero, S., Vicentini, L., Vaira, V., & Corbetta, S. (2021). The Core Stem Genes SOX2, POU5F1/OCT4, and NANOG Are Expressed in Human Parathyroid Tumors and Modulated by MEN1, YAP1, and β-catenin Pathways Activation. Biomedicines, 9(6), 637. https://doi.org/10.3390/biomedicines9060637






