A Guide to the Generation of a 6-Hydroxydopamine Mouse Model of Parkinson’s Disease for the Study of Non-Motor Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
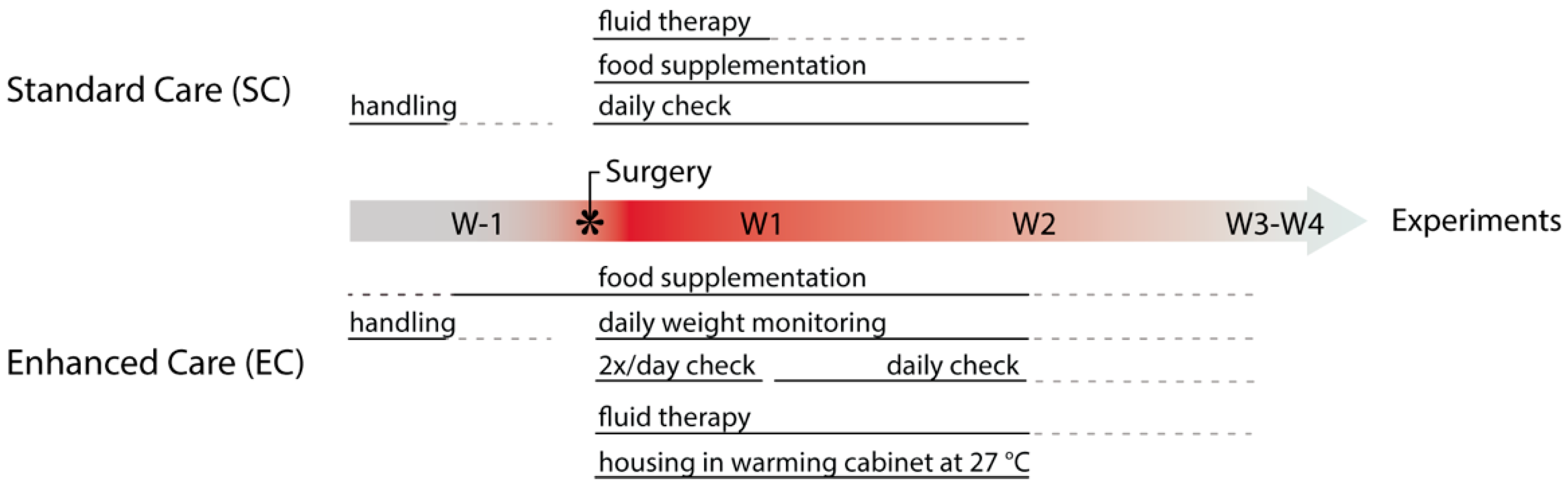
2.2. Pre-Operative Care
2.3. 6-OHDA Surgery
2.4. Post-Operative Care
2.5. Immunohistochemistry and Image Analysis
2.6. Western Blotting
2.7. Statistics
| Type | Identification | Treatment | Reference |
|---|---|---|---|
| General Health | Appearance and activity pattern of the animal in home cage including interaction with environment, cage mates and nest building. | Further hands-on examination. | [54] |
| Body Condition | Visual assessment of body shape and hands-on examination by passing the fingers over sacroiliac bones. | Assess body condition score and further examine with attention to humane endpoints. | [55] |
| Pain or Distress | Mouse shows reluctance to move, failure to groom and unkempt appearance of fur coat, lack of appetite and thin body condition, loss of nest-building behavior, in some cases vocalization. Determined by mouse facial expression orbital tightening (squinting), nose bulging, cheek bulging, drawing of the ears back behind the head. | Administration of buprenorphine and consideration of euthanasia based on humane endpoints. | [56,57] |
| Dehydration | Weak appearance with recessed eyes and fuzzy fur. Skin turgor is reduced and pinched skin over the back remains tented. | Subcutaneous or intraperitoneal fluid replacement therapy with warmed Lactated Ringer’s solution, sterile saline (0.9%) or glucose (5%). | [47] |
| Hypothermia | Animals are cool at touch and have a body temperature lower than 36.5 °C. | Increase in ambient temperature of housing, cages placed in warming cabinet or on a thermal blanket, administration of warm fluids. | [58] |
| Aphagia and Adipsia | Measurement of food and water consumption. Body weight monitoring. | Easy access to palatable food supplementation, hydration complemented with glucose solution. | [59,60] |
| Penile Prolapse (Paraphimosis) * | Swollen, distended and reddened penis. | Lubrication and placement of soft bedding to decrease swelling, hydration support. | [49,54] |
| Urethral Obstruction (urethral plugs) * | Firm, cream-colored proteinaceous material observed at tip of penis. | Remove plug with lubrication and local analgesics or short anesthesia. | [61,62] |
3. Results
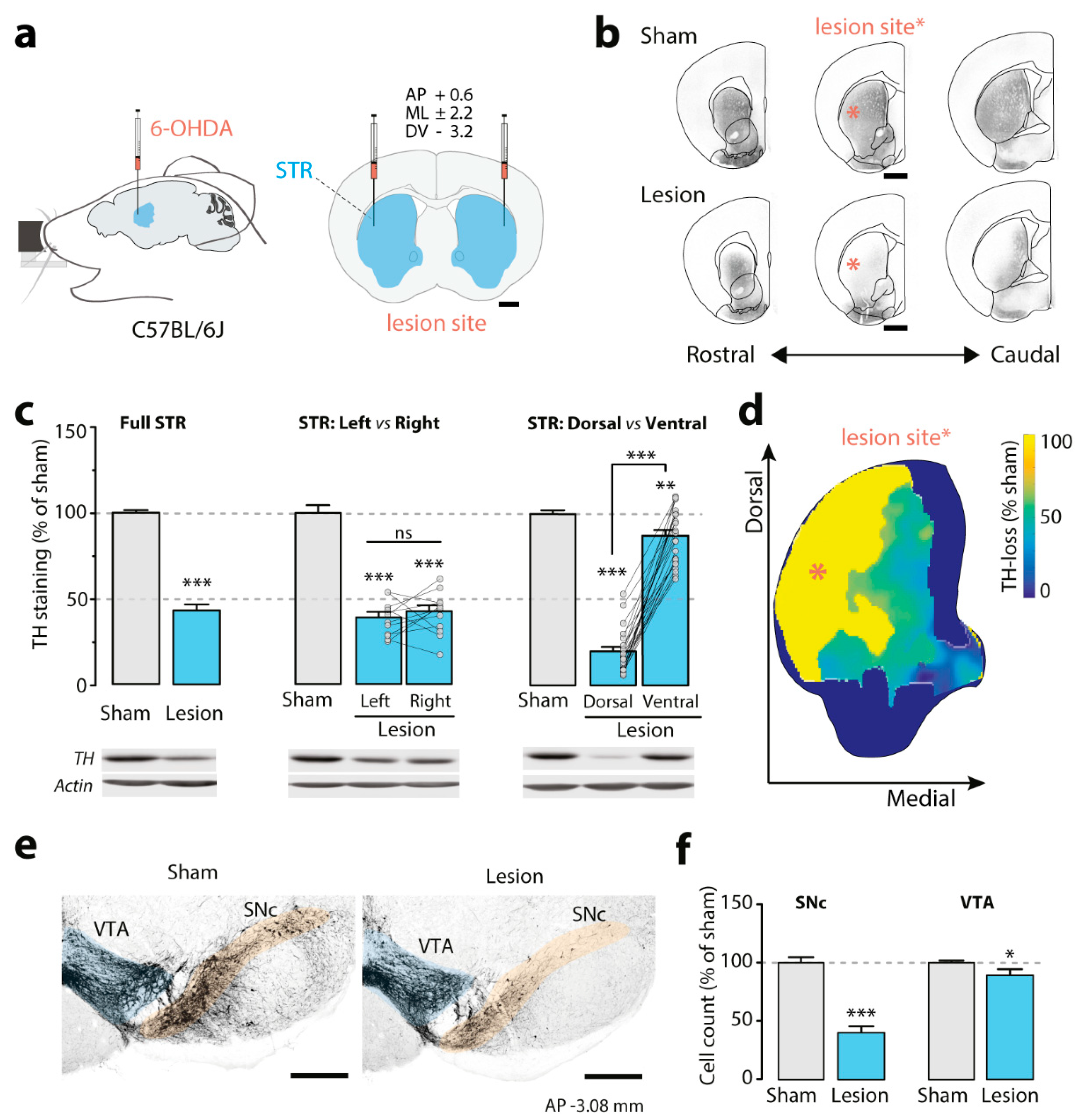
3.1. Effect of the Bilateral Partial 6-OHDA Lesion on TH Immunoreactivity
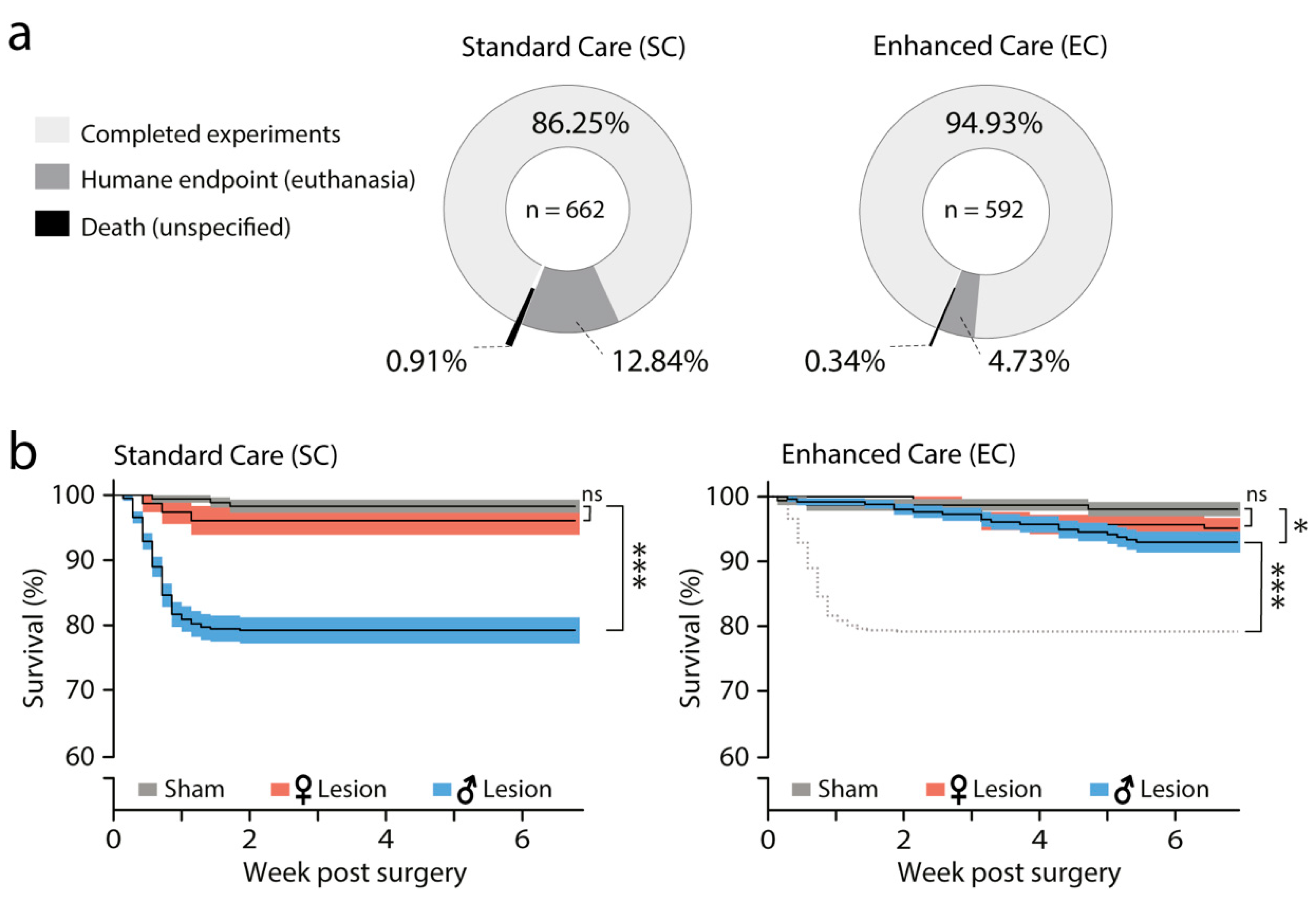
3.2. Effect of SC and EC Protocols on Survival
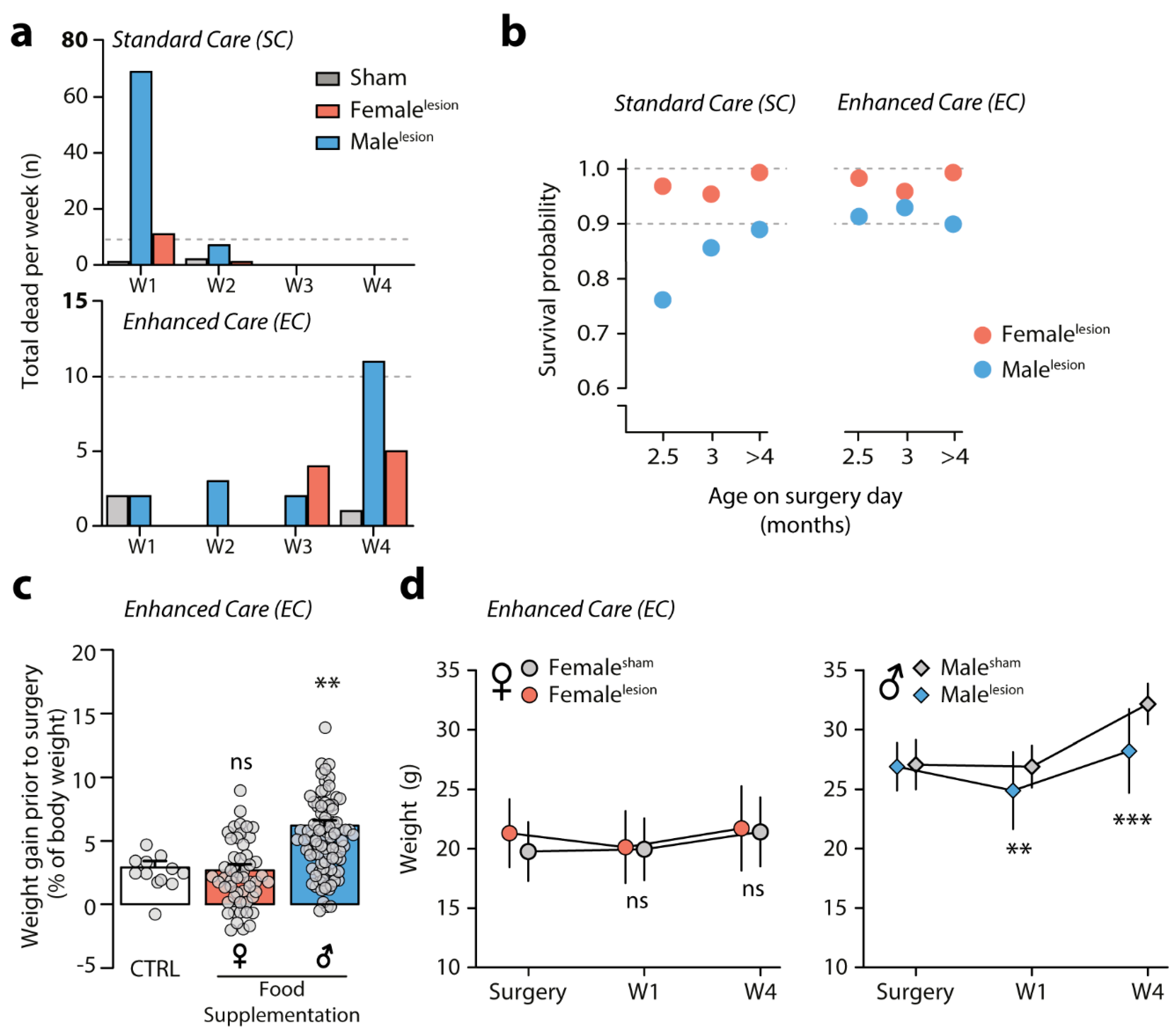
3.3. Survival of 6-OHDA Malelesion Mice Depends on Age and Can Be Predicted by Weight
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Tieu, K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect Med. 2011, 1, a009316. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.M.; Ko, H.S.; Dawson, V.L. Genetic animal models of Parkinson’s disease. Neuron 2010, 66, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E. A critical appraisal of the premotor symptoms of Parkinson’s disease: Potential usefulness in early diagnosis and design of neuroprotective trials. Mov. Disord. 2011, 26, 775–783. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rub, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Blandini, F.; Armentero, M.T. Dopamine receptor agonists for Parkinson’s disease. Expert Opin. Investig. Drugs 2014, 23, 387–410. [Google Scholar] [CrossRef]
- Mercuri, N.B.; Bernardi, G. The ‘magic’ of L-dopa: Why is it the gold standard Parkinson’s disease therapy? Trends Pharmacol. Sci. 2005, 26, 341–344. [Google Scholar] [CrossRef]
- Tranzer, J.P.; Thoenen, H. An electron microscopic study of selective, acute degeneration of sympathetic nerve terminals after administration of 6-hydroxydopamine. Experientia 1968, 24, 155–156. [Google Scholar] [CrossRef]
- Ungerstedt, U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 1968, 5, 107–110. [Google Scholar] [CrossRef]
- Buhidma, Y.; Rukavina, K.; Chaudhuri, K.R.; Duty, S. Potential of animal models for advancing the understanding and treatment of pain in Parkinson’s disease. NPJ Parkinson Dis. 2020, 6. [Google Scholar] [CrossRef]
- Simola, N.; Morelli, M.; Carta, A.R. The 6-hydroxydopamine model of Parkinson’s disease. Neurotox. Res. 2007, 11, 151–167. [Google Scholar] [CrossRef]
- Luthman, J.; Fredriksson, A.; Sundstrom, E.; Jonsson, G.; Archer, T. Selective lesion of central dopamine or noradrenaline neuron systems in the neonatal rat: Motor behavior and monoamine alterations at adult stage. Behav. Brain Res. 1989, 33, 267–277. [Google Scholar] [CrossRef]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.; Benabid, A.L.; Sadoul, R.; Verna, J.M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef]
- Cadet, J.L.; Brannock, C. Free radicals and the pathobiology of brain dopamine systems. Neurochem. Int. 1998, 32, 117–131. [Google Scholar] [CrossRef]
- Glinka, Y.Y.; Youdim, M.B. Inhibition of mitochondrial complexes I and IV by 6-hydroxydopamine. Eur. J. Pharmacol. 1995, 292, 329–332. [Google Scholar] [CrossRef]
- Faivre, F.; Joshi, A.; Bezard, E.; Barrot, M. The hidden side of Parkinson’s disease: Studying pain, anxiety and depression in animal models. Neurosci. Biobehav. Rev. 2019, 96, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Magnard, R.; Vachez, Y.; Carcenac, C.; Krack, P.; David, O.; Savasta, M.; Boulet, S.; Carnicella, S. What can rodent models tell us about apathy and associated neuropsychiatric symptoms in Parkinson’s disease? Transl. Psychiatry 2016, 6, e753. [Google Scholar] [CrossRef]
- McDowell, K.; Chesselet, M.F. Animal models of the non-motor features of Parkinson’s disease. Neurobiol. Dis. 2012, 46, 597–606. [Google Scholar] [CrossRef]
- Medeiros, D.C.; Lopes Aguiar, C.; Moraes, M.F.D.; Fisone, G. Sleep Disorders in Rodent Models of Parkinson’s Disease. Front. Pharmacol. 2019, 10, 1414. [Google Scholar] [CrossRef] [PubMed]
- Bonito-Oliva, A.; Pignatelli, M.; Spigolon, G.; Yoshitake, T.; Seiler, S.; Longo, F.; Piccinin, S.; Kehr, J.; Mercuri, N.B.; Nisticò, R.; et al. Cognitive impairment and dentate gyrus synaptic dysfunction in experimental parkinsonism. Biol. Psychiatry 2014, 75, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Breese, G.R.; Traylor, T.D. Depletion of brain noradrenaline and dopamine by 6-hydroxydopamine. Br. J. Pharmacol. 1971, 42, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Reader, T.A.; Gauthier, P. Catecholamines and serotonin in the rat central nervous system after 6-OHDA, 5-7-DHT and p-CPA. J. Neural Transm. 1984, 59, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.M.; Barbieiro, J.; Lima, M.M.; Dombrowski, P.A.; Andreatini, R.; Vital, M.A. Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson’s disease are predominantly associated with serotonin and dopamine. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.M.; Falquetto, B.; Moreira, T.S.; Takakura, A.C. Orexinergic neurons are involved in the chemosensory control of breathing during the dark phase in a Parkinson’s disease model. Exp. Neurol. 2018, 309, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Hurley, S.W.; Johnson, A.K. The role of the lateral hypothalamus and orexin in ingestive behavior: A model for the translation of past experience and sensed deficits into motivated behaviors. Front. Syst. Neurosci. 2014, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T. The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat. Rev. Neurosci. 2007, 8, 171–181. [Google Scholar] [CrossRef]
- Williams, R.H.; Burdakov, D. Hypothalamic orexins/hypocretins as regulators of breathing. Expert Rev. Mol. Med. 2008, 10, e28. [Google Scholar] [CrossRef]
- Faull, R.L.; Laverty, R. Changes in dopamine levels in the corpus striatum following lesions in the substantia nigra. Exp. Neurol. 1969, 23, 332–340. [Google Scholar] [CrossRef]
- Jeon, B.S.; Jackson-Lewis, V.; Burke, R.E. 6-Hydroxydopamine lesion of the rat substantia nigra: Time course and morphology of cell death. Neurodegeneration 1995, 4, 131–137. [Google Scholar] [CrossRef]
- Sarre, S.; Yuan, H.; Jonkers, N.; Van Hemelrijck, A.; Ebinger, G.; Michotte, Y. In vivo characterization of somatodendritic dopamine release in the substantia nigra of 6-hydroxydopamine-lesioned rats. J. Neurochem. 2004, 90, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Stott, S.R.; Barker, R.A. Time course of dopamine neuron loss and glial response in the 6-OHDA striatal mouse model of Parkinson’s disease. Eur. J. Neurosci. 2014, 39, 1042–1056. [Google Scholar] [CrossRef]
- Przedborski, S.; Levivier, M.; Jiang, H.; Ferreira, M.; Jackson-Lewis, V.; Donaldson, D.; Togasaki, D.M. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience 1995, 67, 631–647. [Google Scholar] [CrossRef]
- Sauer, H.; Oertel, W.H. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: A combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 1994, 59, 401–415. [Google Scholar] [CrossRef]
- Ungerstedt, U.; Arbuthnott, G.W. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970, 24, 485–493. [Google Scholar] [CrossRef]
- Von Voigtlander, P.F.; Moore, K.E. Turning behavior of mice with unilateral 6-hydroxydopamine lesions in the striatum: Effects of apomorphine, L-DOPA, amanthadine, amphetamine and other psychomotor stimulants. Neuropharmacology 1973, 12, 451–462. [Google Scholar] [CrossRef]
- Branchi, I.; D’Andrea, I.; Armida, M.; Carnevale, D.; Ajmone-Cat, M.A.; Pèzzola, A.; Potenza, R.L.; Morgese, M.G.; Cassano, T.; Minghetti, L.; et al. Striatal 6-OHDA lesion in mice: Investigating early neurochemical changes underlying Parkinson’s disease. Behav. Brain Res. 2010, 208, 137–143. [Google Scholar] [CrossRef] [PubMed]
- De Leonibus, E.; Pascucci, T.; Lopez, S.; Oliverio, A.; Amalric, M.; Mele, A. Spatial deficits in a mouse model of Parkinson disease. Psychopharmacology 2007, 194, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Masini, D.; Lopes-Aguiar, C.; Bonito-Oliva, A.; Papadia, D.; Andersson, R.; Fisahn, A.; Fisone, G. The histamine H3 receptor antagonist thioperamide rescues circadian rhythm and memory function in experimental parkinsonism. Transl. Psychiatry 2017, 7, e1088. [Google Scholar] [CrossRef]
- Bonito-Oliva, A.; Masini, D.; Fisone, G. A mouse model of non-motor symptoms in Parkinson’s disease: Focus on pharmacological interventions targeting affective dysfunctions. Front. Behav. Neurosci. 2014, 8, 290. [Google Scholar] [CrossRef]
- Masini, D.; Bonito-Oliva, A.; Bertho, M.; Fisone, G. Inhibition of mTORC1 Signaling Reverts Cognitive and Affective Deficits in a Mouse Model of Parkinson’s Disease. Front. Neurol. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, K.; Hurst, J.L. Optimising reliability of mouse performance in behavioural testing: The major role of non-aversive handling. Sci. Rep. 2017, 7, 44999. [Google Scholar] [CrossRef]
- Hurst, J.L.; West, R.S. Taming anxiety in laboratory mice. Nat. Methods 2010, 7, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Sztainberg, Y.; Chen, A. An environmental enrichment model for mice. Nat. Protoc. 2010, 5, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Kronenberger, J.P.; Medioni, J. Food neophobia in wild and laboratory mice (Mus musculus domesticus). Behav. Processes 1985, 11, 53–59. [Google Scholar] [CrossRef]
- Santini, E.; Valjent, E.; Usiello, A.; Carta, M.; Borgkvist, A.; Girault, J.-A.; Hervé, D.; Greengard, P.; Fisone, G. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J. Neurosci. 2007, 27, 6995–7005. [Google Scholar] [CrossRef]
- Bekkevold, C.M.; Robertson, K.L.; Reinhard, M.K.; Battles, A.H.; Rowland, N.E. Dehydration parameters and standards for laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 233–239. [Google Scholar]
- Rentsch, P.; Stayte, S.; Morris, G.P.; Vissel, B. Time dependent degeneration of the nigrostriatal tract in mice with 6-OHDA lesioned medial forebrain bundle and the effect of activin A on L-Dopa induced dyskinesia. BMC Neurosci. 2019, 20, 5. [Google Scholar] [CrossRef]
- Thiele, S.L.; Warre, R.; Nash, J.E. Development of a unilaterally-lesioned 6-OHDA mouse model of Parkinson’s disease. J. Vis. Exp. 2012, 14, 3234. [Google Scholar] [CrossRef]
- Caggiano, V.; Leiras, R.; Goñi-Erro, H.; Masini, D.; Bellardita, C.; Bouvier, J.; Caldeira, V.; Fisone, G.; Kiehn, O. Midbrain circuits that set locomotor speed and gait selection. Nature 2018, 553, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Ragan, T.; Kadiri, L.R.; Venkataraju, K.U.; Bahlmann, K.; Sutin, J.; Taranda, J.; Arganda-Carreras, I.; Kim, Y.; Seung, H.S.; Osten, P. Serial two-photon tomography for automated ex vivo mouse brain imaging. Nat. Methods 2012, 9, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Renier, N.; Adams, E.L.; Kirst, C.; Wu, Z.; Azevedo, R.; Kohl, J.; Autry, A.E.; Kadiri, L.; Venkataraju, K.U.; Zhou, Y.; et al. Mapping of Brain Activity by Automated Volume Analysis of Immediate Early Genes. Cell 2016, 165, 1789–1802. [Google Scholar] [CrossRef] [PubMed]
- Jager, K.J.; van Dijk, P.C.; Zoccali, C.; Dekker, F.W. The analysis of survival data: The Kaplan-Meier method. Kidney Int. 2008, 74, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, T.; Foltz, C.; Karlsson, E.; Linton, C.G.; Smith, J.M. Health Evaluation of Experimental Laboratory Mice. Curr. Protoc. Mouse Biol. 2012, 2, 145–165. [Google Scholar] [CrossRef]
- Ullman-Cullere, M.H.; Foltz, C.J. Body condition scoring: A rapid and accurate method for assessing health status in mice. Lab. Anim. Sci. 1999, 49, 319–323. [Google Scholar]
- Carstens, E.; Moberg, G.P. Recognizing pain and distress in laboratory animals. ILAR J. 2000, 41, 62–71. [Google Scholar] [CrossRef]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; LaCroix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 2010, 7, 447–449. [Google Scholar] [CrossRef]
- Beale, C.N.; Esmail, M.Y.; Aguiar, A.M.; Coughlin, L.; Merley, A.L.; Alarcon Falconi, T.M.; Perkins, S.E. Use of Air-activated Thermal Devices during Recovery after Surgery in Mice. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 392–400. [Google Scholar] [CrossRef]
- Szczypka, M.S.; Rainey, M.A.; Kim, D.S.; Alaynick, W.A.; Marck, B.T.; Matsumoto, A.M.; Palmiter, R.D. Feeding behavior in dopamine-deficient mice. Proc. Natl. Acad. Sci. USA 1999, 96, 12138–12143. [Google Scholar] [CrossRef]
- Ungerstedt, U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol. Scand. Suppl. 1971, 367, 95–122. [Google Scholar] [CrossRef]
- Taylor, D.M. Urethral plugs and urine retention in male mice. Lab. Anim. 1985, 19, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.; Trower, C.; Hough, T.A.; Stewart, M.; Cheeseman, M.T. Urethral obstruction by seminal coagulum is associated with medetomidine-ketamine anesthesia in male mice on C57BL/6J and mixed genetic backgrounds. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 296–299. [Google Scholar]
- Bendele, A.M. Urologic Syndrome, Mouse. In Urinary System Monographs on Pathology of Laboratory Animals; Jones, T.C., Hard, G.C.H., Mohr, U., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 456–462. [Google Scholar]
- Lundblad, M.; Picconi, B.; Lindgren, H.; Cenci, M.A. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: Relation to motor and cellular parameters of nigrostriatal function. Neurobiol. Dis. 2004, 16, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Boix, J.; Padel, T.; Paul, G. A partial lesion model of Parkinson’s disease in mice–characterization of a 6-OHDA-induced medial forebrain bundle lesion. Behav. Brain Res. 2015, 284, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Glajch, K.E.; Fleming, S.M.; Surmeier, D.J.; Osten, P. Sensorimotor assessment of the unilateral 6-hydroxydopamine mouse model of Parkinson’s disease. Behav. Brain Res. 2012, 230, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Thiele, S.L.; Warre, R.; Khademullah, C.S.; Fahana, N.; Lo, C.; Lam, D.; Talwar, S.; Johnston, T.H.; Brotchie, J.M.; Nash, J.E. Generation of a model of L-DOPA-induced dyskinesia in two different mouse strains. J. Neurosci. Methods 2011, 197, 193–208. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Stafford, J.M.; Pendergast, J.S. High-Fat Feeding Does Not Disrupt Daily Rhythms in Female Mice because of Protection by Ovarian Hormones. Front. Endocrinol. 2017, 8, 44. [Google Scholar] [CrossRef]
- Ichitani, Y.; Okamura, H.; Matsumoto, Y.; Nagatsu, I.; Ibata, Y. Degeneration of the nigral dopamine neurons after 6-hydroxydopamine injection into the rat striatum. Brain Res. 1991, 549, 350–353. [Google Scholar] [CrossRef]
- Bezard, E.; Dovero, S.; Imbert, C.; Boraud, T.; Gross, C.E. Spontaneous long-term compensatory dopaminergic sprouting in MPTP-treated mice. Synapse 2000, 38, 363–368. [Google Scholar] [CrossRef]
- Heikkila, R.E.; Sonsalla, P.K. The MPTP-treated mouse as a model of parkinsonism: How good is it? Neurochem. Int. 1992, 20. [Google Scholar] [CrossRef]
- Johannessen, J.N.; Chiueh, C.C.; Burns, R.S.; Markey, S.P. Differences in the metabolism of MPTP in the rodent and primate parallel differences in sensitivity to its neurotoxic effects. Life Sci. 1985, 36, 219–224. [Google Scholar] [CrossRef]
- Francardo, V.; Recchia, A.; Popovic, N.; Andersson, D.; Nissbrandt, H.; Cenci, M.A. Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkinson’s disease. Neurobiol. Dis. 2011, 42, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Sebastianutto, I.; Maslava, N.; Hopkins, C.R.; Cenci, M.A. Validation of an improved scale for rating l-DOPA-induced dyskinesia in the mouse and effects of specific dopamine receptor antagonists. Neurobiol. Dis. 2016, 96, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Leentjens, A.F.; Moonen, A.J.; Dujardin, K.; Marsh, L.; Martinez-Martin, P.; Richard, I.H.; Starkstein, S.E.; Köhler, S. Modeling depression in Parkinson disease: Disease-specific and nonspecific risk factors. Neurology 2013, 81, 1036–1043. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Falup Pecurariu, C.; Odin, P.; van Hilten, J.J.; Antonini, A.; Rojo-Abuin, J.M.; Borges, V.; Trenkwalder, C.; Aarsland, D.; Brooks, D.J.; et al. Gender-related differences in the burden of non-motor symptoms in Parkinson’s disease. J. Neurol. 2012, 259, 1639–1647. [Google Scholar] [CrossRef]
- Solla, P.; Cannas, A.; Ibba, F.C.; Loi, F.; Corona, M.; Orofino, G.; Marrosu, M.G.; Marrosu, F. Gender differences in motor and non-motor symptoms among Sardinian patients with Parkinson’s disease. J. Neurol Sci. 2012, 323, 33–39. [Google Scholar] [CrossRef]
- Szewczyk-Krolikowski, K.; Tomlinson, P.; Nithi, K.; Wade-Martins, R.; Talbot, K.; Ben-Shlomo, Y.; Hu, M.T. The influence of age and gender on motor and non-motor features of early Parkinson’s disease: Initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat. Disord. 2014, 20, 99–105. [Google Scholar] [CrossRef]
- Yagi, S.; Yoshikawa, E.; Futatsubashi, M.; Yokokura, M.; Yoshihara, Y.; Torizuka, T.; Ouchi, Y. Progression from unilateral to bilateral parkinsonism in early Parkinson disease: Implication of mesocortical dopamine dysfunction by PET. J. Nucl. Med. 2010, 51, 1250–1257. [Google Scholar] [CrossRef]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114, 2283–2301. [Google Scholar] [CrossRef]
- Hirsch, E.; Graybiel, A.M.; Agid, Y.A. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 1988, 334, 345–348. [Google Scholar] [CrossRef]
- Kish, S.J.; Shannak, K.; Hornykiewicz, O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N. Engl. J. Med. 1988, 318, 876–880. [Google Scholar] [CrossRef]
- Alberico, S.L.; Cassell, M.D.; Narayanan, N.S. The Vulnerable Ventral Tegmental Area in Parkinson’s Disease. Basal Ganglia 2015, 5, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol. Chem. Neuropathol. 1991, 14, 153–197. [Google Scholar] [CrossRef] [PubMed]
- Barone, P. Treatment of depressive symptoms in Parkinson’s disease. Eur. J. Neurol. 2011, 18 (Suppl. 1), 11–15. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.H.; Depboylu, C.; Hermanns, G.; Maurer, L.; Windolph, A.; Oertel, W.H.; Ries, V.; Höglinger, G.U. Long-term treatment with L-DOPA or pramipexole affects adult neurogenesis and corresponding non-motor behavior in a mouse model of Parkinson’s disease. Neuropharmacology 2015, 95, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Woodley, S.K.; Baum, M.J. Effects of sex hormones and gender on attraction thresholds for volatile anal scent gland odors in ferrets. Horm. Behav. 2003, 44, 110–118. [Google Scholar] [CrossRef]
- Breen, D.P.; Vuono, R.; Nawarathna, U.; Fisher, K.; Shneerson, J.M.; Reddy, A.B.; Barker, R.A. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014, 71, 589–595. [Google Scholar] [CrossRef]
| Domain | Test | Phenotype | Pharmacological Intervention | References | |
|---|---|---|---|---|---|
| Motor | Spontaneous locomotion | Open field | No effect | - | [40,41] |
| Novel home cage | No effect | - | [21] | ||
| Vertical activity | Cylinder | Reduced rearing | - | [21] | |
| Gait pattern | Treadmill (ventral plane videography) | Impaired hindlimb gait dynamics | - | [40] | |
| Affective | Depression | Porsolt forced swim | Increased immobility | L-DOPA = Pramipexole + Reboxetine + Rapamycin + | [40,41] |
| Tail suspension | Increased immobility | - | [40] | ||
| Anxiety | Open field center zone | Center avoidance (thigmotaxis) | Rapamycin + | [40,41] | |
| Elevated plus maze | Reduced exploration of open arms | L-DOPA = Pramipexole + Reboxetine + Thioperamide = Rapamycin + | [39,40,41] | ||
| Light-dark box | Increased latency to enter the bright chamber | Thioperamide = | [39] | ||
| Cognitive | Novelty detection | Novel object recognition | Deficit in long-term recognition memory | L-DOPA + Pramipexole = Thioperamide + Rapamycin + | [21,39,41] |
| Circadian | Circadian activity | Circadian activity rhythm in social environment | Reduced activity during the active period of the 24 h cycle | Thioperamide + | [39] |
| Endogenous activity cycle in social environment during constant darkness | Disruption of the endogenous circadian rhythm (activity pattern fragmentation) | - | [39] | ||
| Olfactory | Olfactory discrimination | Olfactory habituation/dishabituation | Deficit of olfactory discrimination | - | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masini, D.; Plewnia, C.; Bertho, M.; Scalbert, N.; Caggiano, V.; Fisone, G. A Guide to the Generation of a 6-Hydroxydopamine Mouse Model of Parkinson’s Disease for the Study of Non-Motor Symptoms. Biomedicines 2021, 9, 598. https://doi.org/10.3390/biomedicines9060598
Masini D, Plewnia C, Bertho M, Scalbert N, Caggiano V, Fisone G. A Guide to the Generation of a 6-Hydroxydopamine Mouse Model of Parkinson’s Disease for the Study of Non-Motor Symptoms. Biomedicines. 2021; 9(6):598. https://doi.org/10.3390/biomedicines9060598
Chicago/Turabian StyleMasini, Débora, Carina Plewnia, Maëlle Bertho, Nicolas Scalbert, Vittorio Caggiano, and Gilberto Fisone. 2021. "A Guide to the Generation of a 6-Hydroxydopamine Mouse Model of Parkinson’s Disease for the Study of Non-Motor Symptoms" Biomedicines 9, no. 6: 598. https://doi.org/10.3390/biomedicines9060598
APA StyleMasini, D., Plewnia, C., Bertho, M., Scalbert, N., Caggiano, V., & Fisone, G. (2021). A Guide to the Generation of a 6-Hydroxydopamine Mouse Model of Parkinson’s Disease for the Study of Non-Motor Symptoms. Biomedicines, 9(6), 598. https://doi.org/10.3390/biomedicines9060598






